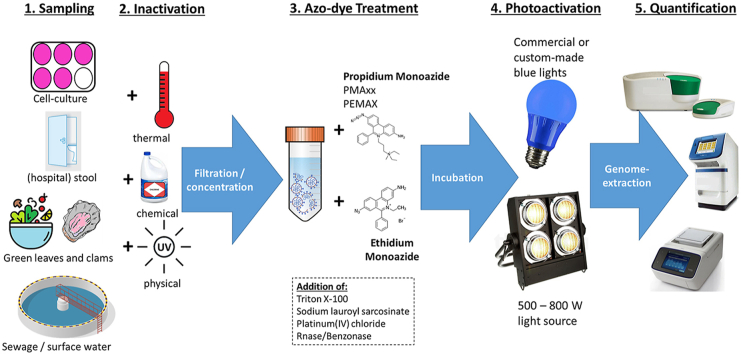
Abstract
Capsid integrity quantitative PCR (qPCR), a molecular detection method for infectious viruses combining azo dye pretreatment with qPCR, has been widely used in recent years; however, variations in pretreatment conditions for various virus types can limit the efficacy of specific protocols. By identifying and critically synthesizing forty-one recent peer-reviewed studies employing capsid integrity qPCR for viruses in the last decade (2009–2019) in the fields of food safety and environmental virology, we aimed to establish recommendations for the detection of infectious viruses. Intercalating dyes are effective measures of viability in PCR assays provided the viral capsid is damaged; viruses that have been inactivated by other causes, such as loss of attachment or genomic damage, are less well detected using this approach. Although optimizing specific protocols for each virus is recommended, we identify a framework for general assay conditions. These include concentrations of ethidium monoazide, propidium monoazide or its derivates between 10 and 200 μM; incubation on ice or at room temperature (20 - 25 °C) for 5–120 min; and dye activation using LED or high light (500–800 Watts) exposure for periods ranging from 5 to 20 min. These simple steps can benefit the investigation of infectious virus transmission in routine (water) monitoring settings and during viral outbreaks such as the current COVID-19 pandemic or endemic diseases like dengue fever.
Keywords: (6) azo dye, EMA, PMA, Microbial contamination, virus infectivity, Water quality
Graphical abstract
Highlights
-
•
PMA/PMAxx have higher efficiency removing false negatives from qPCR for DNA/RNA viruses than EMA.
-
•
One size fits all pretreatment approaches are possible but lead to lower virus signal reduction.
-
•
Capsid integrity qPCR is a valuable tool to adapt existent workflows for improving risk assessment.
-
•
Azo dye pretreatment can help refine significance of qPCR during virus outbreaks.
1. Introduction
Global population expansion and climate change are poised to increase both freshwater demand and wastewater production. Exposure to waterborne and foodborne pathogens through recreational activities, irrigation water, and food consumption, as well as associated occupations, poses a risk to public health in high and low resource environments (Efstratiou et al., 2017; Gibson, 2014). To date, more than 150 enteric viruses have been described to cause waterborne-associated human illnesses, including gastrointestinal and chronic infections (Sinclair et al., 2009). In addition, enteric viruses have shown a significantly higher persistence in the aquatic environment compared to conventional fecal indicator bacteria (Rames et al., 2016). Several enteric viruses relevant to human health could pass conventional sewage treatment in high numbers, thus posing a health risk when partially treated reclaimed sewage is utilized to irrigate fruits and vegetables (Brouwer et al., 2018) or released into the aquatic environment of rivers and lakes (Hellmér et al., 2014). Consequently, viral infectivity measurements have been proposed to be included in guidelines of water reuse for potable and non-potable purposes to demonstrate water reuse safety and evaluate water treatment efficiencies through log-reduction value achievements (Farkas et al., 2020; Gerba and Betancourt, 2019). Due to their high infectivity and transmission rate as well as usually relatively low infectious dose, virus analysis in water and on fomites is frequently used when investigating likelihood of water-/surface borne transmission. This is especially the case in viral outbreaks causing acute and chronic illnesses like the Ebola virus disease, severe acute respiratory syndrome (SARS), Middle Eastern respiratory syndrome (MERS), seasonal dengue outbreaks in (sub-)tropical regions and the current pandemic Coronavirus Disease 2019 (COVID-19) caused by SARS-Coronavirus-2 (SARS-CoV-2)(Corman et al., 2020; Grubaugh et al., 2019)).
While still the gold standard, culture-based methods for propagation of infectious human pathogenic viruses in a laboratory environment require specialized facilities, experienced personnel and appropriate cell lines for virus propagation, and test results may only become available after five to ten days (Rodriguez et al., 2009). Molecular techniques using quantitative polymerase chain reaction (qPCR) are faster and have been successfully used in the past two decades to determine virus loads in the aquatic environment and to comply with food safety regulations (Bosch et al., 2018; Gerba and Betancourt, 2019). While robust, cost-efficient and uniquely sensitive and specific, qPCR has the severe limitation of not being able to differentiate between infectious and non-infectious virus particles, thus overestimating the number of viruses present in a sample (Chhipi-Shrestha et al., 2017). Novel approaches like modifying the targeted gene sequence or the length of the PCR product, or amplifying less stable messenger RNA after reverse transcription to DNA (e.g. (Ho et al., 2016; Ko et al., 2003; Polston et al., 2014; Wu et al., 2019)) generally lack robustness and sensitivity.
One of the most established qPCR modifications to measure infectivity is capsid integrity qPCR, an approach where samples are pre-treated with the intercalating azo dyes propidium monoazide (PMA), ethidium monoazide (EMA) or their derivates PMAxx and PEMAX. First described almost two decades ago by Nogva et al. (2003) to allow the identification of viable but non-cultivable bacteria, this technique has been successfully adapted to remove putatively false-positive qPCR signals deriving from virions with broken capsids in complex matrices like sewage and surface water (Leifels et al., 2016; Randazzo et al., 2018a). Based on the principle that an azo dye can only enter virions with a damaged capsid to covalently and irreversibly bind with viral DNA or RNA, this pretreatment can block amplification of nucleic acids due to the detachment of the polymerase when it encounters the dye-genome complex. Subsequently, only genomic targets are amplified that originate from intact virions while those nucleic acids that are free (outside the virion) or belong to non-infectious viruses are removed from the quantitative qPCR. This indirect viable measurement method has been especially useful for those viruses for which cell cultivation-based detection has been difficult but has yet to be fully validated (Estes et al., 2019). One known limitation of the azo intercalating dyes is their inability to differentiate viruses that have lost their infectivity due to damaged nucleic acids but whose capsid remains intact, a condition often found after UV-C treatment (Leifels et al., 2015). Moreover, there are numerous factors that affect the efficacy of a method, including virus type, inactivation method, type of dye and its concentration. Incubation conditions and light source are also crucial in the applicability of capsid integrity qPCR as reflected by the great range of capsid integrity pretreatment conditions in the literature. Consequently, the objectives of this review were to evaluate the efficiency of azo dye pretreatment conditions as stated in current literature and to establish protocols and considerations of the capsid integrity qPCR methods for virus infectivity monitoring.
