Summary
Implant devices have = proven a successful treatment modality in reconstructive surgeries. However, increasing rates of peri-implant diseases demand further examination of their pathogenesis. Polymicrobial biofilm formation on titanium surfaces has been considered the main risk factor for inflammatory processes on tissues surrounding implant devices, which often lead to implant failure. To overcome microbial accumulation on titanium surfaces biofilm targeting strategies have been developed to modify the surface and incorporate antimicrobial coatings. Because antibiotics are widely used to treat polymicrobial infections, these agents have recently started to be incorporated on titanium surface. This review discusses the biofilm formation on titanium dental implants and key factors to be considered in therapeutic and preventative strategies. Moreover, a systematic review was conducted on coatings developed for titanium surfaces using different antibiotics. This review will also shed light on potential alternative strategies aiming to reduce microbial loads and control polymicrobial infection on implanted devices.
Subject areas: Microbiofilms, Surface Science
Graphical Abstract
Highlights
-
•
Polymicrobial biofilms are the main etiologic factor of implant-related infections
-
•
Titanium (Ti) biomaterial is a substrate for microbial accumulation
-
•
Antibiotic-loaded coating on titanium is a promising strategy this accumulation
-
•
These strategies need to improve drug releasing and are tested by polymicrobial models
Microbiofilms; Surface Science
Introduction
Titanium (Ti) has been used as the main biomaterial for orthopedic and dental implant devices due to its excellent physical-chemical properties and high biocompatibility with host tissues that promote predictable long-term treatment (Spriano et al., 2018). Nonetheless, once exposed to the external environment, implants are also exposed to microbial adhesion and biofilm formation (Lang et al., 1993; Marsh et al., 2011; Mombelli and Décaillet 2011), which ultimately can trigger local inflammation in the surrounding tissues (Arciola et al., 2018).
Polymicrobial biofilm infections are the main reason for dental implant failure, which is associated with an exacerbated inflammatory response followed by significant tissue damage, resulting in loss of supporting structures (Nguyen et al., 2017). Progressive biofilm maturation and polymicrobial community assembly often result in increased virulence (Costerton et al., 1995; Flemming and Wingender, 2010; Bowen et al., 2018), and this process has been implicated as a critical factor in the pathogenesis of biofilm infections (Bowen et al., 2018). Although a host inflammatory response aiming to activate immune functions and control the microbial growth is present, this process can also result in a hypervirulent and multidrug-tolerant biofilm (Marsh and Devine, 2011). Such biofilms are challenging to treat due to their complex microbial composition and tridimensional structure (Flemming and Wingender, 2010; Arciola et al., 2018). This fact may explain the lack of consensus regarding the effective treatment strategies for oral implant-related infections (Heitz-Mayfield and Mombelli, 2014).
Although oral biofilm formation has been widely explored in the literature, it has been focused mainly on dental surfaces (Bowen et al., 2018). However, in recent years, dental implants have been widely used in the replacement of missing teeth, and therefore, their different surface properties, which directly affect biologic responses and microbial adhesion, must be considered in developing therapeutic strategies related to biofilm removal (Song et al., 2015; Souza et al., 2020a, 2020b). Therefore, the knowledge we gained on biofilm formation on oral surfaces cannot be simply transferred to implant surfaces. To overcome microbial accumulation on Ti surfaces some anti-biofilm strategies have been developed, mainly focused on surface properties and antimicrobial coatings (Song et al., 2015; Ferraris and Spriano, 2016).
Several antimicrobial molecules, compounds, and ions were functionalized on Ti material to reduce viable microorganisms in biofilms forming on the surface (Ferraris and Spriano, 2016; Chouirfa et al., 2019). Because antibiotics have been used to treat polymicrobial infections, including oral infections, these agents have also been loaded on Ti surfaces as a coating layer, aiming to provide bactericidal and/or bacteriostatic effect (Darouiche, 2004; Davidson et al., 2015; Stevanović et al., 2020). Even though this may be a promising approach to control microbial accumulation on implanted devices, such strategies must be developed using agents and conditions that do not damage the physical-chemical properties of Ti surface, so that biocompatibility with oral tissues can be maintained. This review aims to discuss the biofilm formation process on Ti material and key factors to be considered by therapeutic strategies using antibiotic agents loaded on surfaces, focusing on current and emergent biofilm control strategies.
Polymicrobial biofilm formation on titanium surfaces
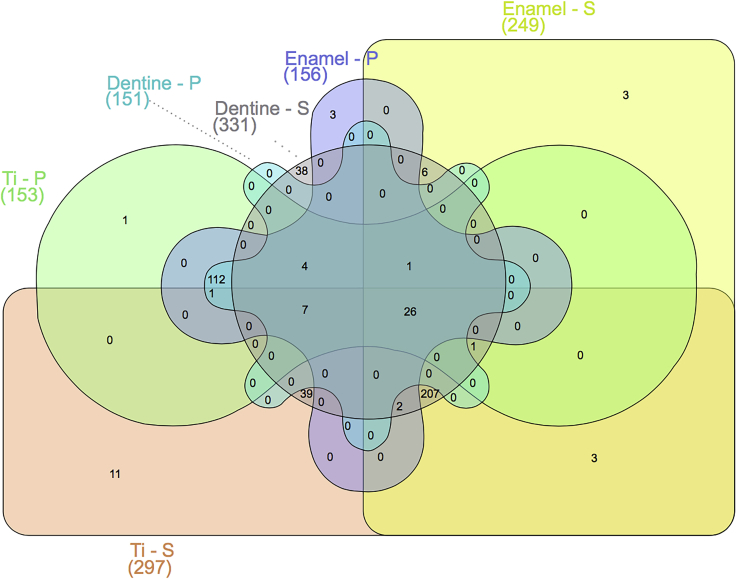
Protein adsorption on implanted biomaterial is considered the first biologic response immediately after implantation in the human body. In the oral environment, both saliva present at the supragingival level and blood plasma at the subgingival level are protein-rich fluids in contact with dental implant devices (Lendenmann et al., 2000; Rabe et al., 2011). The protein layer adsorbed on implant surfaces is responsible for mediating subsequent cellular events, such as microbial and host cell adhesion (Kalasin and Santore, 2009; Rabe et al., 2011; Bürgers et al., 2018) as well as the osseointegration process of dental implants (Romero-Gavilán et al., 2017). Importantly, our group and others have shown that the chemical composition and topography of Ti surfaces affect the proteomic profile of proteins adsorbed from saliva and plasma (Dodo et al., 2013; Pantaroto et al., 2019; Souza et al., 2020a). Moreover, Lima et al. (2008) showed that the pellicle protein composition on Ti surfaces is different compared with hydroxyapatite surfaces (main mineral of tooth enamel), confirming the effect of different material surfaces in the oral environment in modulating protein adsorption. In fact, our unpublished work showed differences in the proteomic profile of proteins adsorbed after 2 h from saliva versus plasma on Ti, enamel, and dentine surfaces (Figure 1). Interestingly, we observed differences not only between saliva and plasma but also among the different substrates tested.
Figure 1.
Venn diagram of proteins adsorbed on titanium (Ti), enamel, and dentine surfaces from saliva (S) and plasma (P)
Surfaces were exposed to human stimulated saliva or human plasma for 2 h at 35°C in an orbital shaker (70 rpm) and evaluated by liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS). Numbers in parentheses are total proteins identified in each surface. Although there are shared proteins among the surfaces and in the different fluids (saliva and plasma), all groups/surfaces showed unique proteins adsorbed, showing the effect of chemical and physical properties of substrate to modulate protein adsorption.
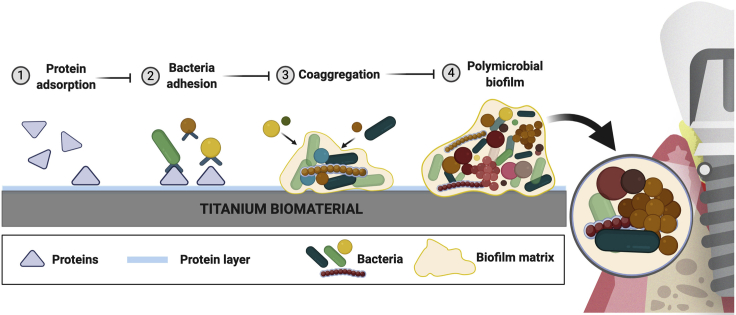
Protein adsorption on surfaces is the first step preceding microbial accumulation and the main mediator of oral microbial adhesion (Whittaker et al., 1996; Rabe et al., 2011). Oral bacteria can adhere on natural oral surfaces or on the surface of any material placed in the mouth, once it is coated with saliva/serum proteins. Thus, the human oral cavity provides a unique environment for microbial adhesion and polymicrobial aggregation hosting close to a thousand of bacterial species detectable by high-throughput 16S rRNA gene sequencing (Dewhirst et al., 2010; Welch et al., 2019). Even though Firmicutes, Proteobacteria, Actinobacteria, Bacteroidetes, and Fusobacteria have been described as the most abundant taxa in the oral cavity (Zaura et al., 2009), it is known that different intraoral niches harbor distinct bacterial communities, with unique sequences for different niches (Zaura et al., 2009). The recognition of protein pellicle receptors by early colonizing bacteria (second step of biofilm formation) allows the selection of certain microbial species accumulation on exposed surfaces (Kolenbrander et al., 2006). These early colonizers, in the dental surfaces, are mostly composed by Streptococcus, Actinomyces, Gemella, Granulicatella, Neisseria, Prevotella, Rothia, and Veillonella species (Kolenbrander et al., 2006). However, in dental implants Streptococcus, Fusobacterium, and Capnocytophaga species have been reported to be more abundant on the surface immediately after implant insertion (Fürst et al., 2007), suggesting some differences when compared with dental surfaces. After initial microbial adhesion, interactions among different species known as co-aggregation (third step of biofilm formation) are proposed to drive the maturation of the biofilm structure. In this way, a specific process of co-aggregation takes place where each strain co-aggregates with a specific set of strains in a well-orchestrated bacterial succession, which results in the incorporation of late colonizers in the biofilm (Kolenbrander et al., 2006). The structure formed is a polymicrobial biofilm, which is characterized by microbial communities enmeshed in a three-dimensional (3D) extracellular matrix attached to a surface (Costerton et al., 1995; Koo et al., 2013). This structure has specific characteristics that promotes microbial growth, creating a well-established and stable “climax community” (Step 4 of biofilm formation) that thrives within its microenvironment in which they have ideal physicochemical conditions, increased virulence, and antibiotic resistance (Socransky and Haffajee, 2002; Marsh et al., 2011) (Figure 2).
Figure 2.
Steps of biofilm formation on titanium biomaterial
Dental implant surfaces made of titanium biomaterial provide the substrate for polymicrobial biofilm formation in the oral environment. Titanium surface is immediately coated by proteins from saliva (supra-mucosal segment) and plasma (sub-mucosal segment) after implant insertion. (1) Protein adsorption on the surface forms a layer with a composition directly affected by the chemical and physical properties of the surface. This layer is the main mediator of microbial adhesion through adhesin-receptor interactions. (2) Initial colonizers, mainly Streptococcus species, adhere on the surface binding to the protein layer. (3) Subsequently, co-aggregation processes and interaction between different species promote biofilm accumulation. (4) This synergistic interaction among organisms continues to contribute to the biofilm structure. These microbial communities are gradually embedded in the extracellular matrix, formed mainly by exopolysaccharides, eDNA, and proteins. This biofilm environment (structure) enhances the microbial interaction and cooperation, antimicrobial resistance, and nutrients/biomolecule retention-diffusion (created by BioRender®).
Even though initial differences are seen for early colonizers in teeth and implants, after 24 h of biofilm formation some similarities were found by 16S rDNA sequencing on Ti and enamel surface, with both surfaces colonized by Firmicutes, Proteobacteria, Fusobacteria, Bacteroidetes, Actinobacteria, and candidate division TM7 (Melo et al., 2017). In spite of the microbiome similarity in biofilms formed in teeth and implant surfaces in health, a cross-arch controlled in vivo study showed that smooth implant surfaces accumulated less biofilm than teeth and showed a more heterogeneous microbiome shift during the absence of oral hygiene, which promoted inflammation around both implants and teeth (Schincaglia et al., 2017). In fact, even with some biogeographic proximity and similar oral microenvironment, dental and implant surfaces can show significant differences in their microbial ecosystems (Dabdoub et al., 2013). These differences may be explained by the higher clinical signs of inflammation at tooth sites compared with implants (Zitzmann et al., 2001; Schincaglia et al., 2017), which may modulate microbiome composition. Because surface properties such as chemical composition and roughness affect the first step of biofilm formation (Souza et al., 2020a), certain differences in microbial attachment are expected even in early stages. However, subsequent environmental pressures, such as local degree of inflammation, may further modulate the late stage of microbial community growth.
Relationship between bacterial microbiota and peri-implant tissue damage
Polymicrobial biofilms forming on dental implant surfaces are considered the main etiologic factor for inflammatory disease processes known as peri-implant mucositis and peri-implantitis (Berglundh et al., 2018). These conditions are the main reason for dental implant treatment failure showing a high prevalence, with more than 40% of implants affected by mucositis and more than 22% affected by peri-implantitis (Salvi et al., 2017). In the oral cavity, peri-implant mucositis is characterized by inflammation in the mucosa around dental implants, but its progression and subsequent progressive loss of supporting bone is known as peri-implantitis (Berglundh et al., 2018). Both conditions are considered as “biofilm-associated pathological conditions,” and therefore, there is a strong evidence that biofilm is the main etiological factor for dental implant-related infections (Berglundh et al., 2018). In fact, biofilms have been described as physical and chemical “stress” factor triggering inflammatory processes on surrounding tissues (Marsh et al., 2011). Initial biofilm growth by early colonizers promotes the colonization and growth of more virulent late colonizers, which increase the overall pathogenicity of the biofilm. This pattern is often associated with an exacerbated inflammatory response followed by significant peri-implant mucosal damage and peri-implant bone resorption (Heitz-Mayfield and Lang, 2010; Nguyen et al., 2017).
