Abstract
Coronavirus disease 2019 (COVID-19) is an emerging infectious disease which has had a rapid surge in cases and deaths since it is first documented in Wuhan, China, in December 2019. COVID-19 is caused by the Betacoronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 uses angiotensin-converting enzyme 2, which is highly expressed in the human lower respiratory tract but also in other tissues, as the cellular entry receptor. Thus, COVID-19 mainly affects the respiratory system but can cause damage to other body systems, including the cardiovascular, gastrointestinal, hepatobiliary, renal, and central nervous systems. We review the pathogenesis and clinical manifestations of the infection, focusing on our current understanding of the disease mechanisms and their translation to clinical outcomes, as well as adverse effects on different body systems. We also discuss the epidemiology pathogenesis, clinical, and multi-organ consequences, and highlight some of the research gaps regarding COVID-19.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Epidemiology, Transmission, Clinical symptoms
Introduction
Early in December 2019, a cluster of patients with pneumonia of unknown origin was successively reported in some hospitals in Wuhan city, China, with a history of exposure to the Huanan Seafood Wholesale Market. A few days later, Chinese health authorities confirmed that this cluster of pneumonia was associated with a novel coronavirus (CoV) infection. The new virus was initially called the 2019 novel coronavirus (2019-nCoV), and the viral infection soon spread widely across the globe [1, 2]. The novel CoV was later named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the Coronavirus Study Group, and the disease was called coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) [3, 4]. The WHO declared this outbreak as a public health emergency of international concern on January 30, 2020, and a pandemic on March 11.
The SARS-CoV-2 is a positive-stranded RNA virus from the Coronaviridae family. Previous outbreaks of other CoVs include severe acute respiratory syndrome (SARS) in 2002 and the Middle East respiratory syndrome (MERS) in 2012. While the overall mortality rate for COVID-19 seems lower than for SARS and MERS, the death rate among severe cases infected by SARS-CoV-2 is alarming [5, 6]. The ultimate scope and effects of this pandemic are unclear at present since the situation is rapidly evolving. The available limited epidemiological and clinical data for SARS-CoV-2 infection suggest that the disease spectrum and transmission efficiency of this virus differ from those reported for SARS-CoV [1, 7–9].
The clinical presentations of SARS-CoV-2 occur across a broad spectrum of severity, ranging from asymptomatic to severe or even fatal in some cases. Currently, there is no US Food and Drug Administration (FDA)-approved treatment available for COVID-19, and numerous clinical trials are ongoing worldwide. The FDA recently granted permission for the emergency use of remdesivir to treat hospitalized patients with severe COVID-19. A recent study has shown that early administration of dexamethasone could reduce the duration of mechanical ventilation and mortality in patients with established moderate-to-severe patients with acute respiratory distress syndrome (ARDS) [10]. The present review aims to summarize the present understanding of SARS-CoV-2 infection, including epidemiology, clinical characteristics, and systemic manifestations, and highlights current research gaps.
Incubation period
The first prediction of the mean incubation period of COVID-19 infection was 5.2 days [95% confidence interval (CI) 4.1–7.0 days] [11]. A large study with 4021 patients reported an incubation period of 4.75 days (interquartile range 3.0–7.2 days) [12], while another study assessed that rarely the incubation period could be as long as 24 days in some cases [13]. However, the incubation period tended to be longer among patients > 70 years (20 days) than those aged < 70 years (11.5 days) [14]. A long incubation period may lead to a high rate of asymptomatic and subclinical infections and increased transmission. The transmission of SARS-CoV-2 may occur with the illness onset and even with mild or no symptoms [15]. In addition, no significant difference in viral loads between symptomatic and asymptomatic patients has been reported, indicating the potential of virus transmission from asymptomatic carriers [16]. However, the transmission of SARS-CoV-2 during the incubation period is yet to be established.
Pathogenesis
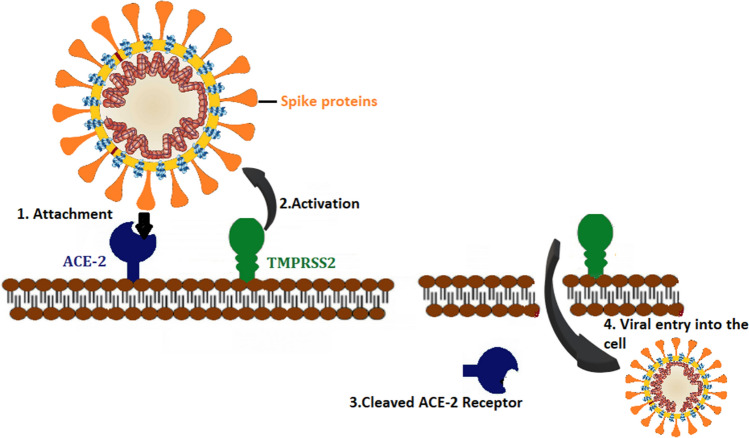
The S protein is a major glycoprotein that involves two subunits, S1 and S2. S1 encompasses a receptor-binding domain (RBD) that is responsible for recognizing and binding to angiotensin-converting enzyme 2 receptor (ACE2), the host cell receptor. The envelope S protein RBD of SARS-CoV-2 is structurally similar to that of SARS-CoV [16]. S2 contains the other basic elements required for membrane fusion.
ACE2 is a type I integral membrane protein mostly found in the lower respiratory tract, such as the lungs' alveolar type II cells that provide the leading entry site for the virus human hosts [17, 18]. ACE2 also has a role in lung protection, and, therefore, viral binding to this receptor contributes to pathogenicity [16]. Furthermore, ACE2 is also expressed in the upper esophagus, cholangiocytes, ileum, colon, kidney proximal tubule cells, myocardial cells, and bladder [19]. A recent study showed that the affinity between S glycoprotein of SARS-CoV-2 and ACE2 is 10–20 fold more significant than that of SARS-CoV, which could explain the highly infectious ability of SARS-CoV-2 [20].
The entry and binding processes of SARS-CoV-2 are then followed by the fusion of the viral membrane and the host cell membrane. After this fusion occurs, the type II transmembrane serine protease (TMPRSS2) that is present on the surface of the host cell will cleave ACE2 and activates the receptor attached S proteins [21]. Activation of the S proteins leads to conformational changes and allows the virus to enter the cells [22]. Thus, both TMPRSS2 and ACE2 are the main determinants of the virus's entry, and activation of TMPRSS2 as a protease is needed for the attachment of the S protein to its cellular ligand (Fig. 1) [23]. Upon cell entry, SARS-CoV-2 releases its genomic material in the cytoplasm and is translated into the nucleus.
Fig. 1.
Pathogenesis and the interaction of the viral spike (S)-protein with angiotensin-converting enzyme 2 receptor (ACE2): (1) S proteins on the surface of
Clinical features of SARS-CoV-2 infection
COVID-19 patients' age and sex
The Chinese National Reporting System data showed that the median age of confirmed cases was 51 years (range: 2 days–100 years), of which 77.8% were 30–69 years old with a predominance of male patients (51.1%) [24]. These findings were confirmed with a recent meta-analysis [25]. The ACE2 gene is found on the X chromosome, and circulating levels of ACE2 are higher in men than women [11], which could account for the differences in severity and mortality between men and women [26].