2. Literature search and analysis strategy
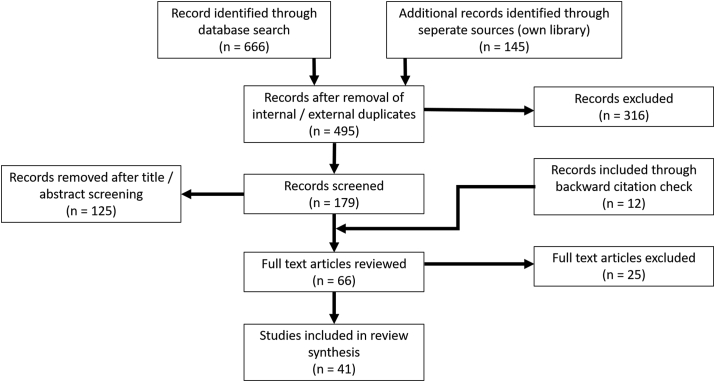
The guidelines for systematic article search and selection as recommended in the PRISMA Statement have been adopted in this work (Moher et al., 2009, 2015). To ensure reproducibility, a search string was constructed in accordance with the Cochrane Handbook (Cochrane, 2019), and a search was conducted in March 2020 (Fig. 1) in relevant databases like Pubmed, Scopus, Ovid, Medline and Web of Knowledge to cover relevant articles in English since the first introduction of the azo dye pretreatment in 2003 (Supplementary Table S1). Articles were screened according to specific criteria.
Fig. 1.
PRISMA flowchart of the literature search strategy and the number of included and excluded articles.
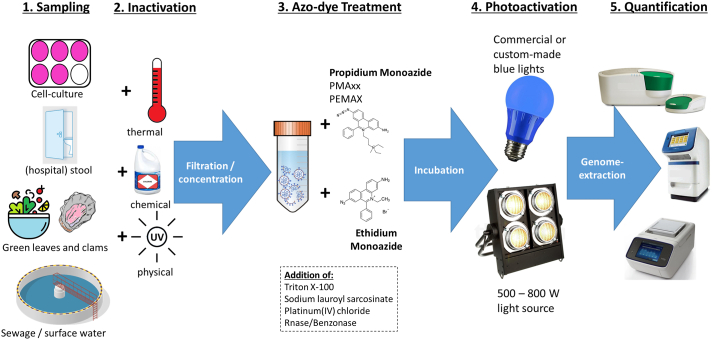
For quality control and to follow the recommendations of (Cochrane, 2019)), two of the authors (M.L. and K.S.) conducted the title and abstract evaluation in parallel, with a third author (E.S.) acting as tie breaker in the case of disagreement. As depicted in Fig. 2, the 41 articles represented here include studies discussing the application of azo dyes (PMA, EMA, PMAxx and PEMAX) to determine virus or bacteriophage infectivity in the context of food safety or environmental virology as well as comparisons of azo dye applications with other established methods such as cell-culture, phage plaque assay or conventional qPCR (Supplementary Table S2). Disinfection methods utilized in water treatment or food safety have also used capsid integrity qPCR to determine the efficiency in virus inactivation (Lee et al., 2018; Leifels et al., 2015; Randazzo et al., 2018b).
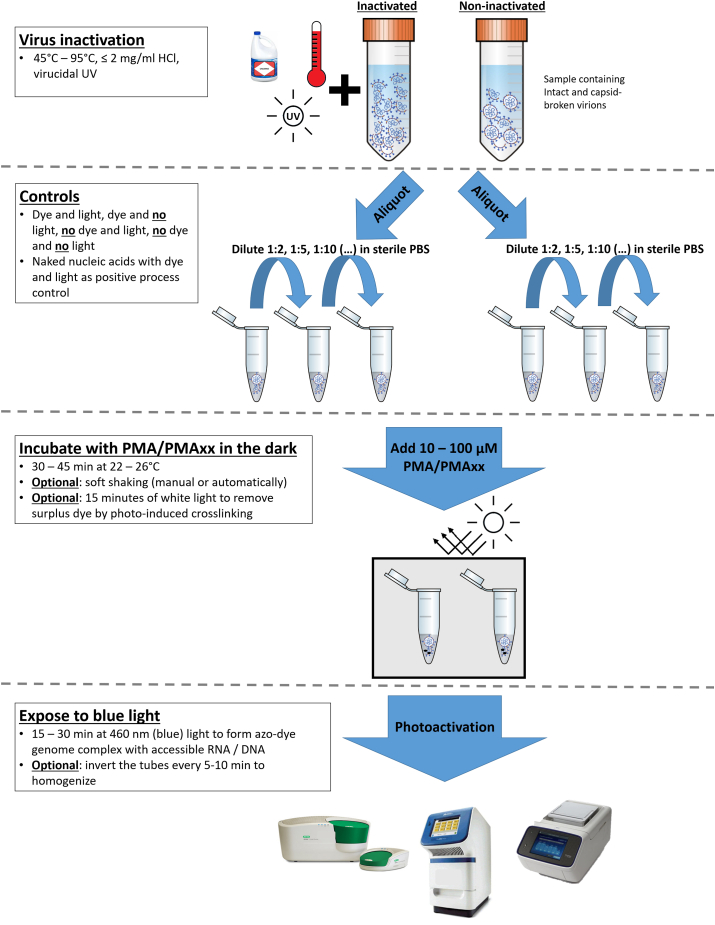
Fig. 2.
