Abstract
RATIONALE
Direct analysis of chemicals on a surface using mass spectrometry is of great importance in forensics, food and drug safety, environmental monitoring, and defense. Solvent extraction-based surface analysis offers a convenient way of controlling the desorption conditions and applying internal standards. To date, it mainly relies on a separate electrospray process to nebulize and ionize the solvents. Here, we report a simple and standalone ionization system for the solvent extraction-based surface analysis without the need of high voltage based on the vibrating sharp-edge spray ionization (VSSI).
METHODS
We modified the original VSSI device and developed a standalone, integrated surface sampling, and ionization system for MS analysis. By incorporating a micropipette-based solvent dispenser with the VSSI device, the new system performs solvent extraction and ionization, and still maintains a small footprint.
RESULTS
We demonstrated 4-order-of-magnitude linear response for glucose spotted on a glass surface with a limit of detection (LOD) of 0.1 pg/mm2. We further characterized the performance of this method with a series of compounds and demonstrated a similar LOD to literature values obtained by desorption electrospray ionization (DESI). Finally, we applied this method to quantitatively measure the concentration of a pesticide ametryn on spinach surfaces. We demonstrated good linearity (R2= 0.99) for ametryn with surface densities in the range of 8–800 pg/mm2 and an LOD of 9 pg/mm2.
CONCLUSIONS
We have demonstrated a simple, effective, direct ambient-ionization method that is highly sensitive to molecules on a wide range of surfaces. The flexibility, small footprint, low cost, and voltage-free nature of this method make it an attractive tool for direct surface sample analysis using MS.
Introduction
Mass spectrometry (MS) is widely used in forensics, food and drug safety1–3, chemical analysis4–6, environmental monitoring7–12, plant sciences13,14, materials and basic life sciences15,16 and defense17,18 as a reliable method to detect and identify minute amounts of chemicals with a high degree of accuracy, precision, and resolution19. In these diverse areas, there is a need for rapid and direct detection of chemicals and biomolecules on various kinds of surfaces20,21 including: tissue surfaces, such as human skin, fruit, vegetable and leaf surfaces, and many other natural and artificial surfaces22. To apply MS analysis to the above applications requires methods that directly sample a surface and subsequently ionize the molecules. To date, several ambient-ionization methods have been reported that can be applied to the direct characterization of molecules on surfaces. Desorption electrospray ionization (DESI)23 and direct analysis in real time (DART)24 are the most widely used direct-ionization methods for surface molecules. They have been used in many applications including: analysis of drug tablets25, detection of explosives26, chemical warfare agents and drugs22,27, toxicological analysis of bio samples28, and food control analysis of pesticides29. In DESI, the desorption and ionization can be optimized independently for improved performance30. Easy sonic spray assisted ionization (EASI) using pressurized gas to generate liquid droplets shares a similar workflow to the DESI without the need of a high voltage supply31,32. Laser ablation electrospray ionization (LAESI) reported by Vertes et al is another surface analysis method using MS33. LAESI is especially useful for studying single cells due to its high spatial resolution and compatibility with aqueous solutions34. Another example is atmospheric pressure matrix assisted laser desorption ionization (APMALDI), which eliminates the need for extensive sample preparation and the vacuum requirements of conventional matrix assisted laser desorption ionization (MALDI)35. APMALDI has the capability to fragment a target molecule and thus provide fragment ion information that can be used for molecular identification36. Recently, Trimpin et al reported matrix assisted ionization (MAI), a technique that does not require a laser for desorption and ionization, making it an attractive method for rapid and convenient surface analysis37–39. Based on the method employed for desorbing surface molecules, there is another surface analysis category, termed solvent extraction-based surface analysis. This method employs a solvent to desorb molecules from the surface with subsequent ionization of the sample analytes. These methods include nano desorption electrospray ionization (nanoDESI)40, probe electrospray ionization (PESI)41, and liquid extraction surface analysis (LESA)42. The major advantage of solvent extraction-based analysis is that internal standards can be easily added to the desorption solvent thereby facilitating the quantification of surface molecules using MS.