Symptoms
The clinical presentations of COVID-19 occur across a broad spectrum, ranging from asymptomatic infection to severe respiratory failure [1, 15, 27, 28]. The main symptoms include fever, dry cough, myalgia, and dyspnea [1, 27]. Headache, diarrhea, fatigue, sore throat, anosmia, ageusia, chest pain, hemoptysis, sputum production, rhinorrhea, nausea, vomiting, skin rash, impaired consciousness, and seizure were also observed [1, 27, 28]. Table 1 shows the most common symptoms in patients with COVID-19 infection. According to the severity of symptoms, patients can be classified as mild, severe, or critical (Table 2) [27].
Table 1.
Clinical symptoms of patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection
| Symptom | Chen et al.[17]. (n = 99) | Huang et al.[1]. (n = 41) | Wang et al.[13]. (n = 138) | Guan et al.[27]. (n = 1099) |
|---|---|---|---|---|
| Fever | 82 (82.8) | 40 (97.6) | 136 (98.6) | 975 (87.9) |
| Cough | 81 (81.8) | 31 (75.6) | 82 (59.4) | 745 (67.7) |
| Dyspnea | 31 (31.3) | 22 (55.0) | 43 (31.2) | 205 (18.6) |
| Sore throat | 5 (5.1) | NR | 24 (17.4) | 153 (13.9) |
| Sputum production | NR | 11 (28.9) | 37 (26.8) | 370 (13.3) |
| Myalgia | 11 (11.1) | 18 (43.9) | 48 (34.8) | 163 (14.8) |
| Headache | 8 (8.1) | 3 (7.9) | 9 (6.5) | 150 (13.6) |
| Diarrhoea | 2 (2.0) | 1 (2.6) | 14 (10.1) | 42 (3.7) |
| Rhinorrhoea | 4 (4.0) | NR | NR | NR |
All data are expressed as n (%)
NR not reported
Table 2.
Classification of clinical types of coronavirus disease 2019 (COVID-19) according to the severity of symptoms
| Clinical types | Symptoms/clinical markers |
|---|---|
| Mild | Non-pneumonia or mild pneumonia |
| Severe | Dyspnea, respiratory frequency ≥ 30/min, blood oxygen saturation ≤ 93%, the partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300, and/or lung infiltrates > 50% within 24/48 h |
| Critical | Respiratory failure, septic shock, and/or multiple organ dysfunction or failure |
Comorbidities
The common comorbidities in patients with COVID-19 are hypertension (HT), diabetes, obesity, respiratory disease, and cardiovascular disease (Table 3). A recent meta-analysis of the comorbidities showed that hypertension was present in approximately 21.1% of patients, respiratory disease in 1.5%, and diabetes in 9.7% of the patients [29]. Moreover, patients who required intensive care unit (ICU) admission were more likely to have these comorbidities than non-ICU patients [24]. In a recent large study (n = 5700) in the US, the most common comorbidities were obesity (41.7%), diabetes (33.8%), and HT (56.6%) [30]. Studies have shown that HT may be linked to an up to 2.5-fold more significant risk of fatal COVID-19, particularly in older persons [31]. ACE2 receptors are vital elements in the renin–angiotensin–aldosterone system (RAAS), a key factor in HT’s pathophysiology. So, inhibition of the RAAS with the ACE2-modulating medications, such as ACE inhibitors or angiotensin II receptor blockers (ARBs), may result in a compensatory increase in tissue levels of ACE2 [31]. Thus there are theories that ACE2-stimulating drugs used to treat HT can increase the risk of developing lethal COVID-19. However, studies have shown that patients on ACE2-modulating medications have significantly better survival than similar patients with HT who are not on these drugs. Moreover, there is currently no clear evidence that ACE inhibitors or ARBs lead to the upregulation of ACE2 in human tissue [32]. Several cardiological groups support the continued use of ACE inhibitors or ARBs in COVID-19 patients with HT [33]. Older persons, men, and those with preexisting HT and/or diabetes were highly prevalent in data reported from China [34]. This pattern was confirmed with a large case series in the US [15]. In particular, obesity is an underappreciated risk factor for COVID-19 [35]. The risk is particularly relevant in the US because the prevalence of obesity is around 40% compared to 6.2% in China, 20% in Italy, and 24% in Spain [36], some of the countries most affected by COVID-19.
Table 3.
Summary of comorbidity history in patients with coronavirus disease 2019 (COVID-19)
| Comorbidity | Huang et al.[1]. (n = 41) | Chen et al.[17]. (n = 99) | Wang et al.[13]. (n = 138) | Guan et al.[27]. (n = 1099) | Richardson et al.[47]. (n = 5700) |
|---|---|---|---|---|---|
| Cardiovascular disease | 6 (14.6) | 40 (40.4) | 20 (14.5) | 42(3.9) | 996 (16.9) |
| Hypertension | 6 (14.6) | NR | 43 (31.2) | 165 (15) | 3026 (56.6) |
| Diabetes | 8 (19.5) | 12 (12.1) | 14 (10.1) | 81 (7.4) | 1808 (33.8) |
| Respiratory disease | 1 (2.4) | 1 (1.0) | 4 (2.9) | 12 (1.1) | 920 (16.1) |
| Malignancy | 1 (2.4) | 1 (1.0) | 10 (7.2) | 10 (0.9) | 320 (6) |
| Chronic kidney disease | NR | NR | 4 (2.9) | 8 (0.7) | 454 (7.9) |
| Chronic liver disease | 1 (2.4) | NR | 4 (2.9) | 23 (21) | 30 (0.5) |
All data are expressed as n (%)
NR not reported
A recent clinical study showed that the levels of various inflammatory factors in the deceased group (CD3 + T cells, CD3 + CD8 + T cells, and CD3 + CD4 + T cells) were higher than in the survivors' group [37]. Besides, in patients with diabetes, the accumulation of activated innate immune cells in metabolic tissues leads to the release of inflammatory mediators, which include interleukin (IL)-1β and tumor necrosis factor-alpha (TNF-α), which promote systemic insulin resistance and β-cell damage [38]. Additionally, metabolic disorders may lead to diminished immune responses by impairing macrophage and lymphocyte functions [39]. Multiple comorbidities are related to the severity of COVID-19 disease progression. Hypertension is the most common and leading comorbidity. In addition, all clinicians are advised to continue ACE2-modulating medications for patients who are already on these drugs for their hypertension. However, patients with comorbidities should take all necessary precautions to avoid getting infected with SARS-CoV-2, as they usually have the worst prognosis.
Intuitively, other vulnerabilities to severe illness from COVID-19 would be believed to be obesity and smoking because these are also well known to increase the risk of severe respiratory infections [40, 41]. Smoking is an established risk factor for developing ARDS [42]. On the other hand, smoking could directly influence the adverse outcomes of COVID-19 patients by impairing endothelial function and enhancing inflammation in the cardiopulmonary systems. A recent meta-analysis with 5960 hospitalized patients noted a pooled prevalence of 6.5% (1.4–12.6%) [43], whereas another analysis of 2986 patients found a high pooled smoking prevalence of 7.6% (3.8–12.4%) [44]. The available data suggest that smokers constituted 1.4–18.5% of hospitalized adult patients with COVID-19 [17, 45, 69]. Smokers are more vulnerable to COVID-19 infections, and COVID-19 patients with a history of smoking had a 14% higher risk of developing COVID-19 induced pneumonia, while the odds to develop severe disease and eventually to death were 14 times higher compared to the nonsmokers [44, 46]. Smokers are more likely to suffer from comorbidities, such as cardiovascular diseases and chronic pulmonary disease compared to nonsmokers. So, COVID-19 patients with a history of smoking may have suffered from comorbidities that could have negatively influenced disease severity[44]. Moreover, smoking increases the expression of ACE2 in the lungs, which may facilitate host cell entry of SARS-CoV-2. Because the levels of the ACE2 protein are not only increased in the bronchial but also in the alveolar epithelium [47]. Available data suggest that nicotine upregulates ACE2, which is recommended as a possible mechanism to increase susceptibility and severity of COVID-19 [46, 48]. In addition, nicotine and cigarette smoke have been described to reduce ACE2, the putative receptor for SARS-CoV-2, in multiple organs. The clinical manifestations of COVID-19 could be explained by a dysfunction of the nicotinic cholinergic system and was postulated that nicotine could also modulate the host immune and inflammatory response by restoring the function of the cholinergic anti-inflammatory pathway [45]. It is important to emphasize that cigarette smoking is detrimental to the lungs in several aspects. So, smokers should be encouraged to quit smoking [46]. However, further studies are necessary to clarify the reasons behind the reported low prevalence of current smoking among hospitalized COVID-19 patients and explain the effects of nicotine on ACE2 expression [46].