Classic animal studies have used biofilm-retaining ligatures to prove cause-effect relation from increased biofilm accumulation and directly correlated this to worse clinical measures, such as bleeding, greater probing depth, loss of attachment, and reduced bone density in natural teeth and implanted devices (Lindhe et al., 1992; Lang et al., 1993; Schou et al., 1993). Interestingly, Schou et al. (1993) also showed that bone loss around implants was significantly greater than around teeth; the study therefore suggested that the presence of marginal inflammation around implants may have more serious clinical implications than does marginal inflammation around teeth with a periodontal ligament. More recently, clinical signs of disease progression were also linked to microbiological changes and transition to a more pathogenic biofilm on dental implant surfaces (Shibli et al., 2008; Padial-Molina et al., 2016). Remarkably, a niche specificity was also shown on dental implant disease sites, in which biofilm composition was different between supra- and sub-mucosal sites, with Veilonella parvula and certain Streptococcus species being more prevalent at supra-mucosal compared with sub-mucosal sites (Shibli et al., 2008). However, in both sites, there was a significantly higher proportion of putative pathogens and reduced abundance of beneficial species during peri-implant infection, when compared with healthy sites (Shibli et al., 2008). Despite some differences, the overall biofilm composition on dental implant surfaces during peri-implant infection still has major similarities with that associated with periodontal diseases. In fact, a systematic review showed that there is insufficient evidence to support specific differences in the microbiota (specific pathogens) between teeth and implants, either in health or in disease on late biofilm states (Retamal-Valdes et al., 2019). This is corroborated by microbiome studies showing a high level of similarity between the microbial profile of periodontitis and peri-implantitis, especially in species that are prevalent in late stages of biofilm formation during disease (Kumar et al., 2012). Interestingly, a similarity between healthy and diseased sites exists in the microbiota that is higher for peri-implant than periodontal diseases (Kumar et al., 2012), showing a strong effect of the implant surface in modulating the microbiome communities formed on its surface in health or disease. In peri-implant disease microbial communities show a lower diversity, compared with healthy sites, supporting a true dysbiotic state, in which lower levels of Prevotella and Leptotrichia and higher levels of Actinomyces, Peptococcus, Campylobacter, nonmutans Streptococcus, Butyrivibrio, and Streptococcus mutans were reported (Kumar et al., 2012). This is in contrast to periodontal diseases where the bacterial diversity in inflammation-associated biofilms is higher than health-associated biofilms (Abusleme et al., 2013).
Moreover, in vivo studies have shown higher total bacteria counts in peri-implantitis biofilms (where not only the smooth but also the rough treated surface of the implant is colonized by biofilm) compared with healthy sites (where only the smooth supra-mucosal implant surface is colonized by biofilms), with increased levels of T. forsythia, T. denticola, F. nucleatum, P. intermedia, P. micros, C. rectus, E. corrodens, Candida albicans, P. nigrescens, C. gracilis, C. ochracea, C. concisus, Streptococcus spp., A. odontolyticus, V. parvula, and E. faecalis (Canullo et al., 2017). Earlier studies also identified T. forsythia in higher abundance in sub-mucosal biofilm samples of peri-implantitis subjects (Shibli et al., 2008).
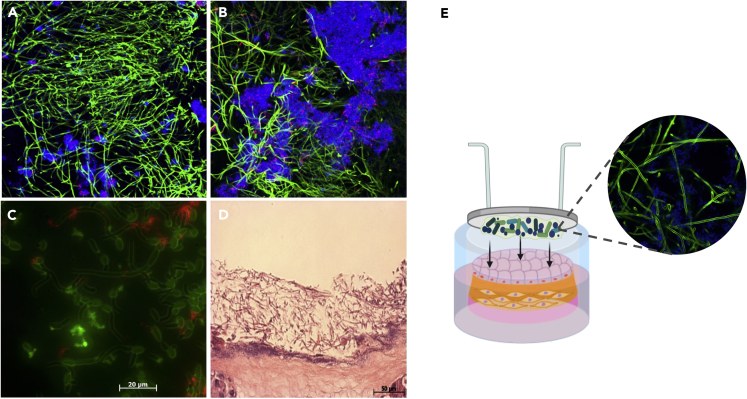
It is interesting to also highlight the increased presence of C. albicans in peri-implantitis biofilms. Although not frequently evaluated in human peri-implant disease studies, C. albicans can grow on Ti surfaces and has been clinically isolated from biofilms associated with peri-implant disease (Leonhardt et al., 1999; Schwarz et al., 2015; Canullo et al., 2017), possibly due to its ability to form robust mixed biofilms with Streptococcus species. Recently our group showed that C. albicans, an oral fungal opportunistic pathogen, increases tissue damage when growing in biofilms at the titanium-mucosal interface (Souza et al., 2020c). Using an in vitro titanium-mucosal interface model we showed that mixed biofilms of C. albicans and Streptococcus species have a mutualistic relationship, promoting bacterial growth and augmenting expression of Candida virulence genes (Souza et al., 2020c). Our studies also showed that cross-kingdom interactions between C. albicans and S. oralis are modulated by different substrates on which the biofilm is growing. Candida stimulates increased glucosyltransferase R expression, an enzyme responsible for the bacterial extracellular matrix, on Ti but not on plastic surfaces (Souza et al., 2020d). Similar mutualistic relationships between C. albicans and Streptococcus species have been described in biofilms growing directly on biotic (oral mucosa) and abiotic (i.e. plastic, titanium) surfaces (Xu et al., 2014a, 2014b; Souza et al., 2020c). These biofilms trigger an exacerbated inflammatory host response and increased tissue damage but have been explored mainly in the context of mucosal infection (Xu et al., 2014b; Bertolini et al., 2019). Although Candida forms biofilms on Ti surfaces (Figure 3), its role on peri-implantitis has not been adequately investigated. In fact, a complete microbiological profile of peri-implant infections including both bacteria and fungi at different stages of disease and their effect on soft and hard tissue damage need further investigation.
Figure 3.
Candida and Streptococcus mixed-biofilms on titanium surface
(A and B) Twenty-four-hour mixed biofilm stained by immuno-FISH of Candida albicans (green) and Streptococcus oralis (stained in blue on A and B and in red on C) growing on (A) titanium surface and (B) polystyrene surface. Bacterial extracellular matrix is stained with Alexa Fluor 647-labeled dextran conjugate probe (red) in figure (B). Images suggest that biofilm growth is modulated by the type of surface.
(C) C. albicans (green) and S. oralis (red) interaction on biofilm growing on the polystyrene surface.
(D) Dual species biofilms growing on the organotypic mucosal construct, which can cause epithelial barrier breach (H&E stain).
(E) Titanium-mucosal interface biofilm model. Biofilm formed on titanium surface suspended 0.5 to 1 mm above the in vitro organotypic mucosal construct surface. Reprinted (adapted) from refs (Souza et al., 2020c, Souza et al., 2020d); Copyright (2020), with permission from American Society for Microbiology and Springer Nature. Figure E created by BioRender®.
Factors affecting microbial diversity in oral biofilms
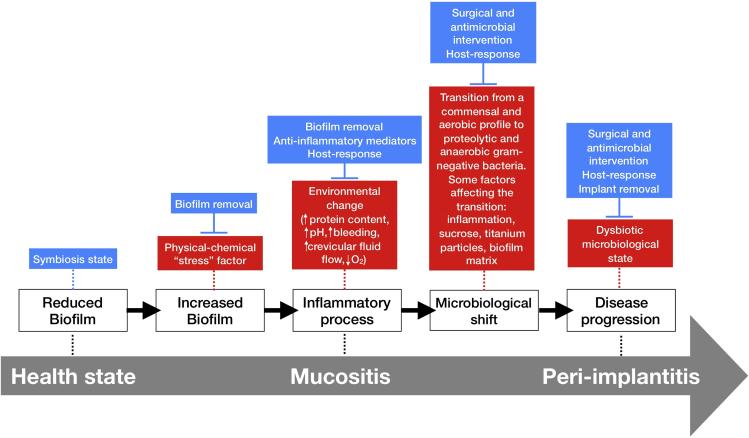
A symbiotic relationship may be established between a polymicrobial biofilm and the host as long as biofilm control methods are routinely applied to maintain low levels of microorganisms on implant surfaces (Schincaglia et al., 2017). However, when left undisturbed biofilms mature, and this may lead to increased inflammation and a rise in the crevicular fluid volume, which provides nutrients for further microbial growth and disruption of host homeostasis (Sultan et al., 2018; Rosier et al., 2018; Naginyte et al., 2019). An adaptation from the “ecological plaque hypothesis” on tooth surfaces may be applied to describe a transition from a healthy state to a disease state in the peri-implant mucosa and bone structures (Figure 4) (Marsh et al., 2011). According to this model, increased biofilm accumulation, mainly due to lack of adequate biofilm removal/disruption (oral hygiene procedures), acts as a “stress” factor triggering an inflammatory process that leads to changes in the local microenvironment, favoring proteolytic and anaerobic gram-negative bacterial overgrowth (Marsh et al., 2011). This inflammatory response may be limited to the peri-implant mucosa and is known as mucositis. A long-standing mucositis can contribute to a microbiological shift favoring disease progression to the alveolar bone leading to peri-implantitis. Alternatively, an anti-inflammatory cytokine feedback loop may control the local inflammatory process and prevent disease progression (Rosier et al., 2018). Although the transition from a healthy oral microbiome to a disease-associated microbiome on biomaterials is still not fully understood, some environmental factors that may contribute to a dysbiosis state in biofilms are carbohydrate exposure, Ti particles and ions released from the implant biomaterial, and presence and composition of an extracellular matrix (Jenkinson, 2011; Bowen et al., 2018; Souza et al., 2019a, 2020e). Thus, even though the initial protein adsorption will mediate the primary and secondary colonizers during bacterial attachment, biofilm formation and maturation is a complex process determined not only by the surface properties, the host-response, and environmental conditions (Cheng et al., 2019).
Figure 4.
Schematic representation of the “ecological plaque hypothesis” in relation to peri-implant disease, adapted from Marsh et al. (2011) and Rosier et al. (2018)
Increased biofilm accumulation on implant surface triggers an inflammatory process that changes the environment leading to microbiological shift and disease progression, as shown by red boxes. Other factors can also favor the microbiological shift on biofilms growing on titanium surfaces, such as carbohydrate (sucrose exposure). However, some factors can control biofilm accumulation and inflammatory response, shown in blue boxes, such as surgical and antimicrobial intervention and host-response.
Sucrose is a unique dietary carbohydrate as it is the only substrate leading to production of streptococcal glucans that constitute the main extracellular matrix component of dental biofilms (Xiao et al., 2012; Koo et al., 2013; Souza et al., 2020d) and promote increased biofilm accumulation on dental surfaces (Ccahuana-Vásquez et al., 2007). Our group recently showed that this carbohydrate can also favor biofilm growth on Ti surface, as evaluated in situ (Souza et al., 2019a). Moreover, increased biofilm accumulation and the ensuing microbial succession on Ti surfaces due to sucrose exposure (20%, four times/day) led to a microbial dysbiotic shift favoring anaerobic putative pathogens by day 7 (Souza et al., 2019a).
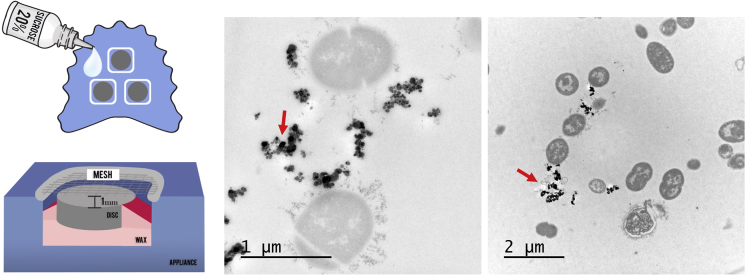
In addition to sucrose, recent work showed that Ti ions and particles commonly released by implants due to mechanical and chemical degradation processes (wear debris from the implant-abutment connection, stripping off from the implant surface while placing, or manipulations during peri-implant surface treatment, ions released from the corrosive environment—acidic pH, microbial accumulation, or chemical agents) also promote a dysbiotic pathogenic biofilm growth on Ti surface (Figure 5) (Souza et al., 2020e). Although Ti itself is not bacteriostatic, in vitro studies have shown a biocidal effect of metal and metal oxide nanoparticles (e.g., zinc oxide, copper oxide, nickel, titanium dioxide) (Yu, 2004; Khan et al., 2015). Nanoparticles cause direct disruption of bacterial cell membrane integrity, as well as induction of oxidative stress by free radical formation and possibly lead to protein and DNA damage (Khan et al., 2015). Because nanoparticle susceptibility and tolerance are species dependent, metal nanoparticles can alter the bacterial biofilm composition and cause a dysbiotic state that favors overgrowth of certain species (Souza et al., 2020e). Other factors that change the microenvironment and may affect the microbiological shift are the extracellular biofilm matrix components. Extracellular polymers in biofilm matrix are responsible for the biofilm three-dimensional architecture (Souza et al., 2020d), generating a highly concentrated nutrient availability and reduced O2 levels (Xiao et al., 2012), which may create a suitable microenvironment for the growth of strict anaerobic putative pathogens. However, the specific effects of extracellular polymers for biofilms growing on Ti surface are still unknown and require further investigation.
Figure 5.
Schematic representation of the in situ model used to form biofilm on titanium surface using palatal appliances
Sucrose is used to promote biofilm accumulation and extracellular matrix formation. Transmission electron microscopy showing biofilms formed in situ on Ti surface and exposed to Ti particles treatment. Red arrows showing Ti particles agglomerated and precipitated Ti ions on extracellular sites. Reprinted (adapted) from Ref. (Souza et al., 2019a); Copyright (2020), with permission from John Wiley and Sons.
Topography properties and current strategies to control polymicrobial biofilms on titanium surfaces
As mentioned earlier, biomaterial surface properties can modify microbial adhesion by selective adsorption of salivary and/or plasma proteins. Both physical and chemical properties of Ti material affect protein adsorption profile (Pantaroto et al., 2019; Souza et al., 2020a). Evidence has suggested that negatively charged surfaces, superhydrophobic and superhydrophilic surfaces, and nm-scale surface characteristics can reduce initial microbial adhesion (Song et al., 2015). Thus, among physical-chemical modifications that have been proposed so far to alter Ti surface properties, the most important are surface-free energy, wettability, charge, and topography in order to limit microbial adhesion. Roughness has been the most evaluated surface property shown to affect microbial adhesion, and although moderate surface roughness may favor microbial adhesion due to a higher contact area between the surface and cells, increased adhesion does not occur on nm-scale or highly roughened surfaces (implant surface with Sa—arithmetical mean height—parameter of more than 2.0 μm) (Albrektsson and Wennerberg, 2004; Whitehead et al., 2006; Puckett et al., 2010; Song et al., 2015). Moreover, the distribution of peaks and valleys affect microbial adhesion directly, because topographic patterns with different shape and size have been shown to inhibit biofilm formation compared with flat surfaces (Song et al., 2015).