Existing solvent extraction-based surface analysis methods require high voltage to nebulize the solution and ionize the targets, resulting in a complex system setup and/or the requirement of well-controlled fluid flow. A potential simple and voltage-free method for solvent extraction-based surface analysis is solvent assisted inlet ionization (SAII), which utilize the vacuum at the mass spectrometer inlet for ionization of liquid samples43. For samples that are difficult to attach to the inlet, this method could require modification of the mass spectrometer inlet to extend the vacuum line. Recently, we reported a new mechanical vibration-based ionization method termed vibrating sharp-edge spray ionization (VSSI)44,45 in which liquid samples are nebulized at the sharp-edges of a vibrating glass slide. Existing acoustics-based methods generate droplets from liquid on a vibrating substrate for ionization46–48. For VSSI, we have demonstrated two working modes: on-substrate ionization and touch ionization. The touch ionization mode is directly compatible with the solvent extraction-based workflow, and it can thus be a convenient method for direct surface MS analysis due to its simplicity and flexibility. However, to date, the potential of VSSI for direct surface analysis has not been explored. In this work, using the VSSI principle, we developed a standalone surface analysis method that has a credit card-sized footprint (Figure 1). We integrated a dispenser to precisely guide solvent onto the surface for analysis without adding extra bulk that affects the performance of the VSSI. This method allows efficient surface sampling and ionization with a high degree of reproducibility, and the quantification capability for MS analysis. We tested the feasibility of this method for the analysis of chemicals ranging from small polar molecules, a small drug molecule, a pesticide and non-polar molecules to a peptide (specifically, we tested acetaminophen, glucose, phenylalanine, caffeine, reserpine, angiotensin II, cholesterol, raffinose, verapamil and ametryn). Our results showed similar sensitivity to recent literature values reported for DESI23,49. Using this VSSI method, a four order-of-magnitude linear response for dried glucose is achieved with a limit of detection (LOD) of 0.1 pg/mm2. More importantly, VSSI enables the direct analysis of chemicals on a surface without the need of complex procedures or external equipment. For example, we demonstrate the direct analysis of pesticides on organic spinach surfaces with a linear response range of two orders of magnitude. This new method offers a new means of direct surface probing on any sample surface without prior sample preparation, which greatly improves the flexibility and biocompatibility of MS surface analysis.
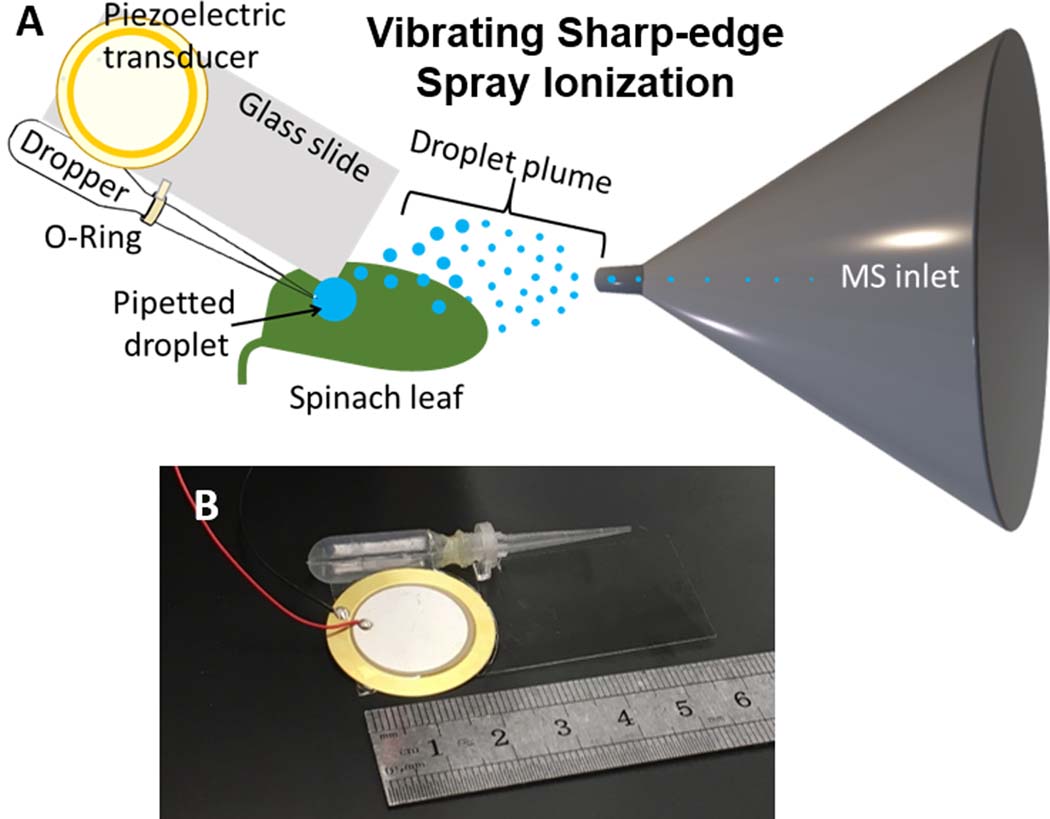
FIGURE 1.