On the other hand, the common comorbidities recorded in reports from different countries included HT, diabetes, and cardiovascular disease, all of which are connected with obesity. Additionally, obesity itself is increasingly well known as both a risk factor and comorbidity [49]. A high prevalence of obesity is described among hospitalized patients with SARS-CoV-2 infection [21]. In a study from the USA, among 1482 hospitalized patients with COVID-19, 48.3% were obese [30]. Another study from China showed that 43% of hospitalized patients with COVID-19 were overweight or obese [51]. Obesity has also been associated with mortality and increased disease severity [52, 53]. Body mass index (BMI) of patients with COVID-19 and cardiovascular disease in the ICU is higher than that of patients without the need for critical care [54].
Moreover, obesity is emerging as a risk factor for outcomes in patients with COVID-19 irrespective of their age and comorbidity. Obesity is an indication that even young patients with COVID-19 are at risk of severe disease if their BMI is elevated. In a USA study with children and adolescents hospitalized with COVID-19, obesity was significantly associated with the disease's severity [55]. Another study from China showed that obesity was the most important critical factor contributing to young patients' death [56]. Obesity is considered a chronic inflammatory disease, and an increase in circulating, pro-inflammatory cytokines, which may worsen COVID-19 outcomes [57]. SARS-CoV-2 induces excessive and prolonged cytokine/chemokine reactions in some infected individuals, known as the cytokine storm [58]. Cytokines and chemokines have an important role in immunity and immunopathology but dysregulated, and exuberant immune responses have been proven to cause ARDS or multiple organ dysfunction [59]. In patients with COVID-19, inflammatory markers such as TNF-α, IL-10, and IL-6 levels rise during the infection phase and decrease during recovery. In addition, patients requiring ICU care have noticeably elevated levels of TNF-α, IL-10, and IL-6 [60]. It is worth highlighting that the elevated level of IL-6 is also observed in obese patients affected by SARS-CoV-2 [57]. So, both obesity and SARS-CoV-2 infection appear to share some common metabolic and inflammatory reaction pathways [57, 58].
On the other hand, obese people also have a higher level of leptin, a pro-inflammatory adipokine, and a lower adiponectin concentration, an anti-inflammatory adipokine [61, 62]. The resultant imbalance between pro-and anti-inflammatory adipokines has been implicated as key to obesity being a significant risk factor for acute lung injury, and it could explain the contribution to complications in COVID-19 patients [52]. In addition, the impaired inflammatory response contributes to the severity of lung injuries observed in patients with influenza and may play a significant role in disease progression in patients with COVID-19 [63]. However, it is necessary to highlight that further studies are required to assess the mechanisms behind increased COVID-19 severity in patients with obesity and also to elucidate the difference between patients with severe and nonsevere COVID‐19.
Clinical manifestations of COVID-19
Cardiovascular involvement in COVID-19
The most common cardiovascular complication in COVID-19 patients is acute myocardial injury (ACI). However, COVID-19 can cause direct and indirect cardiovascular sequelae, including acute coronary syndromes, cardiomyopathy, acute cor pulmonale, arrhythmias, and cardiogenic shock [64]. Clinical involvement and considerations concerning the cardiovascular system in COVID-19 are presented in Box 1. The incidence of ACI has been variable, with approximately 8–12% of the positive cases developing a significant elevation of cardiac troponin I (cTnI), a highly specific marker for cardiac injury [65]. In addition, electrocardiographic and echocardiographic abnormalities are highly prevalent in patients with COVID-19 [25, 29, 32, 34]. Acute cardiac injury incidence was about 13-fold higher in ICU patients with the severe disease than non-ICU patients with the nonsevere disease [64]. In addition, ACI has been steadily shown to be a strong negative prognostic marker in patients with COVID-19 [1, 29]. Patients with ACI are older with more comorbidities, including baseline hypertension, diabetes, coronary heart disease, and heart failure [22]. Two proposed mechanisms have been reported as a reason for myocardial injury occurring with COVID-19. The first proposed mechanism is that ACE2 is expressed at a similar level in the cardiovascular system than in the lungs, which allows viral entry into myocardial cells [66, 67]. Another proposed mechanism is that the cytokine storm triggered by an imbalanced response from type 1 and type 2 T helper cells may lead to myocardial cell injury [1, 38]. High concentrations of IP-10, IFN-g IL-1ß, and monocyte chemotactic protein-1 can be detected in patients infected with COVID-19, which might lead to activated Th1 cell responses. Besides, ICU patients were found to have much higher concentrations of inflammatory factors than non-ICU patients, so these findings suggest that the cytokine storm was associated with disease severity [68].
Cardiac arrhythmias are an additional common cardiovascular manifestation occurring in COVID-19 patients. Arrhythmia was part of the presenting symptomology in 7.3% of 137 patients admitted for COVID-19 disease [69]. A study relating clinical profile and outcomes in 138 patients with COVID-19 reported a 16.7% incidence of arrhythmia, and the incidence was much greater in those requiring ICU admission compared to those who are not (44.4% vs. 8.9%, respectively) [12]. In a case series of 191 patients, heart failure was observed in 23.0% of patients with COVID-19. Notably, heart failure was more common in patients who did not survive the hospitalization (51.9%) compared to survivors (11.7%) [32], although the definition of heart failure was not clearly detailed in that study.
The elevated level of cTnI is an important prognostic marker in patients with COVID-19, even in those without preexisting cardiovascular disease (CVD). The highest mortality is in those with CVD and elevated cTnI, followed by those with elevated cTnI but no CVD. In addition, patients with CVD, but no raised troponins, have lower mortality. So, cTnI levels should be closely monitored. Elevated levels of N-terminal pro-B-type natriuretic peptide and troponin were found in COVID-19 severe cases [27]. Moreover, a significantly higher in-hospital mortality rate was seen in patients with increased troponin levels than those without increased troponin. Patients infected with COVID-19 with acute coronary syndrome often have a poor prognosis [30]. Thus, great attention should be paid to signs of cardiac injury in patients with COVID-19 infection.
Box 1: Cardiovascular involvement in COVID-19.