Schematic overview of a typical case study structure. One or more virus samples (taken from the environment or a culture collection) are split in two and one portion is inactivated, the other not, before filtration and concentration steps. Azo dye pretreatment is then conducted under various incubation conditions and concentrations of PMA, PMAxx, PEMAX or EMA, either in the presence or absence of additives like surfactants and enzymes, before the tubes are exposed to light for photoactivation. While early studies used high-energy light sources (500–800 Watt) to initiate the formation of the light induced dye-genome complex, more recent articles have focused on low energy LED in the blue light spectrum. Virus quantification is done with qPCR or ddPCR for quantitative, or endpoint PCR for qualitative, detection after genome extraction. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Viruses studied
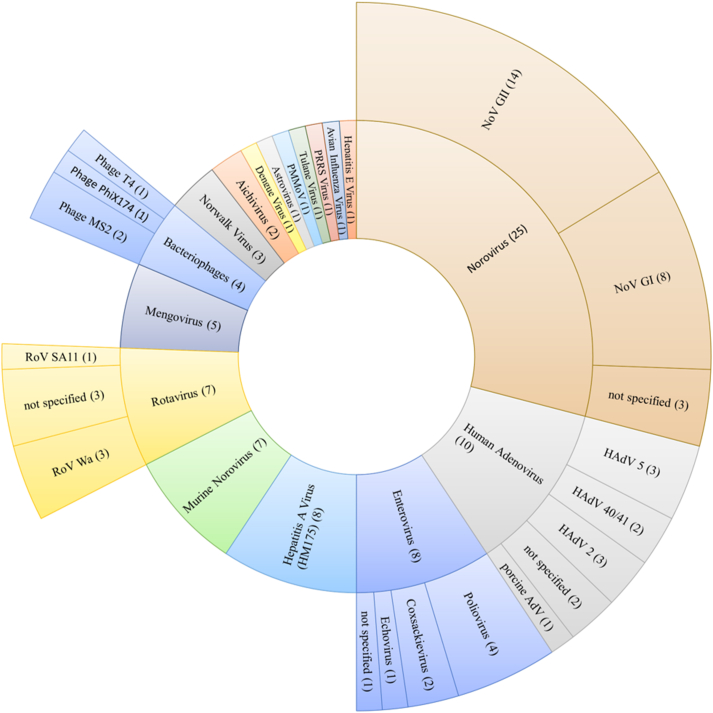
All known types of enteric viruses, whether they contain single- or double-stranded DNA or RNA, have been studied with capsid integrity qPCR methods, with most investigations focusing on non-enveloped viruses (Fig. 3). Most studies investigated two or more viruses (69%) with an emphasis on the detection of norovirus GI and GII (NoV GI/NoV GII) together with hepatitis A virus (HAV). The increase in the number of studies on capsid integrity of NoV and HAV between 2016 and 2020 is most likely associated with the release of ISO/TS 15216-1:2013 (ISO, 2013a) and ISO/TS 15216-2:2013 (ISO, 2013b) that regulate the qualitative analysis and to a certain extent quantification of NoV and HAV in food using qPCR. Both standards have subsequently been replaced with updated versions (ISO, 2017, 2019). The risk associated with norovirus outbreaks due to a low infectious dose and high rate of particle shedding by infected individuals, together with the absence of commercially available animal tissue cell lines, also explains the interest in developing azo dye pretreatments for the detection of NoV (Blanco et al., 2017; Lowther et al., 2019). Murine norovirus (MNV) and mengovirus, two viruses without relevance to human health, have been chosen as both have been reported to be suitable as process controls because they lack human pathogenicity and are easy to propagate on commercial cell lines (Coudray et al., 2013; ISO, 2013a). Of the articles included, only three cover bacteriophages infecting Escherichia coli as their host (phages MS2, PhiX174 and T4). As the laboratory safety requirements, as well as workload for culture-based detection, of those phages are significantly lower than for enteric viruses, the necessity to establish alternative methods to determine phage infectivity is not as urgent as for enteric viruses (Toribio-Avedillo et al., 2020). Their increasing relevance in the context of microbial source tracking will likely lead to more studies in the future (Ogilvie et al., 2018; Wangkahad et al., 2017).
Fig. 3.
Genus and strain number of viruses investigated and frequency of occurrence in case studies (in parentheses). In cases where no strain name was listed, the virus is referred to as ‘not specified’.
The intercalating dye infectivity assay was successfully applied to most viruses tested, with few exceptions. Bacteriophage T4 infecting Escherichia coli required higher temperatures for inactivation than other viruses, with extremely high heat (110 °C) for significant capsid damage; lower temperatures (85 °C) and proteolysis were not effective (Fittipaldi et al., 2012). Moreover, capsid disruption of MNV was more challenging than for other viruses in the same studies, i.e., human adenovirus (HAdV), poliovirus, rotavirus (RoV), and bacteriophages phiX174 and MS2, using heat treatment (Kim and Ko, 2012; Leifels et al., 2015). Lack of efficacy of an azo dye method with avian influenza virus was suspected to be due to natural characteristics of an enveloped virus that make it difficult for EMA to penetrate the compromised capsid (Graiver et al., 2010). However, the PMA dye assay was successfully applied to detect dengue virus, another enveloped, single-stranded RNA virus (Huang et al., 2016), lending further support to the conclusion that PMA is more effective than EMA in removing false positive signals in qPCR while not showing microbicidal effects (Fittipaldi et al., 2012; Gedalanga and Olson, 2009; Leifels et al., 2019).
4. Origin of studied viruses
The viruses analyzed were split evenly between strains obtained from culture-collections and wild types, those obtained from clinical, environmental and food samples (Table 1). While progress has been made in introducing protocols for their propagation in the laboratory, an established cell line to propagate NoV GI/GII has yet to arrive (Estes et al., 2019; Veronica et al., 2018). Subsequently, the studies included in this review analyzed NoV in stool samples obtained from hospitals, in sewage or in surface water. One study evaluated the presence of NoV GI/GII in struvite, a phosphate source used for fertilization, that was reclaimed from wastewater sludge (Yee et al., 2019). For other viruses, direct investigation for effects of strain origins, i.e., laboratory-grown strains and environmentally acquired strains, is limited, and comparisons of results among different studies would lead to biases due to diverse experimental protocols and conditions. Moreover, a limitation of azo dye studies on clinical, environmental and food samples is the absence of information regarding absolute quantification of infectious and non-infectious viruses to evaluate the assay performance, even though known concentrations of mengovirus (Randazzo et al., 2018a, 2018b) or murine norovirus (Leifels et al., 2016, 2019) have been added as internal controls in some studies investigating greywater used for irrigation or freshwater near a recreational bathing site. Other complicating factors include process recovery loss and inhibition effects in qPCR assays that appeared to affect RNA viruses more than DNA viruses regardless of azo dye pretreatment (Leifels et al., 2016). Next, we discuss the assay conditions that influence the efficacy of capsid integrity qPCR in measuring infective viruses.
Table 1.
Study design parameters.