A, Schematic of vibrating sharp‐edge spray ionization (VSSI) for surface analysis and B, an image of a complete VSSI surface analysis device including a piezoelectric diaphragm, a cover glass, and a dropper
Experimental
Materials and reagents.
We used: high performance liquid chromatography (HPLC) grade water, methanol, and chloroform from Fisher Scientific (Waltham, MA, USA); caffeine, acetaminophen, glucose, ametryn, and reserpine from Sigma Aldrich (St. Louis, MO, USA); angiotensin II, phenylalanine, verapamil, and raffinose from Alfa Aesar (Tewksbury, MA, USA); cholesterol from Avanti Polar Lipids (Alabaster, AL, USA); and super-hydrophobic coating (Ultratech International, Jacksonville, FL, USA) An analytical balance (Accuris Instruments, Edison, NJ, USA) was used to measure weights of chemicals and droplets.
MS Instrument.
A Q-Exactive™ Hybrid quadrupole-Orbitrap™ Mass Spectrometer (Thermo Scientific, Bremen, Germany) was used for all MS analyses. The resolving power of the mass spectrometer is 140,000 (at m/z 200). All measurements were performed with an input capillary temperature of 450 °C, injection time of 200 ms, with no sheath gas flow, no auxiliary gas flow, and no spray voltage or spray current. The S-lens value was adjusted between 30 and 50% depending on the molecules being tested.
VSSI Fabrication and Operation.
VSSI devices were fabricated by attaching an O-ring (~5 mm ID, 8 mm OD and 2 mm thickness) to the VSSI device roughly 25 mm from its vibrating sharp edge. The O-ring is attached to the VSSI glass slide using UV-curing photopolymer (Norland optical adhesive, Cranbury, NJ, USA). The modified dispenser is made by attaching the bulb of a commercial 0.2-mL dispenser to a micropipette using epoxy (Devcon, Hartford, CT, USA). A detailed fabrication of the VSSI device is described in our previous work.44 Briefly, a piezoelectric diaphragm (Murata, Kyoto, Japan) is bonded to one end of a 24×60 mm2 No.1 microscope glass slide (VWR, Philadelphia, PA, USA) using epoxy.
The VSSI device is driven with a function generator (RIGOL LXI 2 Channel DG1022, Beaverton, OR, USA) and a power amplifier (Mini-Circuits LZY-22+, Brooklyn, NY, USA) at frequencies between 90 and 100 kHz. For direct MS surface analysis, the modified solvent dispenser is positioned within the O-ring for delivering solvent for sample desorption and subsequent ionization, as shown in Figure 1A. The device was positioned orthogonal to the inlet of the mass spectrometer at ~5 mm for mass spectrometry measurements.
Sample Preparation.
To prepare surfaces with different chemical densities, a super-hydrophobic coating was used to constrain the wetting area for sample deposition on a glass slide. Scotch™ tape was used to mask the sample deposition area and a two-step super-hydrophobic spray process was used to coat the taped front side. After coating, the tape was peeled off, and the glass surface was wiped clean with HPLC grade methanol. This leaves a clean glass area surrounded by a super-hydrophobic boundary. The solutions to be tested were added with the desired concentration (typically, 1 mL was deposited on a 19×30 mm2 surface area). For different areas, to keep the surface density the same, it was normalized to the specific surface area used (e.g., 2 mL was delivered for a larger 19×60 mm2 area). For consistency and simplicity, glass slides were used for all sample deposition as a representative surface. The areas used included 60×19 mm2, 30×19 mm2, 10×19 mm2, 5×5 mm2, and 2.5×2.5 mm2. To ensure deposition of sample analytes on the surface, 30×19 mm2 was preferred as 1 mL of liquid is well constrained within the sample area. The surface density of the analyte to be tested is expressed in g/mm2 by serial dilution of a standard solution to the desired concentration. Thus, when 1 mL of sample was spotted on a 30×19 mm2 area, the surface density (pg/mm2) of the analyte was easily calculated.