Clinical Profile:
Myocarditis and myocardial injury (type 1 and 2)
Arrhythmia
Acute coronary syndrome
Acute cor pulmonale
Cardiomyopathy
Cardiogenic shock
Laboratory markers:
Elevated cardiac-specific troponin and brain natriuretic peptide
Abnormal EKG (Prolonged QTc intervals, elevated ST)
Potential mechanism/pathophysiology:
ACE2 has high expression in cardiovascular tissues that support a direct viral injury
Systemic inflammation
Consideration:
Assess and correct electrolyte imbalance to alleviate the risk of arrhythmia
Clinicians are advised to continue ACE2-modulating medications in patients already on them but evaluate on a case-by-case basis
Respiratory system involvement in COVID-19
Respiratory symptoms are prevalent among hospitalized patients with COVID-19 and range in severity from dyspnea (19–40%) or cough (60–80%) to ARDS (17–42%) [34, 70, 71]. ARDS tends to occur in the first 1–2 weeks of presentation [35, 47]. Older age, high fever, baseline HT, diabetes, chronic kidney disease, preexisting cardiovascular disease, lymphopenia, elevated blood urea nitrogen, prolonged prothrombin time (PT), elevated d-dimer, and elevated C-reactive protein (CRP) were predictors of ARDS [42, 44]. Advanced age (greater than 65 years), neutropenia, elevated d-dimer, low albumin, and elevated lactate dehydrogenase (LDH) are related to higher mortality in those with ARDS [42, 44]. In addition, pro-inflammatory cytokines play an important role in the pathophysiology of lung damage in patients affected by COVID-19. Patients affected by COVID-19 develop a fulminant and damaging immune reaction sustained by cytokines leading to alveolar infiltration by macrophages and monocyte [72]. IL-6 is a key mediator of immune and inflammatory response initiated by infection or injury, and an elevated level of IL-6 is observed in more than one-half of patients with COVID-19 [73]. In addition, levels of IL-6 appear to be correlated with inflammatory response, respiratory failure, needing intubation and/or mechanical ventilation, and mortality in COVID-19 patients [74]. Respiratory failure is a major complication in patients with COVID-19.
Kidney involvement in COVID-19
Clinical involvement and considerations concerning the renal in COVID-19 are presented in Box 2. Preliminary reports suggested that acute kidney injury (AKI) ranged from 3 to 9% in COVID-19 patients [1], but later studies revealed prevalence rates as high as 15% [71]. AKI is more common among patients with severe disease, mostly in the ICU setting, and is considered a negative prognostic factor [75]. Also, renal insufficiency in initial laboratories is considered a prognosticator for severe illness and mortality [76]. Although the apparent impact of COVID-19 on renal tissues is still unclear, it is believed that the mechanisms underlying AKI in these patients are probably explained by the high level of ACE2 expression in the proximal convoluted tubules, the main binding site for COVID-19. Another proposed mechanism is that cytokine overproduction is involved in kidney damage [39]. Moreover, direct SARS-CoV-2 infection of the renal epithelium is estimated to result in mitochondrial dysfunction, acute tubular necrosis, and protein leakage [77]. In severe cases, AKI presents with elevated creatinine, urea, and cystatin-C levels [78]. The severity of AKI in patients infected with SARS-CoV-2 is positively correlated with older age, higher BMI, heart failure history, diabetes mellitus, higher peak airway pressure, and higher progressive organ failure assessment [34]. Moreover, COVID-19 complicates the management of patients on dialysis and with kidney transplantation [79]. Furthermore, hematuria has been reported in nearly half of patients with COVID-19, and proteinuria has been noted in up to 87% of severe or critically ill patients with COVID-19 [80]. Hyperkalemia and acidosis are common electrolyte abnormalities associated with the high cell turnover seen in patients with COVID-19, even among patients without AKI [58].
Box 2: Renal involvement in COVID-19.
Clinical Profile:
Acute kidney injury
Proteinuria
Hematuria
Electrolyte imbalances
Laboratory markers:
Elevated urea, serum creatinine
Elevated cystatin-C
Potential mechanism/pathophysiology:
Direct viral injury
Systemic inflammation
Alterations in renal hemodynamics
Consideration:
Assess the urine analysis to rule out the hematuria and proteinuria
Assess and correct the electrolyte imbalance
Gastrointestinal system involvement in COVID-19
Gastrointestinal (GI) symptoms are common in COVID-19 patients (Fig. 2). Clinical involvement and considerations concerning the GI system in COVID-19 are presented in Box 3. Notably, the first case of COVID-19 infection confirmed in the US reported a history of nausea and vomiting on admission and then diarrhea on day 2 [81]. In a large study of 1,099 patients from 552 hospitals in China, nausea or vomiting was reported in 5.0% and diarrhea in 3.8% of patients [26]. In another study of 140 patients with COVID-19 from Wuhan, GI symptoms were described in up to 39.6% of the patients (17.3% nausea, 12.9% diarrhea, and 5% vomiting) [82]. Several other studies have reported incidences of diarrhea ranging from 2.0 to 10.1% and nausea and/or vomiting ranging from 1.0 to 10.1% [1, 12, 34, 40]. Patients with GI symptoms tend to present later than those without GI symptoms, and there is also usually a delay in disease diagnosis and time to first respiratory symptoms [83]. The incidence of GI symptoms and particularly diarrhea is correlated with a higher rate of stool positivity for viral RNA and a higher viral load for SARS-CoV-2 RNA. The presence of viral RNA in stool was detected in 48.1% of the patients, with a reported duration of 33–47 days after the first onset of disease compared to 9% of patients without GI symptoms [84].
Fig. 2.
Gastrointestinal symptoms in patients with COVID-19
The possible tropism of SARS-CoV-2 for the GI tract is due to the high ACE2 expression in the upper esophagus and stratified epithelial cells and absorptive enterocytes in the ileum and colon [85, 86]. Staining of viral N protein was observed in the cytoplasm of the gastric, duodenal, and rectal epithelium [87]. Also, animal models have shown that the expression of ACE2 was related to colitis, thus suggesting the virus activity may cause enzyme modifications, increasing the susceptibility to intestinal inflammation and diarrhea [88]. These findings have delivered a valuable understanding of the receptor-mediated entry into the host cells and provided the basis for its possible transmission route through fecal material [89]. However, more studies are needed to elucidate the mechanisms underlying diarrhea in this viral infection and describe the association between respiratory and gastrointestinal symptoms [90]. Finally, the GI symptoms are not specific and may develop in patients without COVID-19, especially patients with preexisting GI diseases. It is vitally important that clinicians be aware and alert to GI manifestations in COVID-19 patients, as the onset of new unexplained GI symptoms such as nausea, vomiting, and diarrhea should prompt testing for COVID-19.
Box 3: Gastrointestinal, hepatobiliary, and pancreas involvement in COVID-19.
Clinical Profile:
Abdominal pain
Diarrhea
Nausea and/or vomiting,
Anorexia
Hyperglycemia
Laboratory markers:
Elevated levels of liver transaminases
Elevated levels of bilirubin, alkaline phosphatase, and gamma-glutamyl transferase
Decreased level of albumin
Elevated lipase and amylase
SARS-CoV-2 detection in stool samples
Potential mechanism/pathophysiology:
Direct viral injury
Systemic inflammation
Drug-induced liver injury
Hypoxic medicated dysfunction
Consideration:
In the absence of respiratory symptoms, it is important to consider the gastrointestinal symptoms as a differential diagnosis in COVID-19
Assess the baseline liver tests at hospital admission time
It is important to monitor liver transaminases longitudinally, mainly in patients receiving experimental drugs under clinical evaluation for COVID-19.
Evaluate the preexisting liver diseases to rule out and differentiate the abnormal liver biochemistries.
