Introduction
An abscopal effect, defined as regression of a tumor distant from a localized radiation field, was first described by Mole in 1953.1 This phenomenon has attracted the attention of researchers since the recent emergence of immune checkpoint inhibitors (ICI).2, 3, 4 In 2012, Postow et al5 reported the first case of an abscopal effect in a patient with metastatic melanoma treated by radiation therapy (RT) and ipilimumab, which is a cytotoxic T-lymphocyte-associated antigen 4 inhibitor. This study demonstrated the correlation between abscopal effects and the immune system.5 Since the introduction of ICI, case reports of abscopal effects have increased in several types of cancers, including lymphoma, melanoma, and renal cell carcinoma.6 On the other hand, an abscopal effect without combination ICI is rare. Regarding RT, the optimal dose, fractionation, and target volume in which abscopal effects can occur are unknown regardless of combination ICI.7, 8, 9 We report a first case of endometrial cancer suggesting an abscopal effect after localized intensity modulated RT (IMRT).
Case Report
An 88-year-old woman with postmenopausal bleeding presented to the Department of Obstetrics and Gynecology at our hospital. She underwent hysteroscopy and was found to have pyometra. Biopsy of the endometrium revealed no evidence of malignancy, but endometrial cytology suggested adenocarcinoma. Subsequent endometrial curettage confirmed the diagnosis of adenocarcinoma. Pelvic magnetic resonance imaging detected a localized endometrial mass at the corpus of the uterus. The patient had no evidence of metastasis to a lymph node or distant