For sample testing on realistic agricultural samples, spinach leaves of 10 mm diameter were cut using a cork borer to ensure sample uniformity. The leaves were placed on a piece of double-sided scotch tape affixed to a glass slide. Three μL of ametryn was spotted and, using a small piece of Teflon, the drop was stirred until it dried to ensure a uniform surface concentration.
Results and Discussion
Extraction and Ionization of Analytes on the Surface.
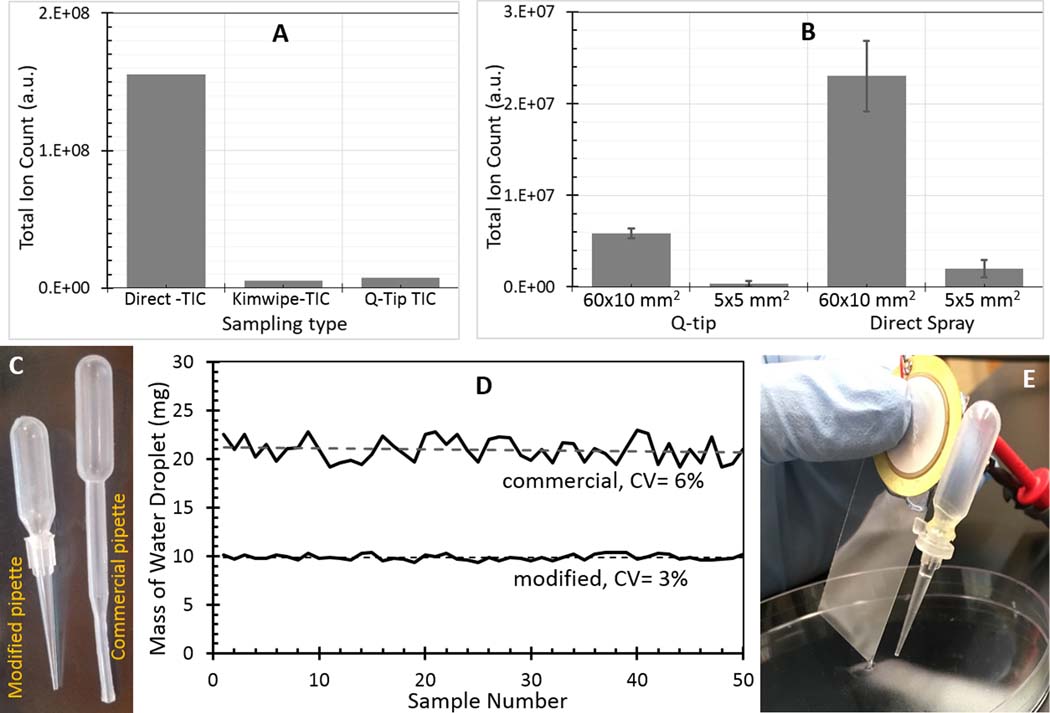
As discussed above, the VSSI-based direct-surface analysis requires a step to extract surface bound molecules into the solvent followed by the nebulization of the solvent for MS analysis. Our previous studies demonstrated that VSSI can nebulize and ionize the solutions it contacts on a wide variety of surfaces44. There are two possible sampling protocols for VSSI-based direct surface analysis. The first is to add a solvent drop onto the surface of interest and nebulize the drop using the VSSI system for MS detection. The other is to use a swab/wipe to collect samples from a wet surface and nebulize the solvent on the swab/wipe with VSSI. The first protocol is straightforward and simple to apply, but the second protocol could enrich target analytes by wiping through a large surface area. To determine the optimal protocol, we compared the ion signals of 1 mL of 100 μM acetaminophen pre-spotted onto a 30×19 mm2 glass surface (surface density 27 ng/mm2) using both protocols. HPLC grade water was used as the extraction solvent for both methods. The ion intensity of acetaminophen using the first protocol is ~10 times that from the samples tested with the second protocol, as shown in Figure 2A. We did not see a significant difference between using a Q-tip® or a Kimwipe® in the second protocol. Because the second protocol may benefit from a larger sampling area, we compared the performance of the two protocols using different sampling areas. It was observed that, for the first protocol, for an increase in area there was a slight but not substantial increase in the total ion count for the target, as expected because the surface density of acetaminophen and the solvent volume was the same in both cases. With the same solvent volume (~10 μL), a larger surface area does not facilitate significantly more analyte extraction. For the second protocol, with the surface area increased from 5×5 mm2 to 60×19 mm2, we observed 10-fold improvement in ion counts as expected due to the concentration effect using a Q-tip®, despite the total ion count still being less than with the direct sampling protocol (see Figure 2B). Thus, further increasing the sampling area should improve the second protocol. However, as 60×19 mm2 is already a large surface area, further increasing the sampling area may not be feasible for many applications. In addition, using larger surfaces could introduce more contaminants into the solution, which may suppress the ionization of target analytes. Therefore, we decided that the first protocol, direct extraction and ionization, was optimal and we use it in the VSSI-based surface analysis.