Hepatobiliary involvement in COVID-19
Liver injury associated with COVID-19 is defined as any liver damage occurring during disease progression and treatment in patients with or without preexisting liver pathologies. Clinical involvement and considerations concerning the hepatobiliary system in COVID-19 are presented in Box 3. Abnormal liver function tests were frequently reported as an extrapulmonary clinical feature, and almost one-half of SARS-CoV-2-infected patients experienced different degrees of liver damage [12, 15]. Overall, the incidence of elevated serum liver biochemical markers primarily elevated aminotransferases and slightly elevated bilirubin gamma-glutamyl transferase (GGT) in hospitalized patients with COVID-19, ranges from 14 to 53% [1, 11, 12, 15, 21, 32]. On the other hand, higher rates of GI symptoms and liver injury with greater elevations of aminotransferases are associated with severe disease and are often seen in ICU patients [45].
The cause of liver injury is likely multifactorial. ACE2 is also expressed in bile duct cells [91], which suggests that SARS-CoV-2 may infect these cells and cause the observed abnormal liver function in these patients. Liver biopsies confirm the presence of viral RNA in liver tissues. Moreover, atypical signs of hepatocyte injury have been presented, such as cell apoptosis along with ballooning, acidophilic bodies, and lobular inflammation, suggesting a mechanism of direct viral injury [52]. However, altered alkaline phosphatase (ALP), the bile duct injury-related index, was not observed in patients with COVID-19 [12, 15]. Current experimental drugs under clinical evaluation for COVID-19, including oseltamivir, lopinavir/ritonavir, ribavirin, remdesivir, and chloroquine phosphate or hydroxychloroquine sulfate, are metabolized in the liver. The likelihood that the liver impairment observed in patients with COVID-19 infection could, in part, be due to drug hepatotoxicity may explain the considerable variation in the prevalence of liver injury observed across treatment cohorts in clinical studies. In addition, liver injury can impair metabolism, dosing, and expected concentrations of the medications, which can increase the risk of toxicity. Other proposed hypotheses are that immune-mediated inflammation, such as cytokine storm and pneumonia-associated hypoxia, may also contribute to liver injury or even lead to liver failure in patients with COVID-19 who are critically ill [47]. Patients with preexisting chronic liver disease may be more susceptible to liver damage from SARS-CoV-2 [92]. The impact of COVID-19 in patients with preexisting chronic liver diseases, such as alcohol-related liver disease, nonalcoholic fatty liver disease, and viral hepatitis, remains to be evaluated. Given the significant prevalence of liver dysfunction in patients with COVID-19, it is important to check the baseline liver enzymes and liver function tests at the time of hospital admission.
Pancreatic involvement in COVID-19
Clinical involvement and considerations regarding the pancreatic system in COVID-19 are presented in Box 3. In a recent study of 52 patients with COVID-19 pneumonia from Wuhan, 9 (17%) experienced a pancreatic injury, defined by an abnormality in pancreatic enzyme levels. Of the nine patients with pancreatic injury, six patients had abnormal blood glucose [93]. Pancreatic injury in COVID-19 may be directly caused by the cytopathic effect mediated by local SARS-CoV-2 replication. In addition, the pancreatic injury might be secondarily due to injurious immune response induced by a viral infection or systemic responses to respiratory failure that leads to damage in multiple organs. Moreover, most patients were on antipyretics medication, which could also cause drug-related pancreatic injury [57]. ACE2 is also expressed in pancreas islets, and previous work showed that SARS-CoV infection caused islet damage and subsequent development of acute diabetes [94].
Neurological involvement in COVID-19
The common neurologic symptoms in patients with COVID-19 are headache, anosmia, and ageusia. Other neurological findings include stroke, impairment of consciousness, coma, seizure, Guillain–Barre syndrome, and encephalopathy [59]. Clinical involvement and considerations concerning the neurological system in COVID-19 are presented in Box 4. In a recent study of 214 patients with COVID-19 infection, 36.4% had neurological manifestations [59]. Patients with severe COVID-19 infection were older and had a higher prevalence of hypertension but had fewer classic symptoms such as fever and cough. Nerve pain, skeletal muscle weakness, pain, tingling, or numbness in the hands and feet are also observed. Neurological features among patients in the ICU with ARDS included encephalopathy, agitation, and confusion [95]. Patients with severe infection were more likely to develop neurologic manifestations like skeletal muscle injury, acute cerebrovascular disease, and conscious disturbance.
The distribution and expression of ACE2 in the nervous system, especially in the cerebral cortex and brain stem, and skeletal muscles, may cause some degree of neurologic manifestations through direct or indirect mechanisms. It is known that cytokine storm syndrome and immunosuppression are important factors accelerating the progression of COVID-19 [96]. Autopsy results showed that the brain tissue of COVID-19 deceased patients was hyperemic and edematous, and some neurons degenerated. A study showed that lymphocyte counts were lower for patients with central nervous system symptoms than those without these symptoms [97]. Also, there is evidence of ocular surface infection in patients with COVID-19, and SARS-CoV-2 RNA was detected in eye secretions of a patient [98]. However, it is still unclear if SARS-CoV-2 is neurotropic in humans and whether it can penetrate the CNS via retrograde neuronal route [99]. Olfactory dysfunction is one of the most common symptoms in COVID-19. Viral neuroinvasion could plausibly be achieved by several routes that include entry via the olfactory nerve, leukocyte migration across the blood–brain barrier, transsynaptic transfer across infected neurons, or infection of vascular endothelium [59, 100].
Box 4: Neurological involvement in COVID-19.
Clinical Profile:
Headache, dizziness, fatigue
Stroke
Confusion, seizures,
Anosmia, ageusia
Conjunctivitis, myalgias, and skeletal muscle weakness
Tingling, or numbness in the hands and feet
Encephalopathy, Guillain-Barre syndrome
Laboratory markers:
Elevated serum creatine kinase(Rhabdomyolysis)
Potential mechanism/pathophysiology:
Direct viral injury
Systemic inflammation
Consideration:
Assess the changes in the neurological examination during the hospitalization
Closely monitor the high-risk populations such as elderly patients and patients with hypertension
Skin involvement in COVID-19
Skin manifestations have rarely been described in patients with COVID-19. The frequency of the skin lesions associated with COVID-19 infection varies according to the series. In a study from China with 1099 patients with COVID-19, the incidence was only 0.2%, while in an observational study in Italy with 88 patients, it was 20.4% [101]. Approximately 44% of the patients had cutaneous findings at disease onset, while the remaining patients developed these during the course of their illness [102]. The average time for the lesions occurred on a median of 9.92 days (range: 1–30) after the onset of systemic symptoms like fever, cough, dyspnea, fatigue, and diarrhea [103]. Erythema is the most common skin manifestation in patients with COVID-19. The cutaneous manifestations included erythematous rash, urticaria like lesions, chickenpox-like vesicles, maculopapular rash, vesicular lesions, and livedo/necrotic lesions. The most commonly affected area is the trunk. The lesions are distributed in flexural regions, the face, and mucous membranes. The erythematous lesions were confined to specific sites, such as the heels, without other triggers such as exposure [104]. However, it is unclear whether these skin manifestations are caused directly by the virus invasion or secondary to host immune response or treatment administration. The available studies were linked to the expression of ACE2 in skin tissue because ACE2 immunoreactivity was detected by immunohistochemistry in the cells of the basal layer of the epidermis extending to the basal cell layer of hair follicles, sebaceous glands, and also the smooth muscle cells surrounding the sebaceous glands [105]. Other proposed mechanisms for COVID-19-related cutaneous manifestations include an immune hypersensitivity response to SARS-CoV-2 RNA and cytokine-release syndrome [106]. In addition, histopathological examination of the lesions has shown inflammatory features with diffuse and dense lymphoid infiltrate [107]. Most cutaneous lesions of COVID-19 have been self-resolving in a few days. In addition, skin manifestations do not correlate with the severity of COVID-19. However, further studies are needed to increase our understanding of the cutaneous manifestations caused by COVID-19.