It is important to highlight that topographical patterns of titanium surfaces may play distinct roles in the dynamics of biofilm growth especially for surfaces with a nm-scale pattern (Puckett et al., 2010; Lorenzetti et al., 2015; Cao et al., 2018). For example, Cao et al. (2018) showed that the spear- and pocket-type nanostructures synthesized via hydrothermal treatment on Ti have shown different antimicrobial potential. The spear-type surface presented little space between the spears, and therefore, the bacteria are only able to position themselves at the tips of the nano-spears. This not only allows a low initial bacterial load but also causes membrane damage and perforations, leading to cell death. However, this anti-biofilm mechanism is unsustainable because the dead bacteria can cover the nano-spears, which would otherwise prevent killing of new arrivals, and allow the formation of a thick biofilm over a period of 6 days. In contrast, the more promising anti-biofilm effect of pocket-type nanostructure is associated with an open porous mesh structure of randomly oriented spears that permit bacterial localization inside the pockets. In this case, bacteria are killed either by direct membrane penetration or by severe membrane deformation. As the biofilm develops over time and the bacterial load increases, more cells accumulate in the inner voids, resulting in cell death by direct penetration/compression from the spears. As a result, only small clusters of live bacteria are identified on the rim of the pockets after 6 days. Importantly, when it comes to these two nanostructures, it seems that wettability properties do not appear to play an important role on anti-biofilm properties. At a nanoscale, surface topography (diameter of nano-spears and spacing between them) rather than surface roughness (spear height) may be the dominant factor in bacterial attachment and survival on the implant surface.
However, at micro-scale patterns, highly roughened surfaces (Sa > 2.0 μm) (Albrektsson and Wennerberg, 2004) have increased hydrophobicity, which can reduce initial bacterial adhesion. Interestingly, we showed that a superhydrophobic coating (Souza et al., 2020b) modified the salivary pellicle composition at the proteomic level, leading to differences in the biofilm bacterial composition as compared with control surfaces. In fact, a superhydrophobic profile on Ti surfaces was shown to not only reduce bacterial and fungal adhesion and biofilm accumulation but also change the composition of adhered micro-organisms favoring a more host-compatible or symbiotic profile (Souza et al., 2020b). Thus, we hypothesized that the anti-biofilm property of the superhydrophobic surface may be related to a direct reduction of microbial adhesion due to hydrophobicity and also by an indirect effect conferred by the specific protein coating composition.
Based on evidence that surface properties directly affect biofilm formation, modifications by means of surface treatments have been proposed to enhance biological responses. Some treatments, such as sandblasted, large grit, and acid-etched (SLA) surface, are commonly available as commercial dental implants due to enhanced primary stability and optimized osseointegration process when compared with machined implants (Li et al., 2002). However, the increased surface roughness obtained by this type of treatment also favors biofilm accumulation if this surface becomes exposed in the oral cavity (Schmidlin et al., 2013). A promising strategy to control biofilm colonization is the development of surface coatings on Ti (Costa et al., 2020a). This approach has been developed using different technologies, such as glow discharge, electrolytic plasma, and photocatalytic materials, all resulting in bioactive surfaces with antibacterial properties (Ferraris and Spriano, 2016). Several antimicrobials, active molecules, compounds, and ions have been incorporated on Ti surface as biofilm-targeting approaches in order to reduce microbial attachment on the surface. Importantly, cell cytotoxicity and short-term efficiency are currently the main limitations of coatings developed so far for Ti surface (or Ti alloys) (Matos et al., 2017; Chang et al., 2018). Moreover, newly developed surface treatments or coatings should consider that bactericidal and/or bacteriostatic effect might not be enough to control biofilms in the long-term since dead cells remaining attached to the implant surface and can still act as binding sites for microorganism coaggregation, promoting colonization by live pathogens (Koo et al., 2017).
After a dysbiotic polymicrobial biofilm forms on implant surfaces leading to peri-implantitis, there is no consensus regarding the effective treatment for these infections (Heitz-Mayfield and Mombelli, 2014). Non-surgical and surgical therapies have been proposed to control or attempt to eradicate biofilm accumulated on implant surfaces to restore a state of peri-implant health (Heitz-Mayfield and Mombelli, 2014). Adjunct treatments using antimicrobial agents have been proposed in combination with mechanical debridement (Figuero et al., 2014). In fact, some locally delivered antimicrobial agents (such as citric acid, povidone iodine, chlorhexidine) have shown to be very effective on reducing viable bacteria on contaminated Ti surfaces by in vitro and in situ studies (Souza et al., 2018, 2019b).
Locally or systemically administered antibiotics have also been used as an adjunctive approach to mechanical therapy for peri-implant infections (Van Winkelhoff, 2012). Because they have shown only a moderate efficacy in the treatment of peri-implant infections, there is still no consensus regarding their application. Interestingly, the effect of systemic antibiotics in the treatment of severe cases of peri-implantitis may be enhanced by previous surface decontamination procedure, as shown by a clinical trial indicating that an improved outcome was found when systemic amoxicillin was used after the implant surface was debrided with the use of adjunct locally applied chlorhexidine agent (Carcuac et al., 2016). Other antibiotics such as doxycycline (Mercado et al., 2018) and minocycline (Renvert et al., 2006) have also showed a promising effect on implant-related infections. Interestingly, a clinical trial showed that the combination of amoxicillin and metronidazole, which is commonly used to treat periodontitis (Feres et al., 2015), did not show a significant effect in peri-implantitis management as adjunct with local mechanical debridement (Shibli et al., 2019). This result may be explained by a limited diffusion of antibiotics in biofilms forming within Ti surface irregularities and underscores the potential importance of surface topography. This supports the idea that slow release of drug-delivery surface coating methods may be more appropriate than systemic antibiotics in the treatment or prevention of peri-implantitis these infections.
Moreover, it is important to highlight that the anti-microbial effect of newly developed treatments should be evaluated mainly by in vivo models, because mucositis and peri-implantitis are chronic diseases and the host response can affect the growth and susceptibility of these biofilms, as well as the rate of drug delivery by surface coatings. Therefore, studies that evaluate only initial steps of microbial adhesion or initial biofilm formation may not show sustainable long-term results, which is an important aspect to consider in the chronic pathogenesis process in vivo. Although most treatments/coatings have been tested using mainly single-species biofilms, which may be useful for initial screening purposes, proper evaluations should take into account the diversity of the oral microbiome. In this regard, microcosm models using saliva or dental biofilm organisms as initial microbial inoculum or in situ models in the oral environment (Souza et al., 2018, 2019a) are more appropriate pre-clinical models. In addition, based on our current knowledge regarding the diversity and composition of health- and disease-associated biofilms, quantitative data regarding total bacterial loads are not sufficient to fully understand the effects of antimicrobials. The composition of biofilms in response to antimicrobials should also be evaluated using high-throughput methods such as DNA-DNA checkboard hybridization and metagenomics.
Emerging evidence for drug delivery coatings as a biofilm control strategy
More recently, controlled drug delivery systems have been proposed as coatings onto Ti surfaces (Fenton et al., 2018). These systems have certain advantages, such as controlled rate of release and the potential to use target-specific agents as surface coatings. However, antimicrobial surface coatings need to be developed considering characteristics such as (1) maintaining or enhancing the chemical, physical, and mechanical properties of the material; (2) non-cytotoxic effect to host tissues; (3) predictable long-term drug release; and (4) incorporation of proper drugs as target agents (Fenton et al., 2018). In this sense, dental implants covered by antimicrobial agents may act as a monolithic system, because the drug release is expected to be homogeneous over the entire surface (Pokrowiecki, 2018). This system must also provide an effective and stable drug concentration to avoid bacterial resistance on the peri-implant site (Pokrowiecki, 2018). However, because dental implants are expected to be functional for decades, the drug release coating needs to allow recharge/re-deposition when needed, or it may act only during initial healing process and initial biofilm formation. Although some drug delivery agents such as polylactide acid (PDLA) increase the release of drugs, the short-term release and inability to be reloaded are still the biggest limitations of this coating approach (Pokrowiecki, 2018).
A local drug delivery system composed of minocycline microspheres has shown some promising clinical results in treating peri-implant infections (Renvert et al., 2006), which has also been used in teeth with periodontal disease for more than two decades (Williams et al., 2001). However, more recent engineering approaches have provided modified materials for surface coating loaded with antibiotics as a new generation of biomaterials to control biofilm accumulation and, consequently, implant-related infections (Koo et al., 2017). The incorporation of antibiotics within a surface coating has been widely applied for Ti materials in the recent years (Nie et al., 2016; Zhang et al., 2019; Thompson et al., 2019). Although these coatings continue to be evaluated, their application in vivo has been challenging, due to short-term release characteristics, and possibly reduced release due to the protein layer adsorbed over the antibiotic coating, as well as cytotoxicity (Koo et al., 2017). Moreover, these treatments should consider surface topography properties to achieve an appropriate antimicrobial activity. In fact, currently there are no commercially available dental implants made of Ti loaded with antibiotics for clinical use. To the best of our knowledge there is no consensus regarding the optimal antibiotic and coating technique used on Ti material to achieve a reduced microbial load with the potential to control implant-related infections. In the following section we conduct a systematic review on this topic, which supports this conclusion.
Results
Study selection
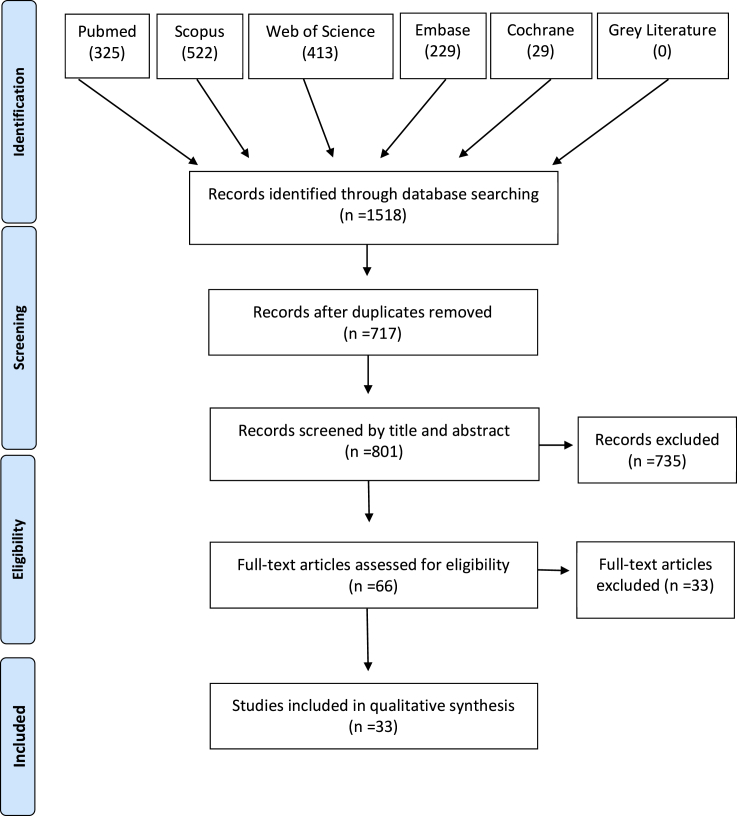
A diagram of the source and selection procedures, according to the PRISMA guidelines, is shown in Figure 6. The initial search identified a total of 1,518 references, including 325 in PubMed, 522 in Scopus, 413 in Web of Science, 229 in Embase, 29 in Cochrane, and 0 in Gray Literature. Of this total, 717 duplicates were removed, with 801 studies remaining. Title and abstract screening resulted in exclusion of 735 records according to the eligibility criteria. The agreement between the two reviewers regarding the titles and abstracts selection was κ = 0.73. Consequently, 66 studies were selected for full-text reading. From that, 33 articles were excluded: two full text were not available (even after three attempts of contact with authors), six were excluded for other reasons (abstract, conference paper, and in vitro model) and 23 did not meet the eligibility criteria. Out of these 23 studies, 18 did not evaluate microbial colonization quantitatively on implant surface, four only evaluated microbial load in bone tissue surrounding the implant, two did not use Ti-based implants, and one human study was excluded due to the absence of a control group (references on Supplemental information). At this stage, the agreement between the two reviewers was κ = 0.87. A total of 33 records were included in the qualitative synthesis, and the main reason for each article exclusion was detailed in Supplemental information (Table S2, related to Figure 6). Importantly, based on our inclusion criteria no study with humans was included, with all studies using animal models.
Figure 6.
Diagram of the source and selection procedures, according to the PRISMA guidelines
Study characteristics
The implant and animal characteristics of the included studies are detailed in Table 1. All included articles were published between 2003 (Lucke et al., 2003) and 2020 (Auñón et al., 2020; Zeng et al., 2020), in twenty-four different countries. Among the implant materials, pure Ti, and titanium-aluminum-vanadium (Ti-Al-V), and titanium-aluminum-niobium (TiAlNb) alloys were used, and different surface treatments were evaluated. Anodized nanotubes and organic and biodegradable coatings were the most investigated surface treatments in association with the antibiotics. With regard to the antibiotic used, gentamicin (n = 11) and vancomycin (n = 11) were the most commonly tested, alone or combined, followed by rifampicin (n = 3), tobramycin (n = 2), doxycycline (n = 2), fosfomycin (n = 1), clindamycin (n = 1), teicoplanin (n = 1), ciprofloxacin (n = 1), minocycline (n = 1), enoxacin (n = 1), bacitracin (n = 1), and daptomycin (n = 1), as shown in Figure 7. Different technologies of deposition such as sol-gel, covalent immobilization, spraying, electrophoretic, polyelectrolyte, and dip coating were applied for antibiotic loading on the Ti surface. All retrieved papers adopted the in vivo design using different animal models (rats: n = 15, rabbits: n = 13, mice: n = 5). Implants were commonly located in tibia or femur. With regard to the infection model, Staphylococcus aureus was the most used (31 studies) with a wide range in bacterial inoculum size (102–109 CFU). P. aeruginosa (Badar et al., 2015) and C. albicans associated with S. aureus (Kucharíková et al., 2016) were also used as microbial inoculum. In addition, the effects of antibiotic-loaded coatings were evaluated in a broad range of follow-ups, varying from 4 h up to 1 month after implant inoculation at different time points. The long-term effect of antibiotic-loaded coatings was analyzed based on the longest follow-up time in each study.
Table 1.