| Parameter | Description | Total number of studies | Percentage of studies |
|---|---|---|---|
| Virus origin | Culture collection | 28 | 49 |
| Wild type (taken from aquatic environment or stool samples) | 29 | 51 | |
| Inactivation method | Natural decay | 6 | 11 |
| High temperature | 25 | 45 | |
| UV light | 7 | 12 | |
| Chemical disinfection | 12 | 21 | |
| Other (e.g. proteolysis) | 6 | 11 | |
| Type of azo dye | PMA | 32 | 53 |
| PMAxx | 10 | 17 | |
| PEMAX | 3 | 5 | |
| EMA | 15 | 25 | |
| Azo dye concentration | ≤10 μM | 8 | 13 |
| 25 μM | 6 | 10 | |
| 50 μM | 20 | 32 | |
| 100 μM | 17 | 27 | |
| 125–150 μM | 1 | 2 | |
| ≥200 μM | 10 | 16 | |
| Incubation time | 5 min | 14 | 34 |
| 10 min | 13 | 32 | |
| 15 min | 1 | 2 | |
| 30 min | 10 | 24 | |
| 60 min | 2 | 5 | |
| 120 min | 1 | 2 | |
| Incubation temperature | On ice | 2 | 5 |
| 4 °C–6 °C | 7 | 18 | |
| 20 °C–25 °C (or room temperature) | 29 | 76 | |
| Light source | LED Active Blue (commercial system) | 22 | 55 |
| Other LED | 7 | 17 | |
| High energy light (500–800 W) | 11 | 28 | |
| Light exposure time | ≤5 min | 6 | 15 |
| 10 min | 6 | 15 | |
| 15 min | 27 | 66 | |
| ≥20 min | 2 | 5 | |
| Azo dye add-ons | Triton X-100 | 5 | 38 |
| Lauroyl sarcosinate | 2 | 15 | |
| Platinum (IV) chloride (PtCl₄) | 3 | 23 | |
| RNase | 1 | 8 | |
| Benzonase | 1 | 8 | |
| Cis-dichlorodiammineplatinum (CDDP) | 1 | 8 |
5. Azo dye type and concentration range
Both EMA and PMA can permeate the bacterial cell membrane and virus capsid to intercalate with nucleic acids when activated with light emitted by high-energy lamps or blue light emitting diodes (LEDs). These azo dyes became available for research purposes in the early 2000s, but a number of studies reported that the more charged EMA tended to enter bacterial cells with intact membranes, thus potentially resulting in false-negative qPCR results and cytotoxic effects (Fittipaldi et al., 2012; Gedalanga and Olson, 2009). Seventy percent of the articles we researched used PMA or the derived PMAxx, and a third of the studies compared them to EMA and/or the derivate PEMAX (Table 1). In general, PMA and PMAxx were found to be more suitable for capsid integrity qPCR, most likely due to their higher ability to enter thermally and chemically inactivated virions while not penetrating intact capsids or showing microbicidal effects (Jeong et al., 2017; Kim et al., 2017; Moreno et al., 2015), when compared to infectious virus titers determined by cell culture (Leifels et al., 2019).
Azo dye concentrations are quite evenly distributed, ranging from less than 10 μM to greater than 200 μM. More than half of the articles under investigation reported either 50 μM or 100 μM (Table 1), which is in accordance with the recommendations by the manufacturer for bacterial cultures (Biotium Inc., 2019). However, some works indicated optimal reduction rates of presumably false positive signals (as shown in the cell culture) with dye concentrations between 4 μM and 10 μM (Karim et al., 2015; Lee et al., 2016; Leifels et al., 2016, 2019; Prevost et al., 2016). The effect of high azo dye concentration appeared to vary with virus type; for example, two-log reduction in viability of phage MS2 has been reported with >125 μM PMA (Kim and Ko, 2012), while 250 μM PMA successfully removed signals of inactivated viruses for MNV and NoV GII.4 without causing an adverse inactivation effect (Jeong et al., 2017; Lee et al., 2015).
6. Incubation conditions
Approximately two thirds of the studies included in this review applied an incubation time with the azo dye of 5 or 10 min (Table 1). A quarter incubated for 30 min and less than one in ten of the studies reported periods lasting longer than one hour. A similarly clear trend is observed for incubation temperature; most studies incubated at temperatures between 20 °C and 25 °C or indicated that room temperature was used (Table 1). The remainder of the articles described the samples being stored on ice or at 4–6 °C; none of them used higher temperatures as sometimes discussed for bacterial assays (Codony et al., 2019). Studies that applied a long incubation time tended to use low temperatures (Coudray-Mounier et al., 2013; Sangsanont et al., 2014; Prevost et al., 2016; Leifels et al., 2016, 2019; Canh et al., 2018, 2019; Oristo et al., 2018; Fraisse et al., 2018).
7. Dye activation conditions
Early publications utilizing azo dyes for the removal of non-infectious virus particles exclusively applied 500- to 800-Watt halogen light sources used in stage lighting (Bellehumeur et al., 2015; Canh et al., 2018; Escudero-Abarca et al., 2014; Graiver et al., 2010; Leifels et al., 2015; Parshionikar et al., 2010; Sangsanont et al., 2014). Besides their operational hazards such as light bulbs exploding due to long running times (a maximum of 5 min is recommended by the manufacturer), both the heat and light emission in the ultraviolet and infrared spectra could potentially harm the sample, thus subverting the purpose of the pretreatment altogether. Wider availability of consumer-grade LED light technology in general and the introduction of dedicated azo dye activation light sources by companies like GenIUL, Spain, allowed for a much more precise and reproducible activation of PMA, EMA, PEMAX and PMAxx. Not surprisingly, 72% of the records included in this review utilized either the commercial LED Active Blue light system (GenIUL, Spain) or LEDs emitting blue (around 460 nm) light (Lee et al. (2015); Fongaro et al. (2016)) as depicted in Table 1. A similar trend towards uniformity in the protocol design is apparent in the length of time a sample is exposed to the light source. While the operational requirements of the 500- to 800-W halogen lamps severely limited the exposure time to very short periods, the standard configuration of the commercial light systems allows for 15 min of intense blue light. Two thirds of the studies therefore employed 15 min as the light exposure time.
8. Additional reagents for azo dye pretreatment
Addition of non-ionic surfactants like Triton X-100 (Coudray-Meunier et al., 2013; Moreno et al., 2015) and sodium lauroyl sarcosinate (Lee et al., 2018, 2019) have been described as beneficial for the determination of virus infectivity and were most frequently used in the studies evaluated (Table 1). Inclusion of non-ionic surfactant enable the azo dye molecules to enter partially or completely ruptured capsids, thus improving their binding properties in the virus genome. Palladium and platinum compounds such as Platinum(IV) chloride (PtCl4) and Cis-dichlorodiammineplatinum (CPPD) are long known to chelate in mammalian cells by nucleic acid ligands (Rosenberg et al., 1965), and have recently been adopted for the qPCR based discrimination between live and dead bacteria such as E. coli and Cronobacter skazakii (Soejima et al. (2016). Attempts to use them to determine virus infectivity have been successful but their associated health risk limits potential applications in routine environmental microbiology (Fraisse et al., 2018; Randazzo et al., 2018b).