Pregnant women with COVID-19
The physiological changes during pregnancy, such as diaphragm elevation, reduced functional residual volumes, and altered cell immunity, lead to increased susceptibility to COVID-19 and worse outcomes than non-pregnant adults [108]. The clinical features reported in pregnant women with confirmed SARS-CoV-2 infections are similar to those of non-pregnant women with COVID-19 pneumonia. The most common symptoms reported by pregnant women with suspected or confirmed COVID-19 were fever, cough, and dyspnea. Lymphopenia, raised CRP levels and elevated liver enzymes were the most common laboratory findings [69, 81].
Pregnant women with COVID-19 are at increased risk of preterm delivery, requiring their babies to be admitted to the neonatal unit. A recent systematic review including over 11,580 pregnant women with COVID-19 reported a rate of preterm birth before 37 weeks of gestation of 17% and a cesarean delivery rate of 65%. Only 6 percent of patients with COVID-19 had spontaneous deliveries [68]. Pregnant women with COVID-19 seem to be at increased risk of requiring admission to an ICU or requiring mechanical ventilation. Preexisting maternal comorbidity was associated with the need for ICU care and also mechanical ventilation. Pregnant women with chronic HT and preexisting diabetes have a higher risk of death with COVID-19 infection, which are well-identified risk factors in the general population [68]. Preterm prelabor rupture of membranes occurred in 18.8% of patients, while the rate of pregnancies experiencing pre-eclampsia was 13.6%, with no reported cases of fetal growth restriction [69, 70]. The perinatal death rate was 7%, including one stillbirth and one neonatal death; 43% of fetuses had fetal distress, and 8.7% of newborns were admitted to the neonatal ICU. The frequency of Apgar score < 7 at 5 min was 4.5%, and no case of neonatal asphyxia was reported. There is no clinical evidence of vertical transmission, mainly when COVID-19 infection occurs later in pregnancy [109].
In addition, more than 90% of hospitalized pregnant women infected by SARS-CoV-2 infections presented with radiological signs indicative of pneumonia that was detected either by chest X-ray or computerized tomography (CT). Animal models have demonstrated transient ACE2 overexpression and increased activity in the placenta and reproductive organs during pregnancy [110]. In contrast, another study described as very low expression of ACE2 in almost all human cell types of the early maternal–fetal interface, suggesting the placenta had virtually no cells susceptible to virus infection [111]. These findings present conflicting evidence regarding the interaction of ACE2 and SARS-CoV-2 at the placental level. However, further studies are needed to assess this interaction. Compared with the general population, pregnant women with COVID-19 are at increased risks of adverse obstetrical outcomes and overall outcomes. We thus need better management strategies to avoid unnecessary exposure of pregnant women to the virus and strategies to decrease the impact of viral infection to improve maternal and fetal outcomes.
Laboratory findings
Blood investigations
Standard blood testing has shown that most patients with COVID-19 have leukopenia, lymphocytopenia with depletion of CD4 and CD8 lymphocytes, and decreased platelet counts. Also, prolonged PT with extended activated thromboplastin time, elevated LDH, elevated liver aminotransferase levels, elevated neutrophils, eosinopenia, and elevated CRP were noted [1, 12, 15]. COVID-19 patients have a high level of IL1β, interferon gamma (IFN-γ), interferon gamma-induced protein 10 (IP-10), and monocyte chemoattractant protein-1 (MCP-1). Severe disease was associated with higher white blood cell count (WBC) count, CRP, d-dimer, LDH, aspartate transaminase, lower lymphocyte count, platelet count, and hemoglobin [1, 16, 32]. Patients admitted to the ICU often have elevated plasma levels of a granulocyte-colony stimulating factor (G-CSF), IP-10, MCP-1, macrophage inflammatory protein-1 α (MIP-1α), and TNF-α [1]. Compared to patients who survived, the patients who died had elevated blood urea, creatinine, white blood cell, neutrophil counts, d-dimer, cTnI, serum ferritin, and LDH, IL-10, and IL-6. In addition, IL-6 levels were more than three times higher in patients with complicated COVID-19 than those with the non-complicated disease [50]. IL-6 levels seem to be a good prognosticator for negative outcomes and an indicator of the progression to severe disease and/or in-hospital mortality. Moreover, elevated CRP levels, lower CD4 + T cell count, decreased C3 levels, lower platelet levels, and severe lymphopenia also common among patients who died [1, 32, 112]. Additionally, decreased serum level of C3 is an immunity-related factor predicting mortality of patients with COVID-19. Although in vitro, diagnostic testing is still the gold standard diagnosis with regard to the typical clinical diagnosis. However, the lab findings could help the physicians to understand the progression and the diagnostic value of patients' prognosis. Moreover, the biochemical markers help differentiate between nonsevere and severe COVID-19 to stratify patients at an early stage correctly.
Radiological findings
Radiological findings are variable and serve as a surrogate to diagnose SARS-CoV-2. The finding may vary with patients' age, disease progression, immunity status, comorbidities, and initial medical intervention [113]. Older patients show more diffuse and extensive pathological imaging findings than those of younger patients [114]. The most common findings in lung CT scans of patients with moderate and severe infection were patchy bilateral shadows or ground-glass opacity (GGO) [36]. More than 75% of patients presented with bilateral lung involvement [1, 24, 36], while multilobe involvement was noted in 71% of infected subjects [115]. Chest CT sensitivity appears to be high in patients with positive RT-PCR (86–97%, based on different studies) and lower in patients with only constitutional and non-respiratory symptoms (about 50%) [116]. On the other hand, chest X-ray sensitivity is lower in detecting typical features of SARS-CoV-2 pneumonia, around 59%, and chest X-ray abnormalities are present in 33–60% of patients [117]. In the early stage of SARS-CoV-2 infection, GGO is the main radiological finding, mostly involving the bilateral lungs and the lungs' peripheral area. The infection can involve all the lobes and mostly the bilateral lower lobes. In addition, bilateral lung involvement is more frequently noticed in the disease's intermediate and late course than earlier in the clinical course [118]. If the infection continues to progress, bilateral multiple lobular and subsegmental areas of consolidation are found at later stages [29]. Consolidation rarely presents without GGO [52, 96]. The opacifications are typically rounded and present peripherally in the subpleural area [29, 52, 53]. In addition to GGO and consolidation, other abnormal changes in the lungs are reported, including crazy-paving, air bronchogram, linear opacities, local or bilateral patchy shadowing, and interlobular septal thickening. Other common CT features in severe cases include pleural effusions, distortion of architecture, bronchiectasis of traction, lymph node enlargement, and tree-in-bud nodularity [29, 53, 119]. Surprisingly, changes in CT scans were noticed in patients before the onset of their symptoms and before the viral RNA detection in specimens from the upper respiratory tract [120].