Summary of included studies
| Author | Implant |
Animal |
Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Material | Surface treatment | Antibiotics | Deposition technology | Type | N∗ | Surgical site | Infection model | ||
| Adams et al. (2009) | Ti6Al4V | Anodized | Vancomycin | Sol-gel | Rat | 11 | F | S. aureus (NR strain origin) | 1, 2, 3, 4w |
| Alt et al. (2014) | Ti6Al4V | Machined | Rifampicin + Fosfomycin |
Ink-jet | Rab | 22 (11 MSSA+ 11 MRSA) | T |
S. aureus (MSSA EDCC5055) (MRSA T6625930) |
4w |
| Antoci et al. (2007) | Ti6Al4V | Machined | Vancomycin | Covalently link | Rat | 9 | F | S. aureus (ATCC 25923) | 1, 2, 3w |
| Auñón et al. (2020) | Ti6Al4V | TiO2 nanotubes | Gentamicin + Vancomycin |
Drug adsorption | Rab | 20 | F | S. aureus (Human Sa5) | 4w |
| Aykut et al. (2010) | cpTi | Si-sandblasted | Clindamycin or Teicoplanin | Spraying | Rab | 30 | T | S. aureus (ATCC 29123) | 1w |
| Badar et al. (2015) | Ti6Al4V | Porous | Ciprofloxacin | Layered double hydroxides suspension | Mic | 12 | S | P. aeruginosa (PAO1 CTX::lux) | 4h |
| Croes et al. (2018) | cpTi | Porous Porous + CS |
Vancomycin | Electrophoretic deposition | Rat | 18 | T | S. aureus (ATCC 49230) | 4w |
| Darouiche et al. (2007) | Ti6Al4V | Si-sandblasted | Minocycline + Rifampin |
Spraying | Rab | 25 | F | S. aureus (P1 – variation of ATCC 25923) | 1w |
| Diefenbeck et al. (2016) | Ti6Al4V | Plasmachemical oxidation | Gentamicin | Immobilization (TA or SDS) | Rat | 15 | T | S. aureus (ATCC 49230) | 4w |
| Ghimire et al. (2019) | Ti6Al4V | Dopamine methacrylate + PEGDMA-Oligo HYD | Vancomycin | Covalently bond | Mic | 22 | F | S. aureus (Xen 29) | 3w |
| Grohmann et al. (2019) | cpTi | Sandblasted and etched | Gentamicin | Polyelectrolyte adsorption (PEM + + PGA/HEP) | Rat | 30 | T | S. aureus (ATCC 49230) | 4w |
| Janson et al. (2019) | cpTi | Anodization + alkaly treatment + HA | Tobramycin | Soaking method | Rab | 5 | F | S. aureus (ATCC 6538) | 9d |
| Jennings et al. (2016) | cpTi | Machined | Vancomycin | Manual application (PH) | Rab | 9 | R | S. aureus (UAMS-1 strain) | 1w |
| Kälicke et al. (2006) | cpTi | PLLA | Rifampicin + Fusidic acid |
Solvent-casting | Rab | 36 | T | S. aureus (V 8189-94) | 4w |
| Kucharíková et al. (2016) | cpTi | Beadblasted and etched | Vancomycin | Covalent immobilization | Mic | NR | S |
S. aureus (SH1000) C. albicans (SC5314) |
2d (fungal) 4d (bacterial) |
| Liu et al. (2017) | cpTi | Machined and nanotubular anodized surface | Gentamicin | Soaking method | Rab | 36 | T | S. aureus (ATCC 25923) | 6w |
| Lucke et al. (2003) | cpTi | PDLLA | Gentamicin | NR | Rat | 30 | T | S. aureus (ATCC 49230) | 6w |
| Lucke et al. (2005) | cpTi | Machined or PDLLA | Gentamicin | PDLLA suspension | Rat | 30 | T | S. aureus (ATCC 49230) | 6w |
| Ma et al. (2017) | cpTi | Machined + NIR light |
Gentamicin | Vacuum drying process onto PEG-MoS2 coating + CS | Rat | 18 | S | S. aureus (NR strain origin) | 1d, 3d, 1w |
| Metsemakers et al. (2015) | cpTi | Anodized + PLEX |
Doxycycline | Spraying | Rab | 28 (12 MSSA +16 MRSA) | H |
S. aureus MSSA (JAR60131) S. aureus MRSA (LUH15101) |
4w |
| Moskowitz et al. (2010) | cpTi | Layer-by-layer | Gentamicin | Polyelectrolyte deposition | Rab | 27 | F | S. aureus (ATCC 49230) | 4d, 1w |
| Neut et al. (2015) | Ti6Al4V | Al-blasted + HA | Gentamicin | Spraying + PLGA | Rab | 14 | F | S. aureus (ATCC 25923) | 2d, 1w |
| Nie et al. (2016) | Ti6Al4V | Machined | Enoxacin | Covalent immobilization | Rat | 24 | F | S. aureus (ATCC 43300) | 3w |
| Nie et al. (2017) | Ti6Al4V | Machined | Bacitracin | Immobilization | Rat | 10 | F | S. aureus (ATCC 25923) | 3w |
| Song et al. (2017) | cpTi | Nanofiber | Doxycycline | Coaxial electrospinning | Rat | 48 | T | S. aureus (ATCC 49230) | 4, 8, 16w |
| Stavrakis et al. (2019) | cpTi | PEG-PPS | Vancomycin or Tigecycline | Encapsulation in PEG-PPS solution | Mic | 18 | F | S. aureus (Xen36) | 6w |
| Thompson et al. (2019) | TiAlNb | Ca-P | Gentamicin | Dip coating | Rat | 18 | T | S. aureus (JAR060131) | 1w |
| Yang et al. (2016) | cpTi | Nanotubes | Gentamicin | Lyophilization + Vacuum-drying | Rat | 9 | F | S. aureus (ATCC 25923) | 6w |
| Yuan et al. (2018) | cpTi | Machined | Vancomycin | Soaking method on nanotubes coating + catechol functionalization | Rat | 6 | F | S. aureus (ATCC 25923) | 4w |
| Zeng et al. (2020) | cpTi | Machined +NIR light |
Daptomycin | Immobilization with IR820 dye on PDA nanocoating | Rat | NR | T | S. aureus (ATCC 25923) | 2w |
| Zhang et al. (2018) | Ti6Al4V | Plasma-sprayed | Vancomycin | Impregnated on the plasma-sprayed coating | Rab | 20 | T | S. aureus MRSA (ATCC 43300) | 6w |
| Zhang et al. (2019) | Ti6Al4V | Machined | Vancomycin | Covalently bond | Mic | 14 | F | S. aureus (Xen29) | 3w |
| Zhou et al. (2017) | Ti6Al4V | PDLLA | Tobramycin | Impregnated on PDLLA coating | Rab | 12 | T | S. aureus (ATCC 25923) | 8w |
Implants and animals data from included experimental studies (author, year; implant: material, surface treatment, antibiotics, deposition technology of the antibiotic; animal: type, sample number, surgical site, infection model; follow-up).
Notes: Implant surface treatment (TiO2, titanium dioxide; Si,Silica; CS, chitosan; PEGDMA, polyethylene glycol dimethacrylate; Oligo, oligonucleotide; HYD, hydrogel; HA, hydroxyapatite; PLLA, poly-L-Lactide; PDLLA, poly(D,L-lactide); NIR, near-infrared light; PLEX, polymer-lipid encapsulation matrix; Al, aluminum; PEG, poly(ethylene glycol); PPS, poly(propylene sulfide); Ca-P, calcium and phosphorus). Deposition technology (TA, tannic acid; SDS, sodium dodecyl sulfate; PEM, polyelectrolyte multilayer; PL, polycation; PGA, polyanion; HEP, heparin; PH, phosphatidylcholine; NR, not reported; PDLLA, poly(D,L-lactide); PEG, polyethylene glycol; MoS2, molybdenum disulfide; CS, chitosan; PLGA, poly(lactic-co-glycolic acid); PPS, poly(propylene sulfide); PDA, polydopamine); Animals (Rat, rats; Mic, mices; Rab, rabbits), Sample number (N) (MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; NR, not reported), Surgical site (T, tibia; F, femur; S, subcutaneous; H, humerus; R, radius), Follow-up (h, hour; d, day; w, week). ∗Sample number reported is the total number of infected animals used for microbiological assessments of non-loaded and antibiotic-loaded surfaces.
Figure 7.
Word clouds of the antibiotics used on titanium coating in the studies included
The font size represents the frequency of antibiotics used in which bigger words mean more frequent antibiotics used.
Antibiotic-loaded coatings reduced microbial load on Ti-implants
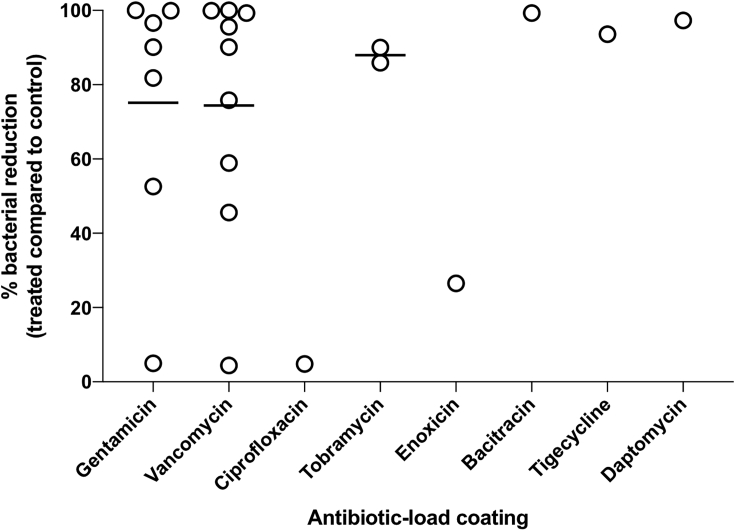
The microbiological assessments of the included studies are detailed in Table 2. Among the quantitative tests for biofilm analysis, the viability of bacterial cells is reported by colony count units (CFU) after implant sonication and agar plating or rolling out the contaminated implant directly on agar plates. Overall, quantitative results on bacterial loads showed greater bacterial implant surface contamination in the control groups (i.e. machined surface or coating without antibiotic). In this systematic review we used the bacterial load (control and test groups) to calculate the percentage of biofilm reduction on antibiotic-loaded surfaces in each study. In addition, the long-term effect of biofilm reduction of antibiotic-loaded coatings was analyzed taking into account the longest follow-up time for each study. Based on this we found a highly variable % of biofilm reduction in different studies, ranging from 0% to >90% reduction (Figure 8). For gentamicin-loaded coatings, the reduction showed a variation of ∼5% (Moskowitz et al., 2010) up to ∼99.9% (Thompson et al., 2019), whereas for vancomycin-loaded coatings minimum and maximum values were ∼45.3% (Kucharíková et al., 2016) and ∼99.2% (Stavrakis et al., 2019), respectively. In contrast, three studies showed higher bacterial load on the test group as well as the absence of reduction with vancomycin (Ghimire et al., 2019), doxycycline (Metsemakers et al., 2015), or gentamicin (Neut et al., 2015) coatings. In addition, the infection rates (presence of contaminated implants) also were commonly smaller in test groups in terms of bacterial counts on agar plates when compared with control groups (Table 2). However, a long-term antibiotic release is not expected, because several studies showed more than 50% of released drugs taking place within hours or days (Lucke et al., 2005; Darouiche et al., 2007; Neut et al., 2015; Yuan et al., 2018; Thompson et al., 2019) (Table 2), with some treatments even showing more than 95% of the deposited drug released within short time periods (Neut et al., 2015; Ghimire et al., 2019; Thompson et al., 2019).
Table 2.