FIGURE 2.
A, Comparison of signal intensity of 100 μM acetaminophen spotted and dried on the same sample surface area over direct Kimwipe swipe and Q‐tip swipe sampling; B, comparison of different sample surface areas and sampling types for 100 μM acetaminophen spotted and tested using 1 μM caffeine as the internal standard; C, the commercial 0.2‐mL pipette versus the modified pipette tip used to dispense solvent; D, co‐variance of droplets dispensed by the two pipettes; and E, VSSI device in use for surface analysis showing the plume generated
To develop a standalone device for the direct extraction protocol, we integrated a simple solvent dispensing unit into the VSSI device. We attached an O-ring to the side of a VSSI glass slide roughly 25 mm from the vibrating edge, which effectively incorporates a small dropper into the VSSI device. By squeezing the dropper, solvent is added onto the target surface close to the vibrating sharp-edge of the VSSI device. We studied the volume and the consistency of the dropper dispenser. For solvent extraction-based surface analysis, using small volumes results in less target dilution, which benefits the sensitivity of the analysis. However, if the sample volume is too small, the variation of the droplet volume and the effect of solvent evaporation will introduce extraction error into the analysis. We tested and compared two microdroppers for dispensing extraction solvent, as shown in Figure 2C. The first dropper consists of a commercial 0.2-mL disposable transfer pipette. The second dropper is a fabricated by using a P10 micropipette tip (0.5 μL-10 μL volume) attached to the bulb of 0.2-mL commercial pipette to reduce the orifice diameter. To characterize the effect of orifice diameter for the two pipettes, on a plastic disposable weigh boat, a drop of water from each pipette was weighed. The weights of 50 drops of water from each pipette were used to determine the consistency of the dropper. The results showed that the commercial dropper dispensed ~20 mg per drop with a CV of 6%, whereas the customized dropper dispensed ~10 mg per drop with a CV of 3% (see Figure 2D). Based on this smaller volume and improved consistency, this customized micropipette was selected as the dispenser. By applying a ~93 kHz signal to the transducer, we observed plume generation using a driving voltage of ~20 Vpp (Figure 2E).
We also studied the positioning of the vibrating sharp-edge with respect to the inlet of the mass spectrometer and its effect on ion intensity. The range of glass slide positions from planar to perpendicular positioning was tested. It was observed that the best plume generation was obtained for the perpendicular position. Thus, the VSSI device was positioned orthogonal to the inlet at 5 mm from the mass spectrometer to achieve the maximum collection of ions for improved analyte ion signal intensity.
Characterization of VSSI-MS for Detection of Chemicals on a Glass Surface.
With the optimized setup geometry and operational parameters, we characterized both the qualitative and the quantitative performance of the VSSI-based surface MS analysis for a series of molecules. Chemicals were first deposited onto glass surfaces by drying solutions of known analyte concentrations. The spotting area is controlled using a super-hydrophobic coating to confine the solution on the glass surface. We first tested the sensitivity of the VSSI method for the chemicals listed in Table 1. The LODs were calculated as the mean of intensity at the target m/z values in a blank solution plus 3 times the standard deviation for 15 trials.