9. Virus inactivation
Various methods to inactivate viruses were used in the studies included in this review. The intention was either to evaluate disinfection efficiency as it is currently used in food safety and water treatment (Jeong et al., 2017; Langlet et al., 2018; Leifels et al., 2016; Randazzo et al., 2018a) or to act as controls to evaluate the efficacy of the capsid integrity protocol (Canh et al., 2018, 2019; Farkas et al., 2020; Leifels et al., 2015). Addition of chlorine, exposure to heat, and proteolysis are known to damage the viral capsid, thus rendering the genome accessible to azo dyes. Temperatures from moderate to high (45 °C–95 °C) for ten to thirty minutes could reproducibly demonstrate the ability of all dyes to remove virus signals (to varying degrees) from molecular quantification (Fraisse et al., 2018; Jeong et al., 2017; Leifels et al., 2015, 2019; Oristo et al., 2018). Similar effects could be shown for the addition of hypochlorite of up to two milligram per milliliter (Fuster et al., 2016; McLellan et al., 2016; Prevost et al., 2016). Light in the ultraviolet spectrum, on the other hand, affects the hydrogen bonds between nucleic acids, resulting in the inability to reproduce inside the host cell. Capsid integrity qPCR failed to capture the loss of virus infectivity in most UV studies (Karim et al., 2015; Kim et al., 2017; Leifels et al., 2016, 2019); it could detect capsid damage caused by medium-pressure UV lamps, especially at 230–245 nm wavelength (Sangsanont et al., 2014), but not nucleic acid damage caused by other wavelengths in the UV range (Beck et al., 2018; Beck et al., 2014; Sirikanchana et al., 2008a, b). Natural decay and the ability of azo dyes to remove virus signals originating from this die-off have been investigated explicitly only by Prevost et al. (2016), Fongaro et al. (2016) and Coudray-Mounier et al. (2013), while other works discussed effects on virus enumeration in environmental samples (Leifels et al., 2016, 2019; Lee et al., 2016). The viruses assessed in those studies are all classified as enteric and non-enveloped, potentially limiting the applicability of insights obtained to enveloped viruses like SARS-CoV-2 or dengue virus. The varying efficiencies of PMA, PMAxx, PEMAX and EMA in preventing the amplification of DNA/RNA of viruses that have lost their ability to infect their host cells due to exposure to heat and reactive substances like chlorine resemble those that have been described for bacteria, starting with the first publication on the subject (Nogva et al., 2003).
10. Recommendations and potential applications
The utilization of azo dye pretreatment can be recommended for applications where culture assays take too long to inform necessary remedial actions, and under low resource conditions either in developing countries or in laboratories with only basic analytical capabilities and biosafety levels. The recent outbreak of SARS-CoV-2 associated COVID-19 represents an example where knowing the ratio of infectious to non-infectious virions would help in determining whether symptomatic or asymptomatic carriers need to be isolated (Beeching et al., 2020; Kaul, 2020) and could potentially increase the confidence in already standardized molecular quantification methods by removing at least a portion of the false-positive signals. Considering the utility of wastewater-based surveillance during the current SARS-CoV-2 pandemic (Corpuz et al., 2020; Thompson et al., 2020), studies evaluating the use of capsid integrity qPCR to detect this non-enteric, enveloped virus are needed and expected to be published in the coming months. A step-by-step protocol introducing azo dye pretreatment to determine capsid integrity (and thus virus infectivity) into an established qPCR workflow can be developed (Fig. 4) based on parameters suggested in this review like incubation duration, dye concentration as well as light source and exposure time. The key steps in the optimization process are virus- and matrix-specific optimization (e.g. increasing the length of the genome region targeted by the qPCR assay to increase the probability of azo-dye genome interaction and the dilution of extracted environmental samples to reduce the influence of co-concentrated inhibitory substances) as well as optimizing incubation conditions, dye-concentration and photoactivation.
Fig. 4.
Design of a capsid integrity qPCR assay. Depending on the sample origin (especially the complexity of the matrix), the virus genus and strain as well as the molecular detection method used, several factors like dilution of the sample, concentration of the azo dye, incubation conditions and photo activation can be modified and optimized during assay development.
To determine the validity of azo dye pretreatment of environmental samples, a robust set of controls for environmental and foodstuff related samples should be included. Such controls could involve viral targets like pepper mild mottle virus (Symonds et al., 2019) and crAssphage (Farkas et al., 2019) that occur in the majority of samples but are not human pathogenic. Alternatively, the addition of known concentrations of viruses that are not endemic in the water under investigation (e.g. mengovirus and murine norovirus) prior to sample concentration as well as the azo-dye pretreatment have been reported. In addition to allowing the determination of a virus recovery rate, such external controls can also help quantifying the ratio of virions with intact and broken capsid.
11. Conclusions
An evaluation of methods on the application of azo dye pretreatment to determine virus infectivity by qPCR revealed a great diversity of viruses that have been tested under a range of treatment conditions. The systematic literature comparison led to the following conclusions:
-
•
PMA and the derivate PMAxx show a higher efficiency in removing false positive signals from qPCR for both DNA and RNA viruses than EMA and PEMAX.
-
•
Incubation duration and temperature, reagent concentration as well as light source and exposure time need to be optimized and validated for the virus under investigation.
-
•
“One-size-fits-all” pretreatment approaches are possible but might lead to lower signal reduction rates of individual viruses.
-
•
Capsid integrity qPCR can be a valuable tool to adapt existent workflows and qPCR protocols to reflect the ability of viruses to infect humans, thus improving risk assessment and consumer safety derived from these measurements.
-
•
Capsid integrity is a strong indicator of virus infectivity, which allows for the establishment of robust assays to assess the infectivity of novel viruses in the event of outbreaks like the 2014 Ebola virus epidemic and the current COVID-19 pandemic.
Author contributions
M.L. and K. S. conceived and planned the literature review. M. L., E. S. and D. C. S. performed the title and abstract as well as full-text screening. M. L. took lead in writing the manuscript, C. D. and M. L. conceptualized and generated figures. S. W., S. M. and K. S. provided valuable feedback and helped shape the discussion, analysis and narrative. All authors contributed to editing and proofreading of the manuscript.