Summary of microbiological data from included experimental studies (author, year; in vitro: drug concentration in the surface, drug release (related immersion period); microbiological assessments: quantitative test, mean and median of bacterial load (standard deviance); % reduction by authors calculated; infection rate; statistical significance)
| Author |
In vitro |
Microbiological assessments |
||||||
|---|---|---|---|---|---|---|---|---|
| Drug concentration in the surface | Drug release (immersion period) | Quantitative test | Bacterial load |
% Reduction | Infection rate | p value | ||
| Control | Experimental | |||||||
| Adams et al. (2009) | 23.5 μg | 70% (6 d) | CFU/implant + Rolled out on agar plates test (Infection rate) | 1w: 11,230.67 (±299.5) 2w: 2,186.33 (±204.2) 3w: 17,7891.0 (±27,220.1) 4w: 18,765.0 (±4,493.75) |
1w: 3.00 (±1.27) 2w: 1.57 (±0.57) 3w: 12,989.0 (±822.07) 4w: 4,531.5 (±221.2) | ~75.8% | NR | NR |
| Alt et al. (2014) | 307 μg rifampicin and 1,522 μg fosfomycin | NR | Rolled out on agar plates test | NR | NR | NR | C: 100% (5/5) T: 16.7% (1/6) | 0.015 |
| Antoci et al. (2007) | NR | NR | CFU/implant | 1w:1,43,814.43 (±10,051.54) 2w: 87,951.81 (±7,831.33) 3w: 22,6315.79 (±11,403.51) |
1w: 10,458.17 (±1,494.02) 2w: 25,903.61 (±1,807.23) 3w: 1,33,333.33 (±14,912.28) |
~58.9% | NR | 0.046 (1-w follow-up) |
| Auñón et al. (2020) | 1.45 mg of gentamicin and 1.4 mg of vancomycin | 56% gentamicin and 10.66% vancomycin (240 min) | CFU/cm2 (Log10) | 1.52 (±1.5) | 0.15 (±0.47) | ~90.1% | NR | 0.0074 |
| Aykut et al. (2010) | NR | NR | Positive or negative culture | NR | NR | NR | C: 100% (10/10) TEI: 0% (0/10) CLIN: 10% (1/10) |
<.001 (C versus TEI, and C versus CLIN) |
| Badar et al. (2015) | 1.2 mg/cm2 | NP | Bioluminescence intensity | 1w: 100.56 (±28.4) 2w: 99.40 (±37.64) 3w: 289.88 (±21.30) |
1w: 26.47 (±4.67) 2w: 68.45 (±28.03) 3w: 276.19 (±47.43) |
~4.8% | NR | NR |
| Croes et al. (2018) | NR | NR | CFU/implant (Log10) | Uncoated: 5.01 (NR) CS control: 5.87 (NR) |
5.63 (NR) | ~4.4% (no difference) | C: 40% (2/5) CS control: 100% (8/8) T: 40% (2/5) |
0.035 (CS control versus T for infection rate) |
| Darouiche et al. (2007) | 35 μg/cm2 (noncovalently bound) | 94% minocycline and 95% rifampin (6 h) | Positive or negative culture | NR | NR | NR | C: 100% (12/12) T: 38.46% (5/13) | 0.0016 |
| Diefenbeck et al. (2016) | 0.59 mg of G-SDS and 1.11 mg of G-TA | 54.2 μg and 424.9 μg G from G-SDS and G-TA, respectively. (24 h) | Positive or negative culture | NR | NR | NR | C: 100% (10/10) G-SDS: 0% (0/10) G-TA: 10% (1/10) | NR |
| Ghimire et al. (2019) | 90 μg/mm³ hydrogel | ~100% (24 h) | CFU/implant | 3w: 520.0 (±40.0) | 3w: 0 | 100% | NR | ≤.01 |
| Grohmann et al. (2019) | (PL-PGA)-20G = 0.32 (PL-PGA)-30G = 0.45 (PL-HEP)-20G = 0.15 (PL-HEP)-30G = 0.51 Data presented as referenced loads |
(PL-PGA)-20G = 5.52 μg/cm2 (PL-PGA)-30G = 18.62 μg/cm2 (PL-HEP)-20G = 46.89 μg/cm2 (PL-HEP)-30G = 86.21 μg/cm2 (14 d) |
CFU/implant (1/mm2) (rolled over agar) |
19.17∗ | (PL-PGA)-20G = 3.33∗ (PL-PGA)-30G = 12.50∗ (PL-HEP)-20G = 19.17∗ (PL-HEP)-30G = 3.33∗ | (PL-PGA)-20G = ~83.1% (PL-PGA)-30G = ~34.8% (PL-HEP)-20G = ~0% (PL-HEP)-30G = ~82.7% | NR | NR (No difference) |
| Janson et al. (2019) | NR | 758 μg (15 min) | CFU/implant | (BC-104): 3.43 (±2)×105 (BC-105): 2.85 (±1.4)×105 |
(BC-104): 0.48×105 (BC-105): 0 | (BC-104): ~85.9% (BC-105): 100% | C (BC-104): 100% (3/3) C (BC-105): 100% (2/2) T (BC-104): 33% (1/3) T (BC-105): 0% (0/2) |
NR |
| Jennings et al. (2016) | 25% | NR | CFU/implant | 600,000 (NR) | 20 (±21) | ~99.9% | NR | <.001 |
| Kälicke et al. (2006) | 3% Rifampicin and 7% fusidic acid | ~80% (42 d) | Harvesting | NR | NR | NR | C: 83% (10/12) PLLA-C: 83%(10/12) T: 17% (2/12) |
0.033 (controls versus test) |
| Kucharíková et al. (2016) | 35 pmol/cm2 | NR | CFU/implant (Log10) |
S. aureus: 5.37 (±0.41) C. albicans: 4.47 (±0.15) |
S. aureus: 2.92 (±0.25) C. albicans: 3.00 (±0.24) | S. aureus: ~45.6% C. albicans: ~32.9% | NR | <.05 (S. aureus) NR (C. albicans) |
| Liu et al. (2017) | NR | NR | Rolled out on agar plates test | Ti-C: >1000 CFU NTATi-C: >1000 CFU |
Ti-G: 473.75 (±10.69) NTATi-G: 40.5 (±12.36) |
Ti-G: ~52.6% NTATi-G: ~95.9% |
C: 100% (4/4) NTATi-C: 100% (4/4) Ti-G: 100% (4/4) NTATi-G: 100% (4/4) |
<.05 |
| Lucke et al. (2003) | 10% | NR | Rolled out on agar plates test | Ti-C: >1,000 CFU PDLLA-C: >1,000 CFU | 182 (±101) | ~81.8% | Ti-C: 100% (10/10) PDLLA-C: 100% (10/10) T: 70% (7/10) |
<.05 |
| Lucke et al. (2005) | 10% | 60% (24 h) | Rolled out on agar plates test | Ti-C: >1,000 CFU PDLLA-C: >1,000 CFU | NR (range 7–136 colonies) | NR | Ti-C: 100% (10/10) PDLLA-C: 100% (10/10) T: 10% (1/10) | NR |
| Ma et al. (2017) | NR | - NIR: 2.8 mg/mL + NIR: 6.5 mg/mL (25 min) |
Antibacterial ratio (%) | 1d – Ti + NIR: 0.92% (±7.32) 3d – Ti + NIR: 82.42% (±2.75) 1w – Ti + NIR: 88.22% (±4.58) 1d – Ti – NIR: 0% (±6.14) 3d – Ti - NIR: 70% (±4.91) 1w – Ti – NIR: 81.05% (±3.68) |
1d – T + NIR: 99.15% (±1.83) 3d – T + NIR: 99.67% (±0.91) 1w – T + NIR: 99.97% (±0.91) 1d – T – NIR: 60.75% (±4.91) 3d – T – NIR: 88.59% (±2.45) 1w – T – NIR: 99.42% (±1.23) | NR | NR | <.001 (1d and 3d for + NIR; 1d for −NIR); <.01 (7d for –NIR); <.05(1w + NIR; 3d for –NIR) |
| Metsemakers et al. (2015) | 3.4 mg | ~95% (4 w) | CFU/implant (Log10) | MSSA: 5.10∗ MRSA: 4.89∗ |
MSSA: 0∗ MRSA: 0∗ |
At least 3-log lower median numbers of CFUs (no significant difference) | MSSA - C: 80% (4/5) MSSA - T: 0% (0/6) MRSA - C: 100% (8/8) MRSA - T: 43% (3/7) |
<.05 (infection rate) |
| Moskowitz et al. (2010) | 550 μg/cm2 | ~100% (5.5 w) | Rolled out on agar plates test | 4d: >200 CFU 1w: >200 CFU |
4d: <22 CFU 1w: <190 CFU | 4d: ~89.0% 1w: ~5.0% |
4d - C: 83% (5/6) 4d - T: 0% (0/6) 7d - C: 71% (5/7) 7d - T: 0% (0/8) | NR |
| Neut et al. (2015) | 1 mg/cm2 | ~100% (168 h) | CFU/implant (Log10) | 2d: 4.82 (±1.32) 1w: 3.60 (±2.64) |
2d: 0 1w: 0 | 100% | C: 100% (7/7) T: 0% (0/7) | 0.001 |
| Nie et al. (2016) | NR | “the covalent modification of the Ti surfaces does not allow the release of any antibiotic” | CFU/implant (Log10) | 6.20 (±0.18) | 4.56 (±0.16) | ~26.5% | NR | <.05 |
| Nie et al. (2017) | NR | NR | CFU/implant (Log10) | 4.67 (±2.34)×105 | 3.3 (1.67)×103 | ~99.3% | NR | <.05 |
| Song et al. (2017) | NR | NR | Washout of implants (O.D. values) | 4w: 0.17 (±0.24) 8w: 0.29 (±0.30) 16w: 0.64 (±0.06) |
4w: 0.04 (±0.02) 8w: 0.04 (±0.01) 16w: 0.29 (±0.29) |
~54.7% | 8w - C: 100% (5/5) 8w - T: 0% (0/5) 16w - C: 100% (5/5) 16w - T: 40% (2/5) | <.005 |
| Stavrakis et al. (2019) | NR | ~23μg vancomycin (1 w) | CFU/implant (Log10) | 2.8 (±1.5)×102 | Vanco: 2 (±2) Tig: 1.8 (±1.8)×101 | Vanco: ~99.3% Tig: ~93.6% |
NR | NR |
| Thompson et al. (2019) | 311.32 μg/mL | >95% (15 min) | CFU/implant (Log10) | 1.76×106 (NR) | 6.43 ×101 (NR) | ~99.9% | C: 100% (9/9) T: 12.5% (1/8) | <.01 |
| Yang et al. (2016) | NR | 91.45 μg (57 h) | CFU/implant | Ti-C: 6.7×105 (±7.55×104) NT-C: 4.6×105 (±6.24×104) |
1.57×104 (±2.08×103) | NT: ~97.7% T: ~96.6% |
NR | <.01 |
| Yuan et al. (2018) | NR | 81.7% (24 h in the presence of S. aureus) | CFU/implant (Log10) | 3.43 (±0.12) | 0.90 (±0.43) | ~73.8% | NR | <.01 |
| Zeng et al. (2020) | 634.6 μg | 408.3 μg (14 d) | CFU/implant | Ti-C - NIR: 192.5 (±12.5) Ti-C + NIR: 182.5 (±22.5) |
T - NIR: 82.5 (±10.0) T + NIR: 5.00 (±2.5) |
NIR−: ~57.1% NIR+: ~97.3% |
NR | <.01 (Ti-C versus T) <.05 (T - NIR versus T + NIR) |
| Zhang et al. (2018) | NR | NR | CFU/implant | 8.42 (±0.68)×105 | 4.04 (±0.89)×104 | ~95.2% | NR | <.05 |
| Zhang et al. (2019) | NR | NR | CFU/implant | 1,478.26 (±521.74) | 65.22 (±65.21) | ~95.6% | NR | <.05 |
| Zhou et al. (2017) | 25% (4 mg) | 26.03 ng/mL (released in the blood after 2 h implant installation) | CFU/implant | >104 | <10³ | ~90.0% | C: 100% (6/6) T: 16.7% (1/6) | <.05 (infection rate) |
Notes: In vitro (NR, not reported; NP, not possible to extract data from the graph; G, gentamicin; SDS, sodium dodecyl sulfate; TA, tannic acid; PL, polycation; PGA, polyanion; HEP, heparin; d, days; min, minutes; h, hours; w, weeks); Quantitative test (CFU, colony forming unit; O.D., optical density); Microbiological assessments (NR, not reported; d, day; w, week; CS, chitosan; ∗ = median; PL, polycation; PGA, polyanion; HEP, heparin; G, gentamicin; BC, bacterial concentration; CFU, colony forming unit; Ti, titanium; C, control group; NTA, nanotubular anodized surface; PDLLA, poly(D,L-lactide); +NIR, with near-infrared light; −NIR, without near-infrared light; T, test group; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; Vanco, vancomycin; Tig, tigecycline; NT, nanotubes); Infection ratio (NR, not reported; C, control group; T, test group; TEI,teicoplanin; CLIN, clindamycin; CS, chitosan; G, gentamicin; SDS, sodium dodecyl sulfate; TA, tannic acid; BC, bacterial concentration; PLLA, poly-L-lactide; Ti, titanium; C, control group; NTA, nanotubular anodized surface; PDLLA, poly(D,L-lactide); MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin.
Figure 8.
Percentage of reduction in the bacterial load on different antibiotic-loaded coatings on titanium surface in relation to control groups according to the study results included in this systematic review
Circles represent each study included in the review in which it was possible to calculate bacterial load reduction and to compare studies using the same antibiotic coating.
Discussion
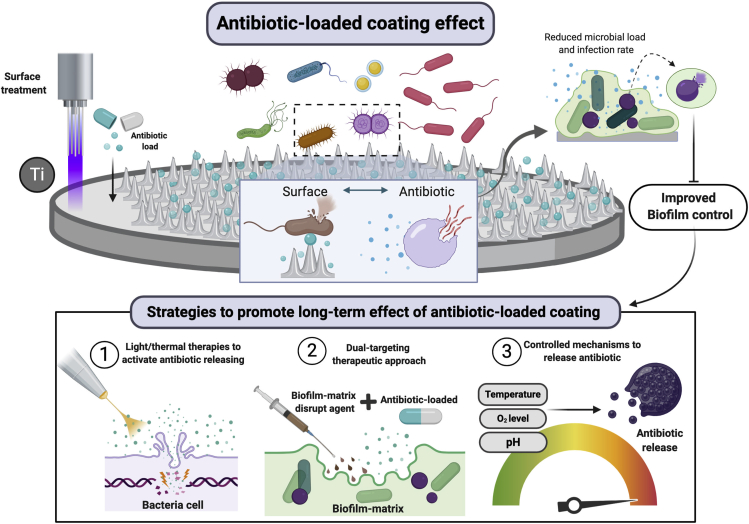
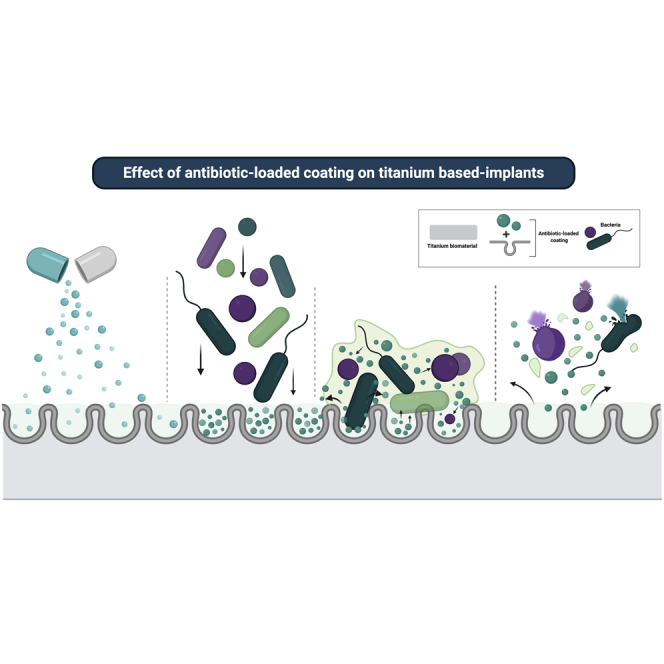
Biofilms provide several advantages to colonizing species such as protection against host defense mechanisms, enhanced microbial co-aggregation, and reduced antimicrobial susceptibility (Costerton et al., 1995; Flemming and Wingender, 2010). These properties enhance microbial accumulation and maturation, which can trigger peri-implant infections. There is currently no consensus regarding the best therapeutic approach to treat dysbiotic biofilms and allow re-establishment of a health-associated microbiome. Newly developed strategies have been focused mainly on surface properties dedicated to non-fouling abilities and incorporation of antibiotics/antimicrobials on the surface as a long-term drug delivery method (Figure 9). Although this seems a promising strategy, no human clinical data are available regarding the efficacy of antibiotic-loaded implants in reducing microbial loads, as shown by our systematic review.
Figure 9.
Schematic representation of the anti-biofilm activity of antibiotic-loaded coatings on titanium surface
Antibiotic loaded on titanium material by surface treatment may have bacteriostatic or bactericidal effect upon microbial contact with the antibiotic incorporated on the surface or by contact with the drug slowly released in the environment. This end result is reduction in microbial load of polymicrobial biofilms and, consequently, infection rates. Although a short-term releasing has been related to antibiotic-loaded coatings on titanium, some strategies (bottom panel) may promote drug releasing and antimicrobial effect, such as light therapies to activate the coating, dual-targeting therapies, and controlled mechanisms to release antibiotic (created by BioRender®).
Although several studies included in this systematic review showed that the biofilms forming on antibiotic-loaded implants were considerably reduced (up to ≥90% biofilm reduction), a large discrepancy was found among studies testing the same antibiotic (Badar et al., 2015). This discrepancy can be ascribed to differences in study design and variable sample sizes that were not supported by power calculations. Badar et al. (2015) was the only study to perform implant placement before infecting the animals and to evaluate the antibacterial efficacy by measuring bacterial luminescence only 4 h after infection with P. aeruginosa. Moreover, the follow-up periods that studies reported were variable, and this may affect outcomes due to variable drug release kinetics.
In studies using the same antibiotic (gentamicin) and same bacterial species for inoculation (S. aureus), one methodology that provided lower values of biofilm reduction was when implants were rolled out on agar plates to quantitatively assess biofilm growth. Although this method is suitable to assess presence or absence of bacteria on the implant surface, it may not be sensitive enough to detect quantitative differences in bacterial loads (Lucke et al., 2003; Moskowitz et al., 2010; Liu et al., 2017; Grohmann et al., 2019). Studies that used a biofilm sonication step prior to plating, instead of the roll over agar method, were able to show a much greater biofilm reduction with antibiotic coating (Yang et al., 2016; Thompson et al., 2019). Sonication is a well-known technique to dislodge biofilms from the substrate and has been validated for the diagnosis of orthopedic implant prosthetic joint infections (Oliva et al., 2016). However, it is important to emphasize that different Ti surface treatments and antibiotic concentrations could also have influenced these differences in results.