Table 1:
List of chemicals tested and solvent systems used for the analysis and their respective calculated LOD values using the mean intensity of the blank solvent plus 3 times the standard deviation at the pertinent m/z values.
| Analyte | VSSI | |||
|---|---|---|---|---|
| LOD (pg/mm2) | Species detected | Surface | Solvent | |
| Acetaminophen | 1 | [M+Na]+ | Glass | H2O |
| Glucose | 0.1 | [M+Na]+ | Glass | H2O |
| Phenylalanine | 10 | [M+Na]+ | Glass | H2O |
| Raffinose | 1 | [M+Na]+ | Glass | H2O |
| Verapamil | 1 | [M+H]+ | Glass | CH3OH |
| Ametryn | 0.1 | [M+H]+ | Glass | CH3OH |
| Caffeine | 10 | [M+Na]+ | Glass | CHCl3:CH3OH (1:1) |
| Cholesterol | 150 | [M+Na]+ | Glass | CHCl3:CH3OH (1:1) |
| Reserpine | 90 | [M+2H]+ | Glass | H2O:CH3OH (1:1) |
| Angiotensin II | 0.05 | [M+2H]+ | Glass | H2O:CH3OH (1:1) |
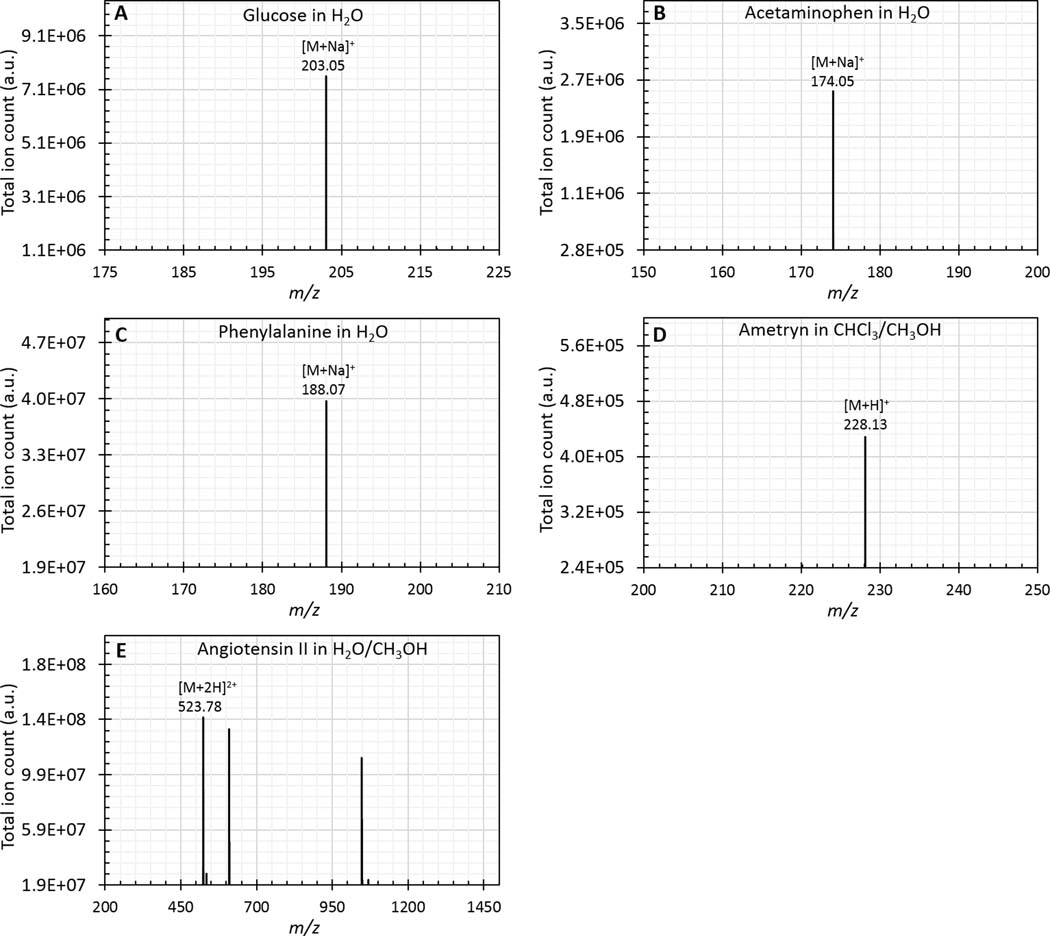
Direct surface analysis with VSSI achieved similar sensitivity (in the pg/mm2 range) to the results obtained with DESI experiments for most of the compounds. Notably, for carbohydrates such as raffinose and glucose (see Figure 3A for glucose mass spectrum), VSSI shows lower LODs of 1 pg/mm2 and 0.1 pg/mm2, respectively, compared with the DESI values of 100 pg/mm2 and 90 pg/mm2, respectively502. These lower LODs probably indicate better desorption efficiency for these highly hydrophilic molecules with VSSI. In contrast, the LOD for the more difficult to protonate molecule, cholesterol, is ~15-times higher for VSSI than for DESI, which could be due to a lack of excess charge carriers available for ionizing cholesterol23. Only the sodium adduct peak for cholesterol was detected with VSSI. In addition to the small molecules, VSSI-based direct surface analysis also works for peptides. We tested angiotensin II samples by depositing them on glass surfaces. A charge state of +3 was the dominant peak in the water/methanol (v/v: 1:1) desorption solvent. The LOD (0.05 pg/mm2) for angiotensin II with VSSI is in a similar range to that obtained with DESI23.
FIGURE 3.
Mass spectra of A, glucose of surface density 1 ng/mm2 in water; B, acetaminophen of surface density 13 μg/mm2 in water; C, phenylalanine of surface density 1 ng/mm2 in water; D, ametryn of surface density 100 pg/mm2 in methanol/chloroform (v/v: 1:1); and E, angiotensin II of surface density 10 ng/mm2 in methanol/water (v/v: 1:1)