Funding
This research was supported by the Singapore National Research Foundation and Ministry of Education under the Research Centre of Excellence Programme.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.wroa.2020.100080.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Beck S.E., Hull N.M., Poepping C., Linden K.G. Wavelength-Dependent damage to adenoviral proteins across the Germicidal UV spectrum. Environ. Sci. Technol. 2018;52:223–229. doi: 10.1021/acs.est.7b04602. [DOI] [PubMed] [Google Scholar]
- Beck S.E., Rodriguez R.A., Linden K.G., Hargy T.M., Larason T.C., Wright H.B. Wavelength dependent UV inactivation and DNA damage of adenovirus as measured by cell culture infectivity and long range quantitative PCR. Environ. Sci. Technol. 2014;48:591–598. doi: 10.1021/es403850b. [DOI] [PubMed] [Google Scholar]
- Beeching N.J., Fletcher T.E., Beadsworth M.B.J. Covid-19: testing times. BMJ. 2020;369:m1403. doi: 10.1136/bmj.m1403. [DOI] [PubMed] [Google Scholar]
- Bellehumeur C., Boyle B., Charette S.J., Harel J., L'Homme Y., Masson L., Gagnon C.A. Propidium monoazide (PMA) and ethidium bromide monoazide (EMA) improve DNA array and high-throughput sequencing of porcine reproductive and respiratory syndrome virus identification. J. Virol. Methods. 2015;222:182–191. doi: 10.1016/j.jviromet.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biotium Inc . Propidium Monoazide Product Information Sheet. 2019. [Google Scholar]
- Blanco A., Guix S., Fuster N., Fuentes C., Bartolomé R., Cornejo T., Pintó R.M., Bosch A. Norovirus in bottled water associated with Gastroenteritis outbreak, Spain, 2016. Emerg. Infect. Dis. 2017;23:1531–1534. doi: 10.3201/eid2309.161489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch A., Gkogka E., Le Guyader F.S., Loisy-Hamon F., Lee A., van Lieshout L., Marthi B., Myrmel M., Sansom A., Schultz A.C., Winkler A., Zuber S., Phister T. Foodborne viruses: detection, risk assessment, and control options in food processing. Int. J. Food Microbiol. 2018;285:110–128. doi: 10.1016/j.ijfoodmicro.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer A.F., Masters N.B., Eisenberg J.N.S. Quantitative microbial risk assessment and infectious disease transmission modeling of waterborne enteric pathogens. Curr Environ Health Rep. 2018;5:293–304. doi: 10.1007/s40572-018-0196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canh V.D., Kasuga I., Furumai H., Katayama H. Impact of various humic acids on EMA-RT-qPCR to selectively detect intact viruses in drinking water. J. Water Environ. Technol. 2018;16:83–93. [Google Scholar]
- Canh V.D., Kasuga I., Furumai H., Katayama H. Viability RT-qPCR combined with sodium deoxycholate pre-treatment for selective quantification of infectious viruses in drinking water samples. Food Environ Virol. 2019;11:40–51. doi: 10.1007/s12560-019-09368-2. [DOI] [PubMed] [Google Scholar]
- Chhipi-Shrestha G., Hewage K., Sadiq R. Microbial quality of reclaimed water for urban reuses: probabilistic risk-based investigation and recommendations. Sci. Total Environ. 2017;576:738–751. doi: 10.1016/j.scitotenv.2016.10.105. [DOI] [PubMed] [Google Scholar]
- Cochrane . 2019. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. (updated July 2019) [Google Scholar]
- Codony F., Dinh-Thanh M., Agustí G. Key factors for removing bias in viability PCR-based methods: a review. Curr. Microbiol. 2019;77:682–687. doi: 10.1007/s00284-019-01829-y. [DOI] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpuz M.V.A., Buonerba A., Vigliotta G., Zarra T., Ballesteros F., Campiglia P., Belgiorno V., Korshin G., Naddeo V. Viruses in wastewater: occurrence, abundance and detection methods. Sci. Total Environ. 2020;745:140910. doi: 10.1016/j.scitotenv.2020.140910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudray-Meunier C., Fraisse A., Martin-Latil S., Guillier L., Perelle S. Discrimination of infectious hepatitis A virus and rotavirus by combining dyes and surfactants with RT-qPCR. BMC Microbiol. 2013;13:216. doi: 10.1186/1471-2180-13-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudray C., Merle G., Martin-Latil S., Guillier L., Perelle S. Comparison of two extraction methods for the detection of hepatitis A virus in lettuces using the murine norovirus as a process control. J. Virol. Methods. 2013;193:96–102. doi: 10.1016/j.jviromet.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Efstratiou A., Ongerth J.E., Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2011–2016. Water Res. 2017;114:14–22. doi: 10.1016/j.watres.2017.01.036. [DOI] [PubMed] [Google Scholar]
- Escudero-Abarca B.I., Rawsthorne H., Goulter R.M., Suh S.H., Jaykus L.A. Molecular methods used to estimate thermal inactivation of a prototype human norovirus: more heat resistant than previously believed? Food Microbiol. 2014;41:91–95. doi: 10.1016/j.fm.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Estes M.K., Ettayebi K., Tenge V.R., Murakami K., Karandikar U., Lin S.-C., Ayyar B.V., Cortes-Penfield N.W., Haga K., Neill F.H., Opekun A.R., Broughman J.R., Zeng X.-L., Blutt S.E., Crawford S.E., Ramani S., Graham D.Y., Atmar R.L. Human Norovirus cultivation in nontransformed stem cell-derived human intestinal enteroid cultures: success and challenges. Viruses. 2019;11:638. doi: 10.3390/v11070638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas K., Mannion F., Hillary L.S., Malham S.K., Walker D.I. Emerging technologies for the rapid detection of enteric viruses in the aquatic environment. Curr. Opin. Environ. Sci. Health. 2020;16:1–6. [Google Scholar]
- Farkas K., Adriaenssens E.M., Walker D.I., McDonald J.E., Malham S.K., Jones D.L. Critical evaluation of CrAssphage as a molecular marker for human-derived wastewater contamination in the aquatic environment. Food Environ. Virol. 2019;11:113–119. doi: 10.1007/s12560-019-09369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittipaldi M., Nocker A., Codony F. Progress in understanding preferential detection of live cells using viability dyes in combination with DNA amplification. J. Microbiol. Methods. 2012;91:276–289. doi: 10.1016/j.mimet.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Fongaro G., Hernández M., García-González M.C., Barardi C.R., Rodríguez-Lázaro D. Propidium monoazide coupled with PCR predicts infectivity of enteric viruses in Swine manure and biofertilized soil. Food Environ Virol. 2016;8:79–85. doi: 10.1007/s12560-015-9225-1. [DOI] [PubMed] [Google Scholar]
- Fraisse A., Niveau F., Hennechart-Collette C., Coudray-Meunier C., Martin-Latil S., Perelle S. Discrimination of infectious and heat-treated norovirus by combining platinum compounds and real-time RT-PCR. Int. J. Food Microbiol. 2018;269:64–74. doi: 10.1016/j.ijfoodmicro.2018.01.015. [DOI] [PubMed] [Google Scholar]