Diagnosis and serology
The detection of SARS-CoV-2 RNA by RT-PCR is used for the diagnosis of COVID-19. Nasopharyngeal swabs or sputum samples of patients are assayed by specific RT-PCR to detect highly conserved genes. These assays target the ORF1a/b, ORF1b-nsp [121], RdRp, S, E, or N genes of SARS-CoV-2 [122]. A correlation study between chest CT and RT-PCR testing of 1014 cases showed that 88% had positive chest CT scans, but only 59% had positive RT-PCR. These findings indicated that chest CT is a sensitive modality to detect SARS-CoV-2 infection [110]. From 60 to 93% of patients in this cohort presented with initial positive chest CT suggesting COVID-19 before the initial RT-PCR test detected SARS-CoV-2. Around 42% of patients with COVID-19 showed improvement in the follow-up chest CT before the RT-PCR results turned negative [75]. The poor sensitivity of the current diagnostic tests makes diagnosis and epidemic control challenging. However, the false-negative results may arise for several reasons, including inadequate sampling technique or low viral load, and thus many patients will require multiple testing to exclude the diagnosis. Repeat testing of nasopharyngeal swabs or sputum samples is recommended in clinically suspected cases with an initially negative result. On the other hand, chest CT may be beneficial for COVID-19 diagnosis in cases of strong clinical suspicion, including individuals showing typical clinical manifestations with negative RT-PCR before any elective procedure. However, it is still very important to determine the ability of chest CT in distinguishing COVID-19 from pneumonia of other causes. It is also recommended that individuals with signs of pneumonia at chest CT self-isolate with serial testing subsequently to exclude the diagnosis definitively to make an accurate diagnosis of COVID-19 [95, 123].
Conclusions
The recent SARS-CoV-2 pandemic outbreak is an ongoing crisis that is causing global uncertainty on an unprecedented scale. This pandemic has become a health threat to the general population and healthcare workers worldwide, with continued increases in the number of cases and deaths. Given the high rate of transmission of the infection among humans, it is important to recognize the basis of its structure replication and pathogenicity, which can lead to the discovery of effective treatments and a vaccine for prevention.
Our understanding of COVID-19 is changing very rapidly, and the discovery of new findings occurs daily. SARS-CoV-2 has been established as a respiratory tract pathogen, but more and more studies have shown that the virus affects many other systems as well. As the disease spreads and new evidence develops, it would be prudent to identify the risk factors for developing systemic complications in patients with COVID-19. Multisystemic involvement is also associated with the disease's severity and might predict worse clinical outcomes and increased mortality. The SARS-CoV-2 virus binds to ACE2 receptors widely expressed in most human cells and can adversely affect every body system. A prospective registry of COVID-19 patients with multisystemic involvement to examine clinical variables and complications relevant to those systems will help characterize organ involvement patterns. The registry would provide significant evidence regarding risk factors, diagnosis, and prognostic assessment to help clinicians identify patients at the highest risk of adverse outcomes. Patients should be closely monitored for organ damage, such as liver, heart, and kidney injury, in addition to respiratory damage, and receive supportive care as needed to reduce the risk of the inflammatory cascade and to improve overall treatment outcomes. It is also important to identify the main drivers of COVID-19 and to examine the factors that account for variability in presentation and severity of the disease, and pursue more research priorities that will help explain several aspects of diseases that remain are poorly understood.
Several preclinical and clinical trials for single drugs or combinations are in progress. However, no drug has demonstrated its clinical utility and could be labeled as a standard of care to date. While we await an effective vaccine, COVID-19 continues to spread, and the death rate to rise exponentially. So, it is vital to investigate the structural and functional variations and patterns of SARS-CoV-2 to understand the basis of its replication, structure, pathogenicity, and molecular mechanisms in different organs and look for potential targets that aid in the development of effective treatments. Controlling the spread of the pandemic and reducing mortality are of greatest priority. At present, there is no FDA-approved treatment or vaccine for patients with COVID-19. Currently, the most effective measures to SARS-CoV-2 are still early detection and quarantine of new sources of infection and early diagnosis and supportive treatments for confirmed patients. Moreover, during the pandemic period of COVID-19, when seeing patients with different systemic manifestations, clinicians should consider SARS-CoV-2 infection in the differential diagnosis to avoid delayed diagnosis or misdiagnosis and prevent transmission.
Author contributions
AK contributed to the conception, drafting the work and revising it critically for important intellectual content. TW contributed to the conception, revising it critically for important intellectual content and final approval of the version to be published. JH and SA contributed to revising it critically for important intellectual content.
Compliance with ethical standards
Conflict of interest
All the authors declare no conflicts of interest.
Ethical approval
This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Arunkumar Krishnan, Email: dr.arunkumar.krishnan@gmail.com.
Tinsay A.Woreta, Email: tworeta1@jhmi.edu.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19.Online: https://www.who.int/dg/speeches/detail/whodirector- general-s-opening-remarks-at-the-media-briefing-on-covid-19. Accessed 14 Aug 2020
- 3.Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novel Coronavirus (2019-nCoV) Situation Report – 22 (World Health Organization); https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf. Accessed 14 Aug 2020
- 5.. World Health Organization. Summary of probable SARS cases with onset of illness Geneva (Switzerland): World Health Organization; 2003 Dec. Available from: https://www.who.int/csr/sars/country/table2004_04_21/en. Accessed 14 Aug 2020
- 6.Zaki AM, Boheemen SV, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Gayle AA, Wilder-Smith A, Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang B, Wang X, Li Q, et al. Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions. J Clin Med. 2020;9:462. doi: 10.3390/jcm9020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villar J, Ferrando C, Martinez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 11.Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127:274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Lu Q, Liu M, et al. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. MedRxiv. 2020 doi: 10.1101/2020.02.10.20021675. [DOI] [Google Scholar]
- 13.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan. China J Med Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 19.Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabi FA, Al Zoubi MS, Kasasbeh GA, et al. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9:231. doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simmons G, Zmora P, Gierer S, et al. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antiviral Res. 2013;100:605–614. doi: 10.1016/j.antiviral.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paules CI, Marston HD, Fauci AS. Coronavirus infections- More than just the common cold. JAMA. 2020;323:707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 24.. World Health Organization(2020). Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Geneva (Switzerland): World Health Organization;. Available from: https://www.who.int/docs/default-source/coronaviruse/who-chinajoint-mission-on-covid-19-final-report.pdf. Accessed 14 Aug 2020
- 25.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin JM, Bai P, He W, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo T, Fan Y, Chen, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fine P, Eames K, Heymann DL. "Herd immunity": a rough guide. Clin Infect Dis. 2011;52:911–916. doi: 10.1093/cid/cir007. [DOI] [PubMed] [Google Scholar]
- 30.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lippi G, Wong J, Henry BM. Hypertension and its severity or mortality in coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130:304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 32.Danser AHJ, Epstein M, Batlle D. Renin–angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin–angiotensin system blockers. Hypertension. 2020;75:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.31713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludwig DS, Malley R. Americans are already too diseased to go back to work right now. https://www.nytimes.com/2020/03/30/opinion/obesity-us-healthcoronavirus.html. Accessed 14 Aug 2020
- 36..WHO. Global Health Obsevatory (GHO) data: overweight and obesity. 2017. who.int/gho/ncd/risk_factors/overweight_obesity/obesity_adults/en. Accessed 14 Aug 2020