VSSI had advantages over current solvent extraction-based methods that require high voltage to nebulize the solution and ionize the target. One advantage of VSSI is that it is a solvent extraction-based surface analysis method so that an internal standard can be easily added directly to the solvent. In additional, in VSSI, extraction (dissolution) of analytes from the sample surface and subsequent nebulization leading to ionization occur in a concerted two-step process. Furthermore, the nebulization efficiency does not strongly depend on the solvent properties for common solvents: water and organic solvents. For small polar molecules (e.g., acetaminophen (Figure 3B), glucose, phenylalanine (Figure 3C) and raffinose, water is used for extraction and ionization. For chemicals with higher octanol-water partition coefficients (e.g., verapamil and ametryn (Figure 3D) methanol is used for extraction and ionization. We can also optimize the extraction-nebulization efficiency using a combination of solvents. Because extraction of chemicals and ionization happens in this two-step process, we can choose a mixture of a solvent that optimizes extraction and one that affects nebulization/ionization. For example, for caffeine and cholesterol, which are highly soluble in chloroform, methanol was added to allow protonation of the analyte, so that it is detected in positive ion mode. Finally, for reserpine and angiotensin II (Figure 3E), methanol: water (1:1) presented a suitable combination of solvents.
Overall, these results indicate that VSSI-based direct surface analysis has similar performance to DESI, the most widely used surface-analysis ionization method. Considering the simplicity in setup and the more controllable process for surface-analyte desorption, VSSI will be advantageous for a number of applications that requires portable analysis platforms especially where irregular surfaces are involved.
We also characterized the quantification potential of the VSSI method for surface samples. A range of surface densities of glucose was deposited on glass slides at concentrations from 0.1 pg/mm2 to 1000 pg/mm2. Direct sampling was employed using water as the solvent. The plot of total ion count vs surface density shows a linear response over 4 orders of magnitude (with R2= 0.982) (Figure 4). This demonstrates the applicability and feasibility of using the VSSI approach for quantitative surface chemical analysis. Notably, here VSSI demonstrated good linear response for a simple sample without using an internal standard, indicating the robust performance of this protocol. For more complex samples, this protocol is also compatible with the use of an internal standard by simply adding a known amount of the standard molecule to the extraction solution. In summary, we have demonstrated that this simple VSSI-based direct-surface MS analysis yields performance that is comparable with the well-established DESI approach, which requires a more complex system setup.
FIGURE 4.