- Fuster N., Pintó R.M., Fuentes C., Beguiristain N., Bosch A., Guix S. Propidium monoazide RTqPCR assays for the assessment of hepatitis A inactivation and for a better estimation of the health risk of contaminated waters. Water Res. 2016;101:226–232. doi: 10.1016/j.watres.2016.05.086. [DOI] [PubMed] [Google Scholar]
- Gedalanga P.B., Olson B.H. Development of a quantitative PCR method to differentiate between viable and nonviable bacteria in environmental water samples. Appl. Microbiol. Biotechnol. 2009;82:587–596. doi: 10.1007/s00253-008-1846-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C.P., Betancourt W.Q. Assessing the occurrence of waterborne viruses in reuse systems: analytical limits and needs. Pathogens. 2019;8 doi: 10.3390/pathogens8030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson K.E. Viral pathogens in water: occurrence, public health impact, and available control strategies. Curr. Opin. Virol. 2014;4:50–57. doi: 10.1016/j.coviro.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graiver D.A., Saunders S.E., Topliff C.L., Kelling C.L., Bartelt-Hunt S.L. Ethidium monoazide does not inhibit RT-PCR amplification of nonviable avian influenza RNA. J. Virol. Methods. 2010;164:51–54. doi: 10.1016/j.jviromet.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Grubaugh N.D., Ladner J.T., Lemey P., Pybus O.G., Rambaut A., Holmes E.C., Andersen K.G. Tracking virus outbreaks in the twenty-first century. Nat Microbiol. 2019;4:10–19. doi: 10.1038/s41564-018-0296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., Bergström T., Norder H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014;80:6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J., Seidel M., Niessner R., Eggers J., Tiehm A. Long amplicon (LA)-qPCR for the discrimination of infectious and noninfectious phix174 bacteriophages after UV inactivation. Water Res. 2016;103:141–148. doi: 10.1016/j.watres.2016.07.032. [DOI] [PubMed] [Google Scholar]
- Huang X., Zhou X., He X., Wang P., Yue S., Wu L., Zhang Y., Xie Q., Zhang B., Zhao W. Detection of infectious dengue virus by selective real-time quantitative polymerase chain reaction. Virol. Sin. 2016;31:342–345. doi: 10.1007/s12250-016-3757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO . 2013. Microbiology of Food and Animal Feed — Horizontal Method for Determination of Hepatitis A Virus and Norovirus in Food Using Real-time RT-PCR — Part 1: Method for Quantification. [Google Scholar]
- ISO . 2013. Microbiology of Food and Animal Feed — Horizontal Method for Determination of Hepatitis A Virus and Norovirus in Food Using Real-time RT-PCR — Part 2: Method for Qualitative Detection. [Google Scholar]
- ISO . 2017. Microbiology of the Food Chain — Horizontal Method for Determination of Hepatitis A Virus and Norovirus Using Real-time RT-PCR — Part 1: Method for Quantification. [Google Scholar]
- ISO . 2019. ISO 15216-2:2019(en), Microbiology of the food chain — Horizontal method for determination of hepatitis A virus and norovirus using real-time RT-PCR — Part 2: Method for detection. [Google Scholar]
- Jeong M.I., Park S.Y., Ha S.D. Thermal inactivation of human norovirus on spinach using propidium or ethidium monoazide combined with real-time quantitative reverse transcription-polymerase chain reaction. Food Contr. 2017;78:79–84. [Google Scholar]
- Karim M.R., Fout G.S., Johnson C.H., White K.M., Parshionikar S.U. Propidium monoazide reverse transcriptase PCR and RT-qPCR for detecting infectious enterovirus and norovirus. J. Virol. Methods. 2015;219:51–61. doi: 10.1016/j.jviromet.2015.02.020. [DOI] [PubMed] [Google Scholar]
- Kaul D. An overview of coronaviruses including the SARS-2 coronavirus – molecular biology, epidemiology and clinical implications. Curr. Med. Res. Pract. 2020;10:54–64. doi: 10.1016/j.cmrp.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-H., Shahbaz H.M., Park D., Chun S., Lee W., Oh J.-W., Lee D.-U., Park J. A combined treatment of UV-assisted TiO2 photocatalysis and high hydrostatic pressure to inactivate internalized murine norovirus. Innovat. Food Sci. Emerg. Technol. 2017;39:188–196. [Google Scholar]
- Kim S.Y., Ko G. Using propidium monoazide to distinguish between viable and nonviable bacteria, MS2 and murine norovirus. Lett. Appl. Microbiol. 2012;55:182–188. doi: 10.1111/j.1472-765X.2012.03276.x. [DOI] [PubMed] [Google Scholar]
- Ko G., Cromeans T.L., Sobsey M.D. Detection of infectious adenovirus in cell culture by mRNA reverse transcription-PCR. Appl. Environ. Microbiol. 2003;69:7377–7384. doi: 10.1128/AEM.69.12.7377-7384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlet J., Kaas L., Croucher D., Hewitt J. Effect of the Shellfish Proteinase K digestion method on Norovirus capsid integrity. Food Environ. Virol. 2018;10:151–158. doi: 10.1007/s12560-018-9336-6. [DOI] [PubMed] [Google Scholar]
- Lee D.-Y., Leung K.T., Lee H., Habash M.B. Simultaneous detection of selected enteric viruses in water samples by multiplex quantitative PCR. Water, Air, Soil Pollut. 2016;227:107. [Google Scholar]
- Lee H.-W., Yoon S.-R., Lee H.-M., Lee J.Y., Kim S.H., Ha J.-H. Use of RT-qPCR with combined intercalating dye and sodium lauroyl sarcosinate pretreatment to evaluate the virucidal activity of halophyte extracts against norovirus. Food Contr. 2019;98:100–106. [Google Scholar]
- Lee H.W., Lee H.M., Yoon S.R., Kim S.H., Ha J.H. Pretreatment with propidium monoazide/sodium lauroyl sarcosinate improves discrimination of infectious waterborne virus by RT-qPCR combined with magnetic separation. Environ. Pollut. 2018;233:306–314. doi: 10.1016/j.envpol.2017.10.081. [DOI] [PubMed] [Google Scholar]
- Lee M., Seo D.J., Seo J., Oh H., Jeon S.B., Ha S.D., Myoung J., Choi I.S., Choi C. Detection of viable murine norovirus using the plaque assay and propidium-monoazide-combined real-time reverse transcription-polymerase chain reaction. J. Virol. Methods. 2015;221:57–61. doi: 10.1016/j.jviromet.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Leifels M., Hamza I.A., Krieger M., Wilhelm M., Mackowiak M., Jurzik L. From lab to lake – evaluation of current molecular methods for the detection of infectious enteric viruses in complex water matrices in an Urban Area. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifels M., Jurzik L., Wilhelm M., Hamza I.A. Use of ethidium monoazide and propidium monoazide to determine viral infectivity upon inactivation by heat, UV- exposure and chlorine. Int. J. Hyg Environ. Health. 2015;218:686–693. doi: 10.1016/j.ijheh.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Leifels M., Shoults D., Wiedemeyer A., Ashbolt N.J., Sozzi E., Hagemeier A., Jurzik L. Capsid integrity qPCR—an azo-dye based and culture-independent approach to estimate adenovirus infectivity after disinfection and in the aquatic environment. Water-Sui. 2019;11:1196. [Google Scholar]