In terms of the infection model, the choice of the appropriate microorganism is one of the most essential steps when evaluating biomaterials in implant-biofilm-related infections. With the exception of one study (Badar et al., 2015), the only experimental pathogen in the studies included in this review was S. aureus. Although S. aureus is highly associated with postoperative infection in orthopedic implants (Oliveira et al., 2018), these results should be interpreted with caution since the presence of other microorganisms in the peri-implant microbiome was not evaluated. In addition, implants were commonly installed in tibia or femur sites, which do not widely represent the environmental conditions and healing of the oral cavity. Thus, the use of a polymicrobial infection model developed in the oral environment could provide more clinically relevant information regarding the effect of antibiotic-loaded coatings in reducing the microbial load. Surprisingly, biofilm-retaining ligature models to study oral multi-species biofilm accumulation on dental implant surfaces loaded with antibiotics are non-existent.
Early microbial colonization may occur during the surgical procedure of implant placement, often associated with immediate pathogen attachment on the implant surface through the surgical site (Hickok and Shapiro, 2012). Therefore, biofilm formation could start during the early stages of post-operative healing and may compromise the osseointegration process (Franz et al., 2011). Thus, drug delivery systems via surface coating may act as effective prevention methods to control microbial accumulation at the time of implant placement (Hickok and Shapiro, 2012). From the studies reporting the quantification of antibiotic release included in our systematic review, approximately 95% of the deposited drug was released within hours or days. Such early, rapid release would allow biofilm prevention in the immediate postoperative window but would not address the chronic peri-implant infection (Salvi et al., 2017; Berglundh et al., 2018), unless the antibiotic coating allows slower drug release so that its effect lasts for a longer period of time. The literature has pointed out that the use of layer by layer assembly might be the most advantageous and intelligent releasing system for long-term release, as the drug is entrapped between layers and is sustained eluted through film-erosion and/or diffusion (Smith et al., 2009; Perni et al., 2020). Further advantage of this system is that the layer can be programmed to degrade under acidic conditions (e.g. infection with aciduric species), such that the drug is available only when necessary. Ultimately, the efficacy of an antibiotic releasing system combined with surface topographic modification approaches (e.g. pocket-type nanostructure Ti surfaces) (Cao et al., 2018) deserves further investigation for implant applications. Experimental work using different bacterial species are required to test the effectiveness of the developed nanostructured surfaces in addition to mechanical tests evaluating structural stability under attrition (during bone insertion and torque). In vivo testing in appropriate animal models (e.g. oral biofilm ligature models) will also need to be conducted in the future.
Another possible way to overcome the limited long-term antimicrobial potential is the use of light-induced photocatalytic coatings, because it can be activated whenever infections are present (Nagay et al., 2019). Our previous study (Nagay et al., 2019) showed that titanium dioxide (TiO2) surfaces doped with nitrogen (N) and bismuth (Bi) exhibited photocatalytic and antimicrobial activity only under visible light activation. Thus, when light activated, the electrons go into the TiO2 molecule and move from the valence band to the conduction band, creating holes. It generates reactive oxygen species that are responsible to promote bacterial cell damage. This proposed surface also exhibited reusability of the photocatalyst, indicating that the surface can be reactivated several times. Jiang et al. (2020) recently developed a similar antibacterial photodynamic therapy where the antibiotics produce reactive oxygen species when under light irradiation. Taking the photocatalytic principle into consideration and the photosensitizer-like antibiotics, the combination of photocatalytic coatings loaded with these antibiotics might be a suitable strategy to reduce microbial accumulation onto implant surfaces under demand and can be activated whenever needed.
Therefore, as future challenges, the antibiotic-loaded coatings on Ti material should consider a long-term and sustained effect, the ability to reload the surface and to keep an effective local bacteriostatic and bactericidal effect at the implant surface without affecting the host-response or causing cytotoxicity to the host tissues surrounding the implants. Moreover, the most used antibiotics on coatings described in our systematic review, gentamicin and vancomycin, are not commonly used for oral infections due to their spectrum of action focusing on aerobic gram-negative bacilli. Thus, for dental implant applications, better targeted antibiotics, such as amoxicillin and metronidazole, should be considered acting against gram negative, anaerobic bacteria that are relevant to peri-implantitis (Costa et al., 2020b).
Therefore, antibiotic-loaded coatings on Ti material are a promising approach to control implant-related infections, but some challenges, such as prolong effectiveness, enhanced surface properties, and effect on microbial load and in the microbial composition, need to be considered by future studies. In a nutshell, this review sheds light on potential alternative strategies aiming to reduce microbial loads and control polymicrobial infection on implanted devices.
Limitations of the study
The systematic review focused only on antibiotic-loaded coatings on titanium surface, but other antimicrobials agents can be used to achieve bacterial killing and prevent microbial infections. Moreover, dental implants can be made with different biomaterials, which was not considered in our systematic review. Finally, the studies included in our systematic review have some limitations. The antibiotic-loaded coatings evaluated require long-term evaluations by in vitro, animal and clinical models to determine their effect on reducing microbial loads and biofilm accumulation. Qualitative analysis of bacterial presence is not enough to assess anti-biofilm activity. Moreover, the effect of newly developed coatings on microbial composition needs to be evaluated, because dysbiotic biofilms are responsible for exacerbated inflammatory responses. In this attempt, microbiological assessment should consider the microbial diversity and environment that mimic the in vivo condition.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This study was supported by the São Paulo Research Foundation (FAPESP) grant number 2020/05231-4 and the Brazilian National Council for Scientific and Technological Development (CNPq) grant number 304853/2018-6 to V.A.R.B., and NIH/NIDCR RO1DE013986 and NIH/NIGMS RO1GM127909 to A.D.B. Graphical abstract was created by BioRender®.
Author contributions
Conceptualization, J.G.S.S., M.B., A.D.B., and V.A.R.B.; Methodology, J.G.S.S., R.C.C., and B.E.N.; Investigation, J.G.S.S., M.B., R.C.C., and B.E.N.; Writing—Original Draft, J.G.S.S. and M.B.; Writing—Review & Editing, M.B., R.C.C., B.E.N., A.D.B., and V.A.R.B.; Supervision, A.D.B. and V.A.R.B.; Funding Acquisition, A.D.B. and V.A.R.B.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2020.102008.
Supplemental information
References
- Abusleme L., Dupuy A.K., Dutzan N., Silva N., Burleson J.A., Strausbaugh L.D., Gamonal J., Diaz P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016–1025. doi: 10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrektsson T., Wennerberg A. Oral implant surfaces: Part 1–review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004;17:536–543. [PubMed] [Google Scholar]
- Adams C.S., Antoci V., Jr., Harrison G., Patal P., Freeman T.A., Shapiro I.M., Parvizi J., Hickok N.J., Radin S., Ducheyne P. Controlled release of vancomycin from thin sol-gel films on implant surfaces successfully controls osteomyelitis. J. Orthop. Res. 2009;27:701–709. doi: 10.1002/jor.20815. [DOI] [PubMed] [Google Scholar]
- Alt V., Kirchhof K., Seim F., Hrubesch I., Lips K.S., Mannel H., Domann E., Schnettler R. Rifampicin-fosfomycin coating for cementless endoprostheses: antimicrobial effects against methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) Acta Biomater. 2014;10:4518–4524. doi: 10.1016/j.actbio.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Antoci V., Jr., Adams C.S., Hickok N.J., Shapiro I.M., Parvizi J. Vancomycin bound to Ti rods reduces periprosthetic infection: preliminary study. Clin. Orthop. Relat. Res. 2007;461:88–95. doi: 10.1097/BLO.0b013e318073c2b2. [DOI] [PubMed] [Google Scholar]
- Arciola C.R., Campoccia D., Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018;16:397–409. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- Auñón Á., Esteban J., Doadrio A.L., Boiza-Sánchez M., Mediero A., Eguibar-Blázquez D., Cordero-Ampuero J., Conde A., Arenas M.Á., de-Damborenea J.J. Staphylococcus aureus prosthetic joint infection is prevented by a fluorine- and phosphorus-doped nanostructured Ti-6Al-4V alloy loaded with gentamicin and vancomycin. J. Orthop. Res. 2020;38:588–597. doi: 10.1002/jor.24496. [DOI] [PubMed] [Google Scholar]
- Aykut S., Oztürk A., Ozkan Y., Yanik K., Ilman A.A., Ozdemir R.M. Evaluation and comparison of the antimicrobial efficacy of teicoplanin- and clindamycin-coated titanium implants: an experimental study. J. Bone Joint Surg. Br. 2010;92:159–163. doi: 10.1302/0301-620X.92B1.22134. [DOI] [PubMed] [Google Scholar]
- Badar M., Rahim M.I., Kieke M., Ebel T., Rohde M., Hauser H., Behrens P., Mueller P.P. Controlled drug release from antibiotic-loaded layered double hydroxide coatings on porous titanium implants in a mouse model. J. Biomed. Mater. Res. A. 2015;103:2141–2149. doi: 10.1002/jbm.a.35358. [DOI] [PubMed] [Google Scholar]
- Berglundh T., Armitage G., Araujo M.G., Avila-Ortiz G., Blanco J., Camargo P.M., Chen S., Cochran D., Derks J., Figuero E. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018;45:S286–S291. doi: 10.1111/jcpe.12957. [DOI] [PubMed] [Google Scholar]
- Bertolini M., Ranjan A., Thompson A., Diaz P.I., Sobue T., Maas K., Dongari-Bagtzoglou A. Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog. 2019;15:e1007717. doi: 10.1371/journal.ppat.1007717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen W.H., Burne R.A., Wu H., Koo H. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26:229–242. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürgers R., Morsczeck C., Felthaus O., Gosau M., Beck H.C., Reichert T.E. Induced surface proteins of Staphylococcus epidermidis adhering to titanium implant substrata. Clin. Oral Investig. 2018;22:2663–2668. doi: 10.1007/s00784-018-2508-9. [DOI] [PubMed] [Google Scholar]
- Canullo L., Radovanović S., Delibasic B., Blaya J.A., Penarrocha D., Rakic M. The predictive value of microbiological findings on teeth, internal and external implant portions in clinical decision making. Clin. Oral Implants Res. 2017;28:512–519. doi: 10.1111/clr.12828. [DOI] [PubMed] [Google Scholar]
- Cao Y., Su B., Chinnaraj S., Jana S., Bowen L., Charlton S., Duan P., Jakubovics N.S., Chen J. Nanostructured titanium surfaces exhibit recalcitrance towards Staphylococcus epidermidis biofilm formation. Sci. Rep. 2018;8:1071. doi: 10.1038/s41598-018-19484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcuac O., Derks J., Charalampakis G., Abrahamsson I., Wennström J., Berglundh T. Adjunctive systemic and local antimicrobial therapy in the surgical treatment of peri-implantitis: a randomized controlled clinical trial. J. Dent. Res. 2016;95:50–57. doi: 10.1177/0022034515601961. [DOI] [PubMed] [Google Scholar]
- Ccahuana-Vásquez R.A., Tabchoury C.P., Tenuta L.M., Del Bel Cury A.A., Vale G.C., Cury J.A. Effect of frequency of sucrose exposure on dental biofilm composition and enamel demineralization in the presence of fluoride. Caries Res. 2007;41:9–15. doi: 10.1159/000096100. [DOI] [PubMed] [Google Scholar]
- Chang Y.Y., Zhang J.H., Huang H.L. Effects of laser texture oxidation and high-temperature annealing of TiV alloy thin films on mechanical and antibacterial properties and cytotoxicity. Materials (Basel) 2018;11:2495. doi: 10.3390/ma11122495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Feng G., Moraru C.I. Micro- and nanotopography sensitive bacterial attachment mechanisms: a review. Front. Microbiol. 2019;10:191. doi: 10.3389/fmicb.2019.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouirfa H., Bouloussa H., Migonney V., Falentin-Daudré C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019;83:37–54. doi: 10.1016/j.actbio.2018.10.036. [DOI] [PubMed] [Google Scholar]
- Costa R.C., Souza J.G.S., Cordeiro J.M., Bertolini M., de Avila E.D., Landers R., Rangel E.C., Fortulan C.A., Retamal-Valdes B., da Cruz N.C. Synthesis of bioactive glass-based coating by plasma electrolytic oxidation: untangling a new deposition pathway toward titanium implant surfaces. J. Colloid Interface Sci. 2020;579:680–698. doi: 10.1016/j.jcis.2020.06.102. [DOI] [PubMed] [Google Scholar]
- Costa R.C., Souza J.G.S., Bertolini M., Retamal-Valdes B., Feres M., Barão V.A.R. Extracellular biofilm matrix leads to microbial dysbiosis and reduces biofilm susceptibility to antimicrobials on titanium biomaterial: an in vitro and in situ study. Clin. Oral Implants Res. 2020;31:1173–1186. doi: 10.1111/clr.13663. [DOI] [PubMed] [Google Scholar]
- Costerton J.W., Lewandowski Z., Caldwell D.E., Korber D.R., Lappin-Scott H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Croes M., Bakhshandeh S., van Hengel I., Lietaert K., van Kessel K., Pouran B., van der Wal B., Vogely H.C., Van Hecke W., Fluit A.C. Antibacterial and immunogenic behavior of silver coatings on additively manufactured porous titanium. Acta Biomater. 2018;81:315–327. doi: 10.1016/j.actbio.2018.09.051. [DOI] [PubMed] [Google Scholar]