organ on enhanced computed tomography (CT). Her serum CA125 level was not measured at that time. She had several past medical conditions, including breast cancer after mastectomy with adjuvant tamoxifen, mild dementia, encephalorrhagia, and parkinsonian syndrome. She underwent laparoscopic-assisted modified radical hysterectomy with bilateral salphingoopherectomy, but did not undergo lymphadenectomy owing to her advanced age and comorbidities. She was pathologically diagnosed with International Federation of Gynecology and Obstetrics stage IA endometrial cancer of histologic grade 3. She received no adjuvant therapy.
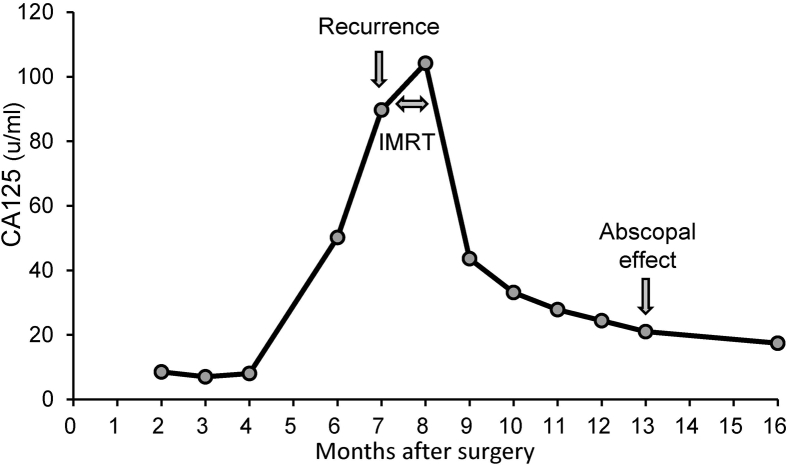
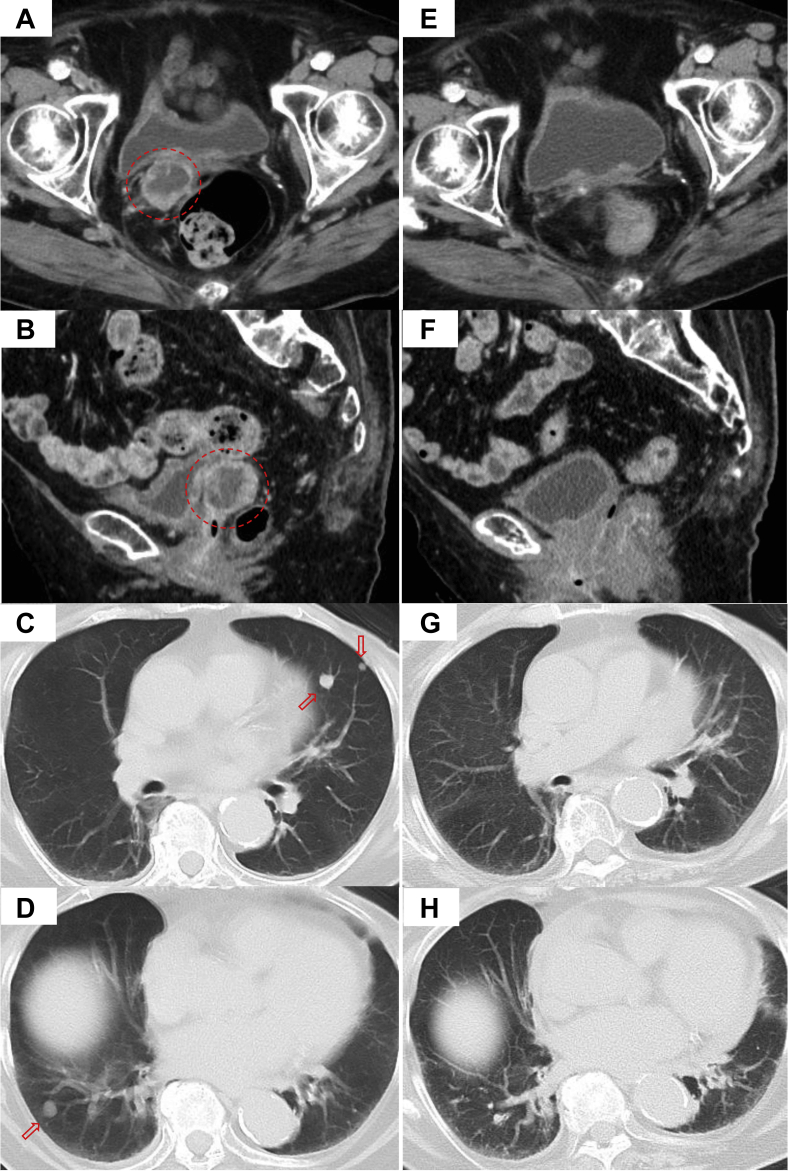
Her serum CA125 level started to increase at 6 months after surgery, as shown in Figure 1. She had no abnormal finding with hysteroscopy. The vaginal cuff cytology revealed no evidence of malignancy. However, the patient developed vaginal cuff recurrence and multiple lung metastases based on enhanced CT 7 months after surgery, as shown in Figure 2A-D. 18F-fluorodeoxyglucose positron emission tomography/CT was not performed because the enhanced CT seemed to definitively demonstrate metastases obviously. As the patient was considered to be unable to tolerate chemotherapy owing to her comorbidities, she received salvage RT only for the local recurrent tumor without systemic therapy.
Figure 1.
Change in the serum CA125 level after surgery.
Figure 2.
Contrast-enhanced computed tomography before radiation therapy (A-D) and at 5 months after radiation therapy (E-H). Local recurrent tumor (A, B, circle). Multiple lung metastases (C, D, arrows).