- Lowther J.A., Bosch A., Butot S., Ollivier J., Made D., Rutjes S.A., Hardouin G., Lombard B., In't Veld P., Leclercq A. Validation of EN ISO method 15216 - Part 1 - quantification of hepatitis A virus and norovirus in food matrices. Int. J. Food Microbiol. 2019;288:82–90. doi: 10.1016/j.ijfoodmicro.2017.11.014. [DOI] [PubMed] [Google Scholar]
- McLellan N.L., Lee H., Habash M.B. Evaluation of propidium monoazide and long-amplicon qPCR as an infectivity assay for coliphage. J. Virol. Methods. 2016;238:48–55. doi: 10.1016/j.jviromet.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A., Group P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno L., Aznar R., Sanchez G. Application of viability PCR to discriminate the infectivity of hepatitis A virus in food samples. Int. J. Food Microbiol. 2015;201:1–6. doi: 10.1016/j.ijfoodmicro.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Nogva H.K., Dromtorp S.M., Nissen H., Rudi K. Ethidium monoazide for DNA-based differentiation of viable and dead bacteria by 5'-nuclease PCR. Biotechniques. 2003;34:804–808. doi: 10.2144/03344rr02. 810, 812-803. [DOI] [PubMed] [Google Scholar]
- Ogilvie L.A., Nzakizwanayo J., Guppy F.M., Dedi C., Diston D., Taylor H., Ebdon J., Jones B.V. Resolution of habitat-associated ecogenomic signatures in bacteriophage genomes and application to microbial source tracking. ISME J. 2018;12:942–958. doi: 10.1038/s41396-017-0015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oristo S., Lee H.-J., Maunula L. Performance of pre-RT-qPCR treatments to discriminate infectious human rotaviruses and noroviruses from heat-inactivated viruses: applications of PMA/PMAxx, benzonase and RNase. J. Appl. Microbiol. 2018;124:1008–1016. doi: 10.1111/jam.13737. [DOI] [PubMed] [Google Scholar]
- Parshionikar S., Laseke I., Fout G.S. Use of propidium monoazide in reverse transcriptase PCR to distinguish between infectious and noninfectious enteric viruses in water samples. Appl. Environ. Microbiol. 2010;76:4318–4326. doi: 10.1128/AEM.02800-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polston P.M., Rodriguez R.A., Seo K., Kim M., Ko G., Sobsey M.D. Field evaluation of an improved cell line for the detection of human adenoviruses in environmental samples. J. Virol. Methods. 2014;205:68–74. doi: 10.1016/j.jviromet.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Prevost B., Goulet M., Lucas F.S., Joyeux M., Moulin L., Wurtzer S. Viral persistence in surface and drinking water: suitability of PCR pre-treatment with intercalating dyes. Water Res. 2016;91:68–76. doi: 10.1016/j.watres.2015.12.049. [DOI] [PubMed] [Google Scholar]
- Rames E., Roiko A., Stratton H., Macdonald J. Technical aspects of using human adenovirus as a viral water quality indicator. Water Res. 2016;96:308–326. doi: 10.1016/j.watres.2016.03.042. [DOI] [PubMed] [Google Scholar]
- Randazzo W., Khezri M., Ollivier J., Le Guyader F.S., Rodriguez-Diaz J., Aznar R., Sanchez G. Optimization of PMAxx pretreatment to distinguish between human norovirus with intact and altered capsids in shellfish and sewage samples. Int. J. Food Microbiol. 2018;266:1–7. doi: 10.1016/j.ijfoodmicro.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Randazzo W., Vasquez-García A., Aznar R., Sánchez G. Viability RT-qPCR to distinguish between HEV and HAV with intact and altered capsids. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01973. 1973-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R.A., Pepper I.L., Gerba C.P. Application of PCR-based methods to assess the infectivity of enteric viruses in environmental samples. Appl. Environ. Microbiol. 2009;75:297–307. doi: 10.1128/AEM.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg B., Van Camp L., Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- Sangsanont J., Katayama H., Kurisu F., Furumai H. Capsid-damaging effects of UV irradiation as measured by quantitative PCR coupled with ethidium monoazide treatment. Food Environ. Virol. 2014;6:269–275. doi: 10.1007/s12560-014-9162-4. [DOI] [PubMed] [Google Scholar]
- Sinclair R.G., Jones E.L., Gerba C.P. Viruses in recreational water-borne disease outbreaks: a review. J. Appl. Microbiol. 2009;107:1769–1780. doi: 10.1111/j.1365-2672.2009.04367.x. [DOI] [PubMed] [Google Scholar]
- Sirikanchana K., Shisler J.L., Marinas B.J. Effect of exposure to UV-C irradiation and monochloramine on adenovirus serotype 2 early protein expression and DNA replication. Appl. Environ. Microbiol. 2008;74:3774–3782. doi: 10.1128/AEM.02049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirikanchana K., Shisler J.L., Marinas B.J. Inactivation kinetics of adenovirus serotype 2 with monochloramine. Water Res. 2008;42:1467–1474. doi: 10.1016/j.watres.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Soejima T., Minami J., Xiao J.Z., Abe F. Innovative use of platinum compounds to selectively detect live microorganisms by polymerase chain reaction. Biotechnol. Bioeng. 2016;113:301–310. doi: 10.1002/bit.25711. [DOI] [PubMed] [Google Scholar]
- Symonds E.M., Rosario K., Breitbart M. Pepper mild mottle virus: agricultural menace turned effective tool for microbial water quality monitoring and assessing (waste)water treatment technologies. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.R., Nancharaiah Y.V., Gu X., Lee W.L., Rajal V.B., Haines M.B., Girones R., Ng L.C., Alm E.J., Wuertz S. Making waves: wastewater surveillance of SARS-CoV-2 for population-based health management. Water Res. 2020;184:116181. doi: 10.1016/j.watres.2020.116181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toribio-Avedillo D., Méndez J., Muniesa M., Blanch A.R. Evaluation of new components in modified Scholten's medium for the detection of Somatic Coliphages. Food Environ. Virol. 2020;12:148–157. doi: 10.1007/s12560-020-09419-z. [DOI] [PubMed] [Google Scholar]
- Veronica C., Esther K.M., Hannah B., Khalil E., Xi-Lei Z., Robert L.A., Mary K.E., Jan V. Human Norovirus replication in human intestinal enteroids as model to evaluate virus inactivation. Emerg. Infect. Dis. J. 2018;24:1453. doi: 10.3201/eid2408.180126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangkahad B., Mongkolsuk S., Sirikanchana K. Integrated Multivariate Analysis with Nondetects for the Development of Human Sewage Source-Tracking Tools Using Bacteriophages of Enterococcus faecalis. Environ. Sci. Technol. 2017;51:2235–2245. doi: 10.1021/acs.est.6b04714. [DOI] [PubMed] [Google Scholar]
- Wu Z., Zeng T., Guo W.-J., Bai Y.-Y., Pang D.-W., Zhang Z.-L. Digital single virus immunoassay for ultrasensitive multiplex avian influenza virus detection based on fluorescent magnetic multifunctional nanospheres. ACS Appl. Mater. Interfaces. 2019;11:5762–5770. doi: 10.1021/acsami.8b18898. [DOI] [PubMed] [Google Scholar]
- Yee R.A., Leifels M., Scott C., Ashbolt N.J., Liu Y. Evaluating microbial and chemical hazards in commercial struvite recovered from wastewater. Environ. Sci. Technol. 2019;53:5378–5386. doi: 10.1021/acs.est.8b03683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.