- Dabdoub S.M., Tsigarida A.A., Kumar P.S. Patient-specific analysis of periodontal and peri-implant microbiomes. J. Dent. Res. 2013;92 doi: 10.1177/0022034513504950. 168S-75S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darouiche R.O., Mansouri M.D., Zakarevicz D., Alsharif A., Landon G.C. In vivo efficacy of antimicrobial-coated devices. J. Bone Joint Surg. Am. 2007;89:792–797. doi: 10.2106/JBJS.F.00414. [DOI] [PubMed] [Google Scholar]
- Darouiche R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- Davidson H., Poon M., Saunders R., Shapiro I.M., Hickok N.J., Adams C.S. Tetracycline tethered to titanium inhibits colonization by gram-negative bacteria. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103:1381–1389. doi: 10.1002/jbm.b.33310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst F.E., Chen T., Izard J., Paster B.J., Tanner A.C., Yu W.H., Lakshmanan A., Wade W.G. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbeck M., Schrader C., Gras F., Mückley T., Schmidt J., Zankovych S., Bossert J., Jandt K.D., Völpel A., Sigusch B.W. Gentamicin coating of plasma chemical oxidized titanium alloy prevents implant-related osteomyelitis in rats. Biomaterials. 2016;101:156–164. doi: 10.1016/j.biomaterials.2016.05.039. [DOI] [PubMed] [Google Scholar]
- Dodo C.G., Senna P.M., Custodio W., Paes Leme A.F., Del Bel Cury A.A. Proteome analysis of the plasma protein layer adsorbed to a rough titanium surface. Biofouling. 2013;29:549–557. doi: 10.1080/08927014.2013.787416. [DOI] [PubMed] [Google Scholar]
- Fenton O.S., Olafson K.N., Pillai P.S., Mitchell M.J., Langer R. Advances in biomaterials for drug delivery. Adv. Mater. 2018:e1705328. doi: 10.1002/adma.201705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feres M., Figueiredo L.C., Soares G.M., Faveri M. Systemic antibiotics in the treatment of periodontitis. Periodontol. 2015;2000(67):131–186. doi: 10.1111/prd.12075. [DOI] [PubMed] [Google Scholar]
- Ferraris S., Spriano S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;61:965–978. doi: 10.1016/j.msec.2015.12.062. [DOI] [PubMed] [Google Scholar]
- Figuero E., Graziani F., Sanz I., Herrera D., Sanz M. Management of peri-implant mucositis and peri-implantitis. Periodontol. 2014;2000(66):255–273. doi: 10.1111/prd.12049. [DOI] [PubMed] [Google Scholar]
- Flemming H.C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Franz S., Rammelt S., Scharnweber D., Simon J.C. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- Fürst M.M., Salvi G.E., Lang N.P., Persson G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implants Res. 2007;18:501–508. doi: 10.1111/j.1600-0501.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- Ghimire A., Skelly J.D., Song J. Micrococcal-nuclease-triggered on-demand release of vancomycin from intramedullary implant coating eradicates Staphylococcus aureus infection in mouse femoral canals. ACS Cent. Sci. 2019;5:1929–1936. doi: 10.1021/acscentsci.9b00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann S., Menne M., Hesse D., Bischoff S., Schiffner R., Diefenbeck M., Liefeith K. Biomimetic multilayer coatings deliver gentamicin and reduce implant-related osteomyelitis in rats. Biomed. Tech. (Berl) 2019;64:383–395. doi: 10.1515/bmt-2018-0044. [DOI] [PubMed] [Google Scholar]
- Heitz-Mayfield L.J., Mombelli A. The therapy of peri-implantitis: a systematic review. Int. J. Oral Maxillofac. Implants. 2014;29:325–345. doi: 10.11607/jomi.2014suppl.g5.3. [DOI] [PubMed] [Google Scholar]
- Heitz-Mayfield L.J., Lang N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol. 2010;2000(53):167–181. doi: 10.1111/j.1600-0757.2010.00348.x. [DOI] [PubMed] [Google Scholar]
- Hickok N.J., Shapiro I.M. Immobilized antibiotics to prevent orthopaedic implant infections. Adv. Drug Deliv. Rev. 2012;64:1165–1176. doi: 10.1016/j.addr.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson O., Sörensen J.H., Strømme M., Engqvist H., Procter P., Welch K. Evaluation of an alkali-treated and hydroxyapatite-coated orthopedic implant loaded with tobramycin. J. Biomater. Appl. 2019;34:699–720. doi: 10.1177/0885328219867968. [DOI] [PubMed] [Google Scholar]
- Jenkinson H.F. Beyond the oral microbiome. Environ. Microbiol. 2011;13:3077–3087. doi: 10.1111/j.1462-2920.2011.02573.x. [DOI] [PubMed] [Google Scholar]
- Jennings J.A., Beenken K.E., Skinner R.A., Meeker D.G., Smeltzer M.S., Haggard W.O., Troxel K.S. Antibiotic-loaded phosphatidylcholine inhibits staphylococcal bone infection. World J. Orthop. 2016;18:467–474. doi: 10.5312/wjo.v7.i8.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q.I., Fangiie E., Tian J., Yang J., Zhang J., Che Y. Light-excited antibiotics for potentiating bacterial killing via reactive oxygen species generation. ACS Appl. Mater. Interfaces. 2020;12:16150–16158. doi: 10.1021/acsami.0c02647. [DOI] [PubMed] [Google Scholar]
- Kalasin S., Santore M.M. Non-specific adhesion on biomaterial surfaces driven by small amounts of protein adsorption. Colloids Surf. B Biointerfaces. 2009;73:229–236. doi: 10.1016/j.colsurfb.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Kälicke T., Schierholz J., Schlegel U., Frangen T.M., Köller M., Printzen G., Seybold D., Klöckner S., Muhr G., Arens S. Effect on infection resistance of a local antiseptic and antibiotic coating on osteosynthesis implants: an in vitro and in vivo study. J. Orthop. Res. 2006;24:1622–1640. doi: 10.1002/jor.20193. [DOI] [PubMed] [Google Scholar]
- Khan S.T., Al-Khedhairy A.A., Musarrat J. ZnO and TiO2 nanoparticles as novel antimicrobial agents for oral hygiene: a review. J. Nanopart. Res. 2015;17:276. [Google Scholar]
- Kolenbrander P.E., Palmer R.J., Rickard A.H., Jakubovics N.S., Chalmers N.I., Diaz P. Bacterial interactions and successions during plaque development. Periodontol. 2006;2000(42):47–79. doi: 10.1111/j.1600-0757.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Koo H., Falsetta M.L., Klein M.I. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J. Dent. Res. 2013;92:1065–1073. doi: 10.1177/0022034513504218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H., Allan R.N., Howlin R.P., Stoodley P., Hall-Stoodley L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017;15:740–755. doi: 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharíková S., Gerits E., De Brucker K., Braem A., Ceh K., Majdic G., Spanic T., Pogorovec E., Verstraeten N., Tournu H. Covalent immobilization of antimicrobial agents on titanium prevents Staphylococcus aureus and Candida albicans colonization and biofilm formation. J. Antimicrob. Chemother. 2016;71:936–945. doi: 10.1093/jac/dkv437. [DOI] [PubMed] [Google Scholar]
- Kumar P.S., Mason M.R., Brooker M.R., O’Brien K. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J. Clin. Periodontol. 2012;39:425–433. doi: 10.1111/j.1600-051X.2012.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N.P., Bragger U., Walther D., Beamer B., Kornman K.S. Ligature-induced peri-implant infection in cynomolgus monkeys. I. Clinical and radiographic findings. Clin. Oral Implants Res. 1993;4:2–11. doi: 10.1034/j.1600-0501.1993.040101.x. [DOI] [PubMed] [Google Scholar]
- Lendenmann U., Grogan J., Oppenheim F.G. Saliva and dental pellicle—a review. Adv. Dent. Res. 2000;14:22–28. doi: 10.1177/08959374000140010301. [DOI] [PubMed] [Google Scholar]
- Leonhardt A., Renvert S., Dahlén G. Microbial findings at failing implants. Clin. Oral Implants Res. 1999;10:339–345. doi: 10.1034/j.1600-0501.1999.100501.x. [DOI] [PubMed] [Google Scholar]
- Li D., Ferguson S.J., Beutler T., Cochran D.L., Sittig C., Hirt H.P., Buser D. Biomechanical comparison of the sandblasted and acid-etched and the machined and acid-etched titanium surface for dental implants. J. Biomed. Mater. Res. 2002;60:325–332. doi: 10.1002/jbm.10063. [DOI] [PubMed] [Google Scholar]
- Lima E.M., Koo H., Vacca Smith A.M., Rosalen P.L., Del Bel Cury A.A. Adsorption of salivary and serum proteins, and bacterial adherence on titanium and zirconia ceramic surfaces. Clin. Oral Implants Res. 2008;19:780–785. doi: 10.1111/j.1600-0501.2008.01524.x. [DOI] [PubMed] [Google Scholar]
- Lindhe J., Berglundh T., Ericsson I., Liljenberg B., Marinello C. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin. Oral Implants Res. 1992;3:9–16. doi: 10.1034/j.1600-0501.1992.030102.x. [DOI] [PubMed] [Google Scholar]
- Liu D., He C., Liu Z., Xu W. Gentamicin coating of nanotubular anodized titanium implant reduces implant-related osteomyelitis and enhances bone biocompatibility in rabbits. Int. J. Nanomedicine. 2017;12:5461–5471. doi: 10.2147/IJN.S137137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti M., Dogša I., Stošicki T., Stopar D., Kalin M., Kobe S., Novak S. The influence of surface modification on bacterial adhesion to titanium-based substrates. ACS Appl. Mater. Interfaces. 2015;7:1644–1651. doi: 10.1021/am507148n. [DOI] [PubMed] [Google Scholar]
- Lucke M., Schmidmaier G., Sadoni S., Wildemann B., Schiller R., Haas N.P., Raschke M. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone. 2003;32:521–531. doi: 10.1016/s8756-3282(03)00050-4. [DOI] [PubMed] [Google Scholar]
- Lucke M., Wildemann B., Sadoni S., Surke C., Schiller R., Stemberg A., Raschke M., Haas N.P., Schmidmaier G. Systemic versus local application of gentamicin in prophylaxis of implant-related osteomyelitis in a rat model. Bone. 2005;36:770–778. doi: 10.1016/j.bone.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Ma K., Cai X., Zhou Y., Wang Y., Jiang T. In vitro and in vivo evaluation of tetracycline loaded chitosan-gelatin nanosphere coatings for titanium surface functionalization. Macromol. Biosci. 2017;17:2. doi: 10.1002/mabi.201600130. [DOI] [PubMed] [Google Scholar]
- Marsh P.D., Devine D.A. How is the development of dental biofilms influenced by the host? J. Clin. Periodontol. 2011;38:28–35. doi: 10.1111/j.1600-051X.2010.01673.x. [DOI] [PubMed] [Google Scholar]
- Marsh P.D., Moter A., Devine D.A. Dental plaque biofilms: communities, conflict and control. Periodontol. 2011;2000(55):16–35. doi: 10.1111/j.1600-0757.2009.00339.x. [DOI] [PubMed] [Google Scholar]
- Matos A.O., Ricomini-Filho A.P., Beline T., Ogawa E.S., Costa-Oliveira B.E., Almeida A.B., Nociti Junior F.H., Rangel E.C., da Cruz N.C., Sukotjo C. Three-species biofilm model onto plasma-treated titanium implant surface. Colloids Surf. B Biointerfaces. 2017;152:354–366. doi: 10.1016/j.colsurfb.2017.01.035. [DOI] [PubMed] [Google Scholar]
- Melo F., Nascimento C., Souza D.O., Albuquerque R.F. Identification of oral bacteria on titanium implant surfaces by 16S rDNA sequencing. Clin. Oral Implants Res. 2017;28:697–703. doi: 10.1111/clr.12865. [DOI] [PubMed] [Google Scholar]
- Mercado F., Hamlet S., Ivanovski S. Regenerative surgical therapy for peri-implantitis using deproteinized bovine bone mineral with 10% collagen, enamel matrix derivative and doxycycline-A prospective 3-year cohort study. Clin. Oral Implants Res. 2018;29:583–591. doi: 10.1111/clr.13256. [DOI] [PubMed] [Google Scholar]
- Metsemakers W.J., Emanuel N., Cohen O., Reichart M., Potapova I., Schmid T., Segal D., Riool M., Kwakman P.H.S., Boer L. A doxycycline-loaded polymer-lipid encapsulation matrix coating for the prevention of implant-related osteomyelitis due to doxycycline-resistant methicillin-resistant Staphylococcus aureus. J. Control Release. 2015;209:47–56. doi: 10.1016/j.jconrel.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Mombelli A., Décaillet F. The characteristics of biofilms in peri-implant disease. J. Clin. Periodontol. 2011;38:203–213. doi: 10.1111/j.1600-051X.2010.01666.x. [DOI] [PubMed] [Google Scholar]
- Moskowitz J.S., Blaisse M.R., Samuel R.E., Hsu H.P., Harris M.B., Martin S.D., Lee J.C., Spector M., Hammond P.T. The effectiveness of the controlled release of gentamicin from polyelectrolyte multilayers in the treatment of Staphylococcus aureus infection in a rabbit bone model. Biomaterials. 2010;31:6019–6030. doi: 10.1016/j.biomaterials.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagay B.E., Dini C., Cordeiro J.M., Ricomini-Filho A.P., Avila E.D., Rangel E.C., Cruz N.C., Barao V.A.R. Visible-light-induced photocatalytic and antibacterial activity of TiO 2 codoped with nitrogen and bismuth: new perspectives to control implant-biofilm-related diseases. ACS Appl. Mater. Inter. 2019;11:18186–18202. doi: 10.1021/acsami.9b03311. [DOI] [PubMed] [Google Scholar]
- Naginyte M., Do T., Meade J., Devine D.A., Marsh P.D. Enrichment of periodontal pathogens from the biofilms of healthy adults. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-41882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neut D., Dijkstra R.J., Thompson J.I., Kavanagh C., van der Mei H.C., Busscher H.J. A biodegradable gentamicin-hydroxyapatite-coating for infection prophylaxis in cementless hip prostheses. Eur. Cell. Mater. 2015;29:42–56. doi: 10.22203/ecm.v029a04. [DOI] [PubMed] [Google Scholar]
- Nguyen Vo., Hao J., Chou J., Oshima M., Aoki K., Kuroda S., Kaboosaya B., Kasugai S. Ligature induced peri-implantitis: tissue destruction and inflammatory progression in a murine model. Clin. Oral Implants Res. 2017;28:129–136. doi: 10.1111/clr.12770. [DOI] [PubMed] [Google Scholar]
- Nie B., Ao H., Long T., Zhou J., Tang T., Yue B. Immobilizing bacitracin on titanium for prophylaxis of infections and for improving osteoinductivity: an in vivo study. Colloids Surf. B Biointerfaces. 2017;150:183–191. doi: 10.1016/j.colsurfb.2016.11.034. [DOI] [PubMed] [Google Scholar]
- Nie B., Long T., Ao H., Zhou J., Tang T., Yue B. Covalent immobilization of enoxacin onto titanium implant surfaces for inhibiting multiple bacterial species infection and in vivo methicillin-resistant Staphylococcus aureus infection prophylaxis. Antimicrob. Agents Chemother. 2016;61 doi: 10.1128/AAC.01766-16. e01766–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A., Pavone P., D'Abramo A., Iannetta M., Mastroianni C.M., Vullo V. Role of sonication in the microbiological diagnosis of implant-associated infections: beyond the orthopedic prosthesis. Adv. Exp. Med. Biol. 2016;897:85–102. doi: 10.1007/5584_2015_5007. [DOI] [PubMed] [Google Scholar]