- 37.Guo L, Wei D, ZhangX WuYJ, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA Score. Front Microbiol. 2019;10:2752. doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odegaard JI, Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harbor Perspect Med. 2012;2:a007724. doi: 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8(4):e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.US centers for disease control and prevention.coronavirus disease 2019 (COVID-19): people with certain medical conditions. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medicalconditions.html. Accessed 14 Aug 2020
- 42.Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8:e35. doi: 10.1371/journal.pone.0241265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farsalinos K, Barbouni A, Niaura R. Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: could nicotine be a therapeutic option? Intern Emerg Med. 2020;15:845–852. doi: 10.1007/s11739-020-02355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han L, Ran J, Mak YW, et al. Smoking and influenza-associated morbidity and mortality: a systematic review and meta-analysis. Epidemiology. 2019;30:405–417. doi: 10.1097/EDE.0000000000000984. [DOI] [PubMed] [Google Scholar]
- 46.Farsalinos K, Barbouni A, Poulas K, et al. Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis. Ther Adv Chronic Dis. 2020;11:2040622320935765. doi: 10.1177/2040622320935765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polverino F. Cigarette smoking and COVID-19: a complex interaction. Am J Respir Crit Care Med. 2020;202:471–472. doi: 10.1164/rccm.202005-1646LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brake SJ, Barnsley K, Lu W, et al. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19) J Clin Med. 2020;9:841. doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sattar N, McInnes IB, McMurray JJV. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;42:4–6. doi: 10.1161/circulationaha.120.047659. [DOI] [PubMed] [Google Scholar]
- 50.Kalligeros M, Shehadeh F, Mylona EK, et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity. 2020;28:1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai Q, Chen F, Wang T, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43:1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 52.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx. New York Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng YD, Meng K, Guan HQ, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:450–455. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 55.Zachariah P, Johnson CL, Halabi KC, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York city New York. JAMA Pediatr. 2020;174:e202430. doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang F, Xiong Y, Wei Y, et al. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J Med Virol. 2020;92:2536–2542. doi: 10.1002/jmv.26039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caci G, Albini A, Malerba M, et al. COVID-19 and obesity: dangerous liaisons. J Clin Med. 2020;9:2511. doi: 10.3390/jcm9082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michalakis K, Ilias I. SARS-CoV-2 infection and obesity: Common inflammatory and metabolic aspects. Diabetes Metab Syndr. 2020;14:469–471. doi: 10.1016/j.dsx.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stapleton RD, Dixon AE, Parsons PE, et al. The association between BMI and plasma cytokine levels in patients with acute lung injury. Chest. 2010;138:568–577. doi: 10.1378/chest.10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leal Vde O, Mafra D. Adipokines in obesity. Clin Chim Acta. 2013;419:87–94. doi: 10.1016/j.cca.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Almond MH, Edwards MR, Barclay WS, Johnston SL. Obesity and susceptibility to severe outcomes following respiratory viral infection. Thorax. 2013;68:684–686. doi: 10.1136/thoraxjnl-2012-203009. [DOI] [PubMed] [Google Scholar]
- 64.Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020;58:1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 66.Wong CK, Lam CWK, Wu AKL, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020;16:308–310. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the “Cytokine Storm” in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang ZL, Hou YL, Li DT, Li FZ. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand J Clin Lab Investig. 2020;80:441–447. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Viro. 2020 doi: 10.1002/jmv.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bahl A, Van Baalen MN, Ortiz L, et al. Early predictors of in-hospital mortality in patients with COVID-19 in a large American cohort. Intern Emerg Med. 2020;15:1485–1499. doi: 10.1007/s11739-020-02509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8:738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alberici F, Delbarba E, Manenti C, et al. Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep. 2020;5:580–585. doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang JJ, Dong X, Cao YY. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 83.Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a hong kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang M, Li H, Zhang Y, et al. Transitional basal cells at the squamous-columnar junction generate Barrett’s oesophagus. Nature. 2017;550:529–533. doi: 10.1038/nature24269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haber AL, Biton M, Rogel N, et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiao F, Tang M, Zheng X, et al. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 90.Gao QY, Chen YX, Fang JY. Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 doi: 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 92.Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 (COVID-19) and prevalence of chronic liver disease: a meta-analysis. Liver Int. 2020;40:1316–1320. doi: 10.1111/liv.14465. [DOI] [PubMed] [Google Scholar]
- 93.Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic injury patterns in patients with COVID-19 pneumonia. Gastroenterology. 2020;159:367–370. doi: 10.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;7:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aiello F, Gabriele Gallo Afflitto GG, Mancino R, et al. Coronavirus disease 2019 (SARS-CoV-2) and colonization of ocular tissues and secretions: a systematic review. Eye. 2020;34:1206–1211. doi: 10.1038/s41433-020-0926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang J, Wang P, Li C, Huang Y, Yang C, Zhang L. Central neurological complications and potential neuropathogenesis of COVID-19. Intern Emerg Med. 2020;29:1–4. doi: 10.1007/s11739-020-02476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zubair AS, McAlpine LS, Gardin T, et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77:1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wollina U, Karadağ AS, Rowland-Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020 doi: 10.1111/dth.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao Q, Fang X, Pang Z, Zhang B, Liu H, Zhang F. COVID-19 and cutaneous manifestations: a systematic review. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:212–213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 105.Grzegrzolka S, Pula B, Zamirska A, et al. ACE and ACE2 expression innormal and malignant skin lesions. Folia Histochem Cytobiol. 2013;51:232–238. doi: 10.5603/FHC.2013.0033. [DOI] [PubMed] [Google Scholar]
- 106.Wei C, Friedman AJ. COVID-19 pandemic: are there unique cutaneous manifestations in patients infected with SARS-CoV-2? J Drugs Dermatol. 2020;19:554–555. [PubMed] [Google Scholar]
- 107.Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34:e346–e347. doi: 10.1111/jdv.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dashraath P, Wong JLJ, Lim MXK, et al. Coronavirus Disease 2019 (COVID-19) Pandemic and Pregnancy. Am J Obstet Gynecol. 2020;222:521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mascio DD, Khalil A, Gabriele Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dong L, Tian J, He S, et al. Possible vertical transmission of SARSCoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;32:1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.da Silva SJR, Silva CTAD, Guarines KM, et al. Clinical and laboratory diagnosis of SARS-CoV-2, the virus causing COVID-19. ACS Infect Dis. 2020;6:2319–2336. doi: 10.1021/acsinfecdis.0c00274. [DOI] [PubMed] [Google Scholar]
- 113.Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.. National health commission of the People’s Republic of China. Diagnosis and treatment of new coronavirus pneumonia (version 7) 2020; http://www.nhc.gov.cn/. Accessed 14 Aug 2020
- 115.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kanne JP, Little BP, Chung JH, Elicker BM, Loren H, Ketai LH. Essentials for radiologists on COVID-19: an update radiology scientific expert panel. Radiology. 2020;296:E113–E114. doi: 10.1148/radiol.2020200527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ng MY, Lee EYP, Jin Yang J, et al. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiol Cardiothorac Imag. 2020;2:e200034. doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nair A, Rodrigues JCL, Hare S, et al. A British Society of Thoracic Imaging statement: considerations in designing local imaging diagnostic algorithms for the COVID-19 pandemic. Clin Radiol. 2020;75:329–334. doi: 10.1016/j.crad.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.. Burrell CJ, Howard CR, Murphy FA (2016). Fenner and White's Medical Virology 5th Edition. United States: Academic Press: ISBN: 9780123751577
- 122.. WHO. Laboratory testing of 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance,.WHO/COVID19/laboratory/2020.5. World Health Organization. Accessed 14 Aug 2020
- 123.Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiology. 2020;296:E46–E54. doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]