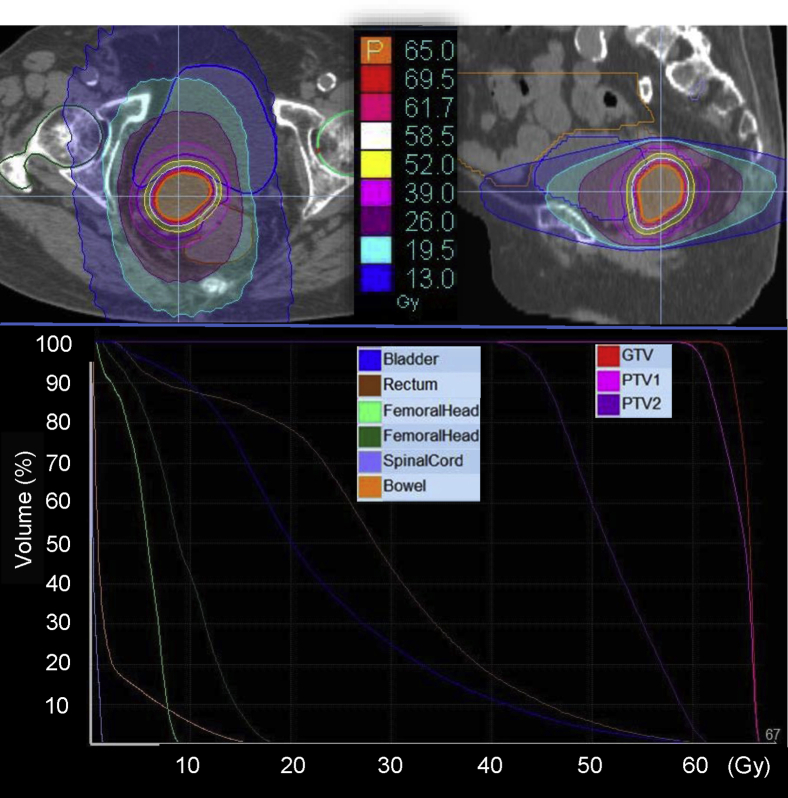
The patient was treated by IMRT using helical tomotherapy. She was immobilized in the supine position with the BodyFIX system (Medical Intelligence, Schwabmuenchen, Germany) and simulated by CT with a 2.5-mm slice thickness. All target volumes and the organs at risk (OAR) were delineated on the RayStation treatment planning system (RaySearch Medical Laboratories AB, Stockholm, Sweden). The gross tumor volume was defined as a gross disease according to enhanced CT. The clinical target volume (CTV) included the gross tumor volume with a margin of 3.0 mm. The planning target volume (PTV) 1 was defined the same as the CTV, and the PTV2 was defined as the CTV plus an 8.0-mm margin excluding the PTV1. The OARs included the rectum, bladder, bilateral femoral head, bowel, and spinal cord. Tomotherapy plans were generated using 6-MV x-ray beams of Radixact X9 (Accuray, Sunnyvale, USA).10 The Precision Treatment Planning System (Accuray) was used with the superposition algorithm for planned calculation. Regarding the tomotherapy treatment conditions, a 2.5-cm field width, pitch of 0.287, and modulation factor of 2.0 were used. The prescription doses of PTV1 and PTV2 were 65 and 52 Gy, respectively, in 26 fractions over 38 days using the simultaneous integrated boost technique. The plan was optimized to achieve a target coverage of 50% (D50%) of the PTV1 and PTV2 receiving 100% of each prescription dose and to minimize the dose to the OAR using dose-volume histogram constraints. The dose-volume histogram and dose distributions are shown in Figure 3. Megavoltage computed tomographic images were acquired for daily set-up verification. IMRT was performed successfully without acute complications or prolonged RT period.
Figure 3.
Dose distribution and dose volume histogram for the patient with recurrent endometrial cancer after surgery. Abbreviations: GTV = gross target volume; PTV = planning treatment volume.
The patient was followed closely without anticancer therapy or late complications. Enhanced CT at 5 months after the completion of IMRT is shown in Figure 2E,F. The patient achieved a complete response at 5 months after IMRT. Serum CA125 decreased to a normal level (ie, ≤ 35.0 u/mL), as shown in Figure 1. Chest CT at 5 months after IMRT is shown in Figure 2G,H. Of note, the multiple lung metastases had disappeared on chest CT. The patient experienced episodes of a drooping eyelid at 8 months after IMRT and was diagnosed with multiple brain metastases. The multiple lung metastases that had existed before pelvic IMRT were no longer there. The patient is still alive today, 11 months after IMRT, with re-emergence of multiple lung metastases.
Discussion
We report the first case of an abscopal effect in a patient with endometrial cancer treated by localized RT alone, which resulted in the disappearance of multiple lung metastases. The patient did not receive anticancer therapy except pelvic IMRT. This phenomenon is rare in both the setting of RT monotherapy and combination therapy with ICI. The increased reproducibility of abscopal effects is of interest. Our present case had 4 features regarding the development of an abscopal effect: prescription of a curable dose by IMRT, no combination of ICI, a markedly elderly patient, and a patient with endometrial cancer.
The patient in our present case was irradiated by a high dose of 65 Gy (2.5 Gy per fraction) in spite of existence of lung metastases because she was considered unable to tolerate systemic anticancer therapy for subsequent recurrence. The dose of 65 Gy corresponded to approximately 68 Gy in equivalent 2-Gy fractions using the linear quadratic model with α/β = 10 Gy. Common prescribed doses were 30 to 40 Gy in previous case reports of abscopal effects because most patients usually receive RT as palliative care.6 There is little known about the optimal dose and fractionation for the development of abscopal effects.7, 8, 9 Dewan et al11 demonstrated inferior abscopal effects by a single 20-Gy dose of radiation compared with regimens of 8 Gy × 3 or 6 Gy × 5 fractions in mouse models when a cytotoxic T-lymphocyte-associated antigen 4 inhibitor was combined with radiation. On the other hand, Morisada et al12 compared the effects using a regimen of 2 Gy × 10 fractions with that of 8 Gy × 2 fractions in mouse models. They found that the addition of ICI after a regimen of 8 Gy × 2 fractions reversed adaptive immune resistance, but this did not occur after a regimen of 2 Gy × 10 fractions. Formenti7 recommended a hypofractionated regimen, ideally with 3 to 5 doses of <10 to 12 Gy each, in future clinical trials of RT combined with immunotherapy. Abuodeh et al6 systematically reviewed a total of 46 cases in 31 articles reported between 1969 and 2014. Their review article included both cases of RT monotherapy and combination therapy with ICI and described treatment, clinical course, and biological findings to gain insight into this rare but evocative phenomenon. The dose and fractionation were wide-ranging, and the median total RT dose was 31 Gy (range, 0.45-60.75 Gy) with a median dose per fraction of 3 Gy (range, 0.15-26 Gy). Both low and high doses of RT can initiate local or abscopal immune responses.9 Our regimen with moderate hypofractionation had the use of high dose in common with the recommendation by Formenti,7 although our patient did not receive ICI. Much remains to be clarified regarding the optimal dose and fractionation for abscopal effects.7, 8, 9
The introduction of ICI has caused a marked change in the treatment of patients with metastatic cancer. Although abscopal effects may be reproducible through effective immunotherapy, those without the combination of ICI are uncommon. In the systematic review mentioned previously,6 the median reported age was 64 years (range, 28-83). The patient in this case may be the oldest among those who have developed abscopal effects, but little is known about the optimal candidates for the development of abscopal effects. There is another case report regarding an abscopal effect in a patient with endometrial cancer.13 A patient with widely metastatic endometrial cancer exhibited abscopal responses after multiple rounds of palliative RT while on nivolumab, which is a programmed death 1 inhibitor. The patient’s primary tumor exhibited DNA mismatch repair deficiency, which is a possible sensitizing factor to RT.14 The U.S. Food and Drug Administration has approved programmed death 1 inhibitors for use against any solid tumors that have mismatch repair deficiencies. Thus, our current report is the first case of a suggested abscopal effect in a patient with endometrial cancer after RT alone.
A literature review reported that abscopal effects occurred after a median time of 2 months (range, 0-24 months) and were maintained for a median time of 6 months (range, 0.7-14 months).6 In the present case, the time required for the documented abscopal effect was 5 months, which is consistent with the reported time. As for the duration of the effect, the effect might have been maintained for 8 months because there was no reappearance of multiple lung metastases that had existed in spite of an occurrence of brain metastases.
One of the limitations of our present case is the absence of pathologic confirmation of multiple lung metastases. However, her clinical course of endometrial cancer and CT findings suggested these tumors were metastases. Another limitation was the lack of data for the immune system, such as CD8+ T lymphocytes, which have been demonstrated to play a key role in abscopal effects.3
In conclusion, we report a first case of endometrial cancer suggesting an abscopal effect after localized IMRT. It is of great interest to improve the reproducibility of abscopal effects. Their mechanism and potency are under investigation in the setting of clinical trials in conjunction with immunotherapy.
Footnotes
Sources of support: JSPS KAKENHI grant number 19K08183.
Disclosures: No conflicts of interest. No disclosures.
The data supporting this study are available upon reasonable request. The data are not public owing to restrictions. For example, they may contain information that can compromise the privacy of the participants.
References
- 1.Mole R.H. Whole body irradiation: Radiobiology or medicine? Br J Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 2.Reynders K., Illidge T., Siva S. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41:503–510. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang R., Zhou T., Liu W. Molecular mechanism of bystander effects and related abscopal/cohort effects in cancer therapy. Oncotarget. 2018;9:18637–18647. doi: 10.18632/oncotarget.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farias V.A., Tovar I., Del Moral R. Enhancing the bystander and abscopal effects to improve radiotherapy outcomes. Front Oncol. 2019;9:1381. doi: 10.3389/fonc.2019.01381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postow M.A., Callahan M.K., Barker C.A. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abuodeh Y., Venkat P., Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40:25–37. doi: 10.1016/j.currproblcancer.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Formenti S.C. Optimizing dose per fraction: A new chapter in the story of the abscopal effect? Int J Radiat Oncol Biol Phys. 2017;99:677–679. doi: 10.1016/j.ijrobp.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Buchwald Z.S., Wynne J., Nasti T.H. Radiation, immune checkpoint blockade and the abscopal effect: A critical review on timing, dose and fractionation. Front Oncol. 2018;8:612. doi: 10.3389/fonc.2018.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theelen W.S., de Jong M.C., Baas P. Synergizing systemic responses by combining immunotherapy with radiotherapy in metastatic non-small cell lung cancer: The potential of the abscopal effect. Lung Cancer. 2020;142:106–113. doi: 10.1016/j.lungcan.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Kraus K.M., Kampfer S., Wilkens J.J. Helical tomotherapy: Comparison of Hi-ART and Radixact clinical patient treatments at the Technical University of Munich. Sci Rep. 2020;10:4928. doi: 10.1038/s41598-020-61499-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewan M.Z., Galloway A.E., Kawashima N. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morisada M., Clavijo P.E., Moore E. PD-1 blockade reverses adaptive immune resistance induced by high-dose hypofractionated but not low-dose daily fractionated radiation. Oncoimmunology. 2018;7 doi: 10.1080/2162402X.2017.1395996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh M.S., Chae Y.K. Repeated abscopal effect with radiotherapy and programmed death 1 blockade in mismatch repair–deficient endometrial cancer. JCO Precision Oncology. 2018;2:1–6. doi: 10.1200/PO.18.00085. [DOI] [PubMed] [Google Scholar]
- 14.Martin L.M., Marples B., Coffey M. DNA mismatch repair and the DNA damage response to ionizing radiation: Making sense of apparently conflicting data. Cancer Treat Rev. 2010;36:518–527. doi: 10.1016/j.ctrv.2010.03.008. [DOI] [PubMed] [Google Scholar]