- Oliveira W.F., Silva P.M.S., Silva R.C.S., Silva G.M.M., Machado G., Coelho L.C.B.B., Correia M.T.S. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018;98:111–117. doi: 10.1016/j.jhin.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Padial-Molina M., Lopez-Martinez J., OValle F., Galindo-Moreno P. Microbial profiles and detection techniques in peri-implant diseases: a systematic review. J. Oral Maxillofac. Res. 2016;7:e10. doi: 10.5037/jomr.2016.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaroto H.N., Amorim K.P., Cordeiro J.M., Souza J.G.S., Ricomini-Filho A.P., Rangel E.C., Barao V.A.R. Proteome analysis of the salivary pellicle formed on titanium alloys containing niobium and zirconium. Biofouling. 2019;35:173–186. doi: 10.1080/08927014.2019.1580360. [DOI] [PubMed] [Google Scholar]
- Perni S., Alotaibi H.F., Yergeshov A.A., Dang T., Abdullin T.I., Prokopovich P. Long acting anti-infection constructs on titanium. J. Control Release. 2020;326:91–105. doi: 10.1016/j.jconrel.2020.06.013. [DOI] [PubMed] [Google Scholar]
- Pokrowiecki R. The paradigm shift for drug delivery systems for oral and maxillofacial implants. Drug Deliv. 2018;25:1504–1515. doi: 10.1080/10717544.2018.1477855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett S.D., Taylor E., Raimondo T., Webster T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials. 2010;31:706–713. doi: 10.1016/j.biomaterials.2009.09.081. [DOI] [PubMed] [Google Scholar]
- Rabe M., Verdes D., Seeger S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011;162:87–106. doi: 10.1016/j.cis.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Renvert S., Lessem J., Dahlen G., Lindahl C., Svensson M. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri-implant infections: a randomized clinical trial. J. Clin. Periodontol. 2006;33:362–369. doi: 10.1111/j.1600-051X.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- Retamal-Valdes B., Formiga M.C., Almeida M.L., Fritoli A., Figueiredo K.A., Westphal M., Gomes P., Feres M. Does subgingival bacterial colonization differ between implants and teeth? A systematic review. Braz. Oral Res. 2019;33:e064. doi: 10.1590/1807-3107bor-2019.vol33.0064. [DOI] [PubMed] [Google Scholar]
- Romero-Gavilán F., Gomes N.C., Ródenas J., Sánchez A., Azkargorta M., Iloro I., Elortza F., Arnáez I.A., Gurruchaga M., Goñi I., Suay I. Proteome analysis of human serum proteins adsorbed onto different titanium surfaces used in dental implants. Biofouling. 2017;33:98–111. doi: 10.1080/08927014.2016.1259414. [DOI] [PubMed] [Google Scholar]
- Rosier B.T., Marsh P.D., Mira A. Resilience of the oral microbiota in health: mechanisms that prevent dysbiosis. J. Dent. Res. 2018;97:371–380. doi: 10.1177/0022034517742139. [DOI] [PubMed] [Google Scholar]
- Salvi G.E., Cosgarea R., Sculean A. Prevalence and mechanisms of peri-implant diseases. J. Dent. Res. 2017;96:31–37. doi: 10.1177/0022034516667484. [DOI] [PubMed] [Google Scholar]
- Schincaglia G.P., Hong B.Y., Rosania A., Barasz J., Thompson A., Panagakos F., Burleson J.A., Dongari-Bagtzoglou A., Diaz P.I. Clinical, immune, and microbiome traits of gingivitis and peri-implant mucositis. J. Dent. Res. 2017;96:47–55. doi: 10.1177/0022034516668847. [DOI] [PubMed] [Google Scholar]
- Schmidlin P.R., Muller P., Attin T., Wieland M., Hofer D., Guggenheim B. Polyspecies biofilm formation on implant surfaces with different surface characteristics. J. Appl. Oral Sci. 2013;21:48–55. doi: 10.1590/1678-7757201302312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou S., Holmstrup P., Hjørting-Hansen E., Kornman K.S. Ligature-induced marginal inflammation around osseointegrated implants and ankylosed teeth. Clin. Oral Implant Res. 1993;4:12–22. doi: 10.1034/j.1600-0501.1993.040102.x. [DOI] [PubMed] [Google Scholar]
- Schwarz F., Becker K., Rahn S., Hegewald A., Pfeffer K., Henrich B. Real-time PCR analysis of fungal organisms and bacterial species at peri-implantitis sites. Int. J. Implant Dent. 2015;1:9. doi: 10.1186/s40729-015-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibli J.A., Melo L., Ferrari D.S., Figueiredo L.C., Faveri M., Feres M. Composition of supra-and subgingival biofilm of subjects with healthy and diseased Implants. Clin. Oral Implants Res. 2008;19:975–982. doi: 10.1111/j.1600-0501.2008.01566.x. [DOI] [PubMed] [Google Scholar]
- Shibli J.A., Ferrari D.S., Siroma R.S., Figueiredo L.C., Faveri M., Feres M. Microbiological and clinical effects of adjunctive systemic metronidazole and amoxicillin in the non-surgical treatment of peri-implantitis: 1 year follow-up. Braz. Oral Res. 2019;33:080. doi: 10.1590/1807-3107bor-2019.vol33.0080. [DOI] [PubMed] [Google Scholar]
- Smith F.R.C., Riollano M., Leung A., Hammond P.T. Layer-by-layer platform technology for small-molecule delivery. Angew. Chem. Int. Ed. Eng. 2009;48:8974–8977. doi: 10.1002/anie.200902782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S.S., Haffajee A.D. Dental biofilms: difficult therapeutic targets. Periodontol. 2002;2000(28):12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- Song F., Koo H., Ren D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015;94:1027–1034. doi: 10.1177/0022034515587690. [DOI] [PubMed] [Google Scholar]
- Song W., Seta J., Chen L., Bergum C., Zhou Z., Kanneganti P., Kast R.E., Auner G.W., Shen M., Markel D.C. Doxycycline-loaded coaxial nanofiber coating of titanium implants enhances osseointegration and inhibits Staphylococcus aureus infection. Biomed. Mater. 2017;12:045008. doi: 10.1088/1748-605X/aa6a26. [DOI] [PubMed] [Google Scholar]
- Souza J.G.S., Bertolini M., Costa R.C., Lima C.V., Barão V.A.R. Proteomic profile of the saliva and plasma protein layer adsorbed on Ti-Zr alloy: the effect of sandblasted and acid-etched surface treatment. Biofouling. 2020;36(4):428–444. doi: 10.1080/08927014.2020.1769613. [DOI] [PubMed] [Google Scholar]
- Souza J.G.S., Bertolini M., Costa R.C., Cordeiro J.M., Nagay B.E., Almeida A.B., Retamal-Valdes B., Nociti F.H., Feres M., Rangel E.C., Barão V.A.R. Targeting pathogenic biofilms: newly developed superhydrophobic coating favors a host-compatible microbial profile on the titanium surface. ACS Appl. Mater. Interfaces. 2020;12:10118–10129. doi: 10.1021/acsami.9b22741. [DOI] [PubMed] [Google Scholar]
- Souza J.G.S., Bertolini M., Thompson A., Barāo V.A.R., Dongari-Bagtzoglou A. Biofilm interactions of Candida albicans and mitis group streptococci in a titanium-mucosal interface model. Appl. Environ. Microbiol. 2020;86 doi: 10.1128/AEM.02950-19. e02950–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza J.G.S., Bertolini M., Thompson A., Mansfield J.M., Grassmann A.A., Maas K., Caimano M.J., Barāo V.A.R., Vickerman M.M., Dongari-Bagtzoglou A. Role of glucosyltransferase R in biofilm interactions between Streptococcus oralis and Candida albicans. ISME J. 2020;14:1207–1222. doi: 10.1038/s41396-020-0608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza J.G.S., Costa-Oliveira B.E., Bertolini M., Lima C.V., Retamal-Valdes B., Faveri M., Feres M., Barāo V.A. Titanium particles and ions favor dysbiosis in oral biofilms. J. Periodontal Res. 2020;55:258–266. doi: 10.1111/jre.12711. [DOI] [PubMed] [Google Scholar]
- Souza J.G.S., Cury J.A., Ricomini Filho A.P., Feres M., Faveri M., Barão V.A.R. Effect of sucrose on biofilm formed in situ on titanium material. J. Periodontol. 2019;90:141–148. doi: 10.1002/JPER.18-0219. [DOI] [PubMed] [Google Scholar]
- Souza J.G.S., Cordeiro J.M., Lima C.V., Barāo V.A.R. Citric acid reduces oral biofilm and influences the electrochemical behavior of titanium: an in situ and in vitro study. J. Periodontol. 2019;90:149–158. doi: 10.1002/JPER.18-0178. [DOI] [PubMed] [Google Scholar]
- Souza J.G.S., Lima C.V., Costa-Oliveira B.E., Ricomini-Filho A.P., Faveri M., Sukotjo C., Feres M., Del Bel Cury A.A., Barão V.A.R. Dose-response effect of chlorhexidine on a multispecies oral biofilm formed on pure titanium and on a titanium-zirconium alloy. Biofouling. 2018;34:1175–1184. doi: 10.1080/08927014.2018.1557151. [DOI] [PubMed] [Google Scholar]
- Spriano S., Yamaguchi S., Baino F., Ferraris S. A critical review of multifunctional titanium surfaces: new frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018;79:1–22. doi: 10.1016/j.actbio.2018.08.013. [DOI] [PubMed] [Google Scholar]
- Stavrakis A.I., Zhu S., Loftin A.H., Weixian X., Niska J., Hegde V., Segura T., Bernthal N.M. Controlled release of vancomycin and tigecycline from an orthopaedic implant coating prevents Staphylococcus aureus infection in an open fracture animal model. Biomed. Res. Int. 2019;2019:1638508. doi: 10.1155/2019/1638508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanović M., Djošić M., Janković A., Kojić V., Vukašinović-Sekulić M., Stojanović J., Odović J., Sakač M.C., Rhee K.Y., Mišković-Stanković V. Antibacterial graphene-based hydroxyapatite/chitosan coating with gentamicin for potential applications in bone tissue engineering. J. Biomed. Mater. Res. A. 2020;108:2175–2189. doi: 10.1002/jbm.a.36974. [DOI] [PubMed] [Google Scholar]
- Sultan A.S., Kong E.F., Rizk A.M., Jabra-Rizk M.A. The oral microbiome: a Lesson in coexistence. PLoS Pathog. 2018;14:e1006719. doi: 10.1371/journal.ppat.1006719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K., Petkov S., Zeiter S., Sprecher C.M., Richards R.G., Moriarty T.F., Eijer H. Intraoperative loading of calcium phosphate-coated implants with gentamicin prevents experimental Staphylococcus aureus infection in vivo. PLoS One. 2019;14:e0210402. doi: 10.1371/journal.pone.0210402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkelhoff A.J. Antibiotics in the treatment of peri-implantitis. Eur. J. Oral Implantol. 2012;5:S43–S50. [PubMed] [Google Scholar]
- Welch J.L.M., Dewhirst F.E., Borusy G.G. Biogeography of the oral microbiome: the site-specialist hypothesis. Annu. Rev. Microbiol. 2019;73:335–358. doi: 10.1146/annurev-micro-090817-062503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead K.A., Rogers D., Colligon J., Wright C., Verran J. Use of the atomic force microscope to determine the effect of substratum surface topography on the ease of bacterial removal. Colloids Surf. B Biointerfaces. 2006;51:44–53. doi: 10.1016/j.colsurfb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Whittaker C.J., Klier C.M., Kolenbrander P.E. Mechanisms of adhesion by oral bacteria. Annu. Rev. Microbiol. 1996;50:513–552. doi: 10.1146/annurev.micro.50.1.513. [DOI] [PubMed] [Google Scholar]
- Williams R.C., Paquette D.W., Offenbacher S., Adams D.F., Armitage G.C., Bray K., Caton J., Cochran D.L., Drisko C.H., Fiorellini J.P. Treatment of periodontitis by local administration of minocycline microspheres: a controlled trial. J. Periodontol. 2001;72:1535–1544. doi: 10.1902/jop.2001.72.11.1535. [DOI] [PubMed] [Google Scholar]
- Xiao J., Klein M.I., Falsetta M.L., Lu B., Delahunty C.M., Yates J.R., Heydorn A., Koo H. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012;8:e1002623. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Sobue T., Thompson A., Xie Z., Poon K., Ricker A., Cervantes J., Diaz P.I., Dongari-Bagtzoglou A. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell. Microbiol. 2014;16:214–231. doi: 10.1111/cmi.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Jenkinson H.F., Dongari-Bagtzoglou A. Innocent until proven guilty: mechanisms and roles of Streptococcus-Candida interactions in oral health and disease. Mol. Oral Microbiol. 2014;29:99–116. doi: 10.1111/omi.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Ao H.Y., Yang S.B., Wang Y.G., Lin W.T., Yu Z.F., Tang T.T. In vivo evaluation of the anti-infection potential of gentamicin-loaded nanotubes on titania implants. Int. J. Nanomedicine. 2016;11:2223–2234. doi: 10.2147/IJN.S102752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T.S. Effect of titanium-ion on the growth of various bacterial species. J. Microbiol. 2004;42:47–50. [PubMed] [Google Scholar]
- Yuan Z., Huang S., Lan S., Xiong H., Tao B., Ding Y., Liu Y., Liu P., Cai K. Surface engineering of titanium implants with enzyme-triggered antibacterial properties and enhanced osseointegration in vivo. J. Mater. Chem. B. 2018;6:8090–8140. doi: 10.1039/c8tb01918e. [DOI] [PubMed] [Google Scholar]
- Zaura E., Keijser B.J.F., Huse S.M., Crielaard W. Defining the healthy "core microbiome" of oral microbial communities. BMC Microbiol. 2009;9:259. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., Wang Y., Sun Z., Chang H., Cao M., Lin K., Xie Y. A novel biocompatible PDA/IR820/DAP coating for antibiotic/photodynamic/photothermal triple therapy to inhibit and eliminate Staphylococcus aureus biofilm. Chem. Eng. 2020;394:1250172. [Google Scholar]
- Zhang B., Braun B.M., Skelly J.D., Ayers D.C., Song J. Significant suppression of Staphylococcus aureus colonization on intramedullary Ti6Al4V implants surface-grafted with vancomycin-bearing polymer brushes. ACS Appl. Mater. Interfaces. 2019;11:28641–28647. doi: 10.1021/acsami.9b07648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wang G., Liu P., Tong D., Ding C., Zhang Z., Xie Y., Tang H., Ji H. Vancomycin-loaded titanium coatings with an interconnected micro-patterned structure for prophylaxis of infections: an in vivo study. RSC Adv. 2018;8:9223–9231. doi: 10.1039/c7ra12347g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Liu Q., Zhou Z., Lu W., Tao J. Efficacy of tobramycin-loaded coating K-wire in an open-fracture rabbit model contaminated by staphylococcus aureus. Int. J. Clin. Exp. Med. 2017;10:6004–6016. [Google Scholar]
- Zitzmann N.U., Berglundh T., Marinello C.P., Lindhe J. Experimental peri-implant mucositis in man. J. Clin. Periodontol. 2001;28:517–523. doi: 10.1034/j.1600-051x.2001.028006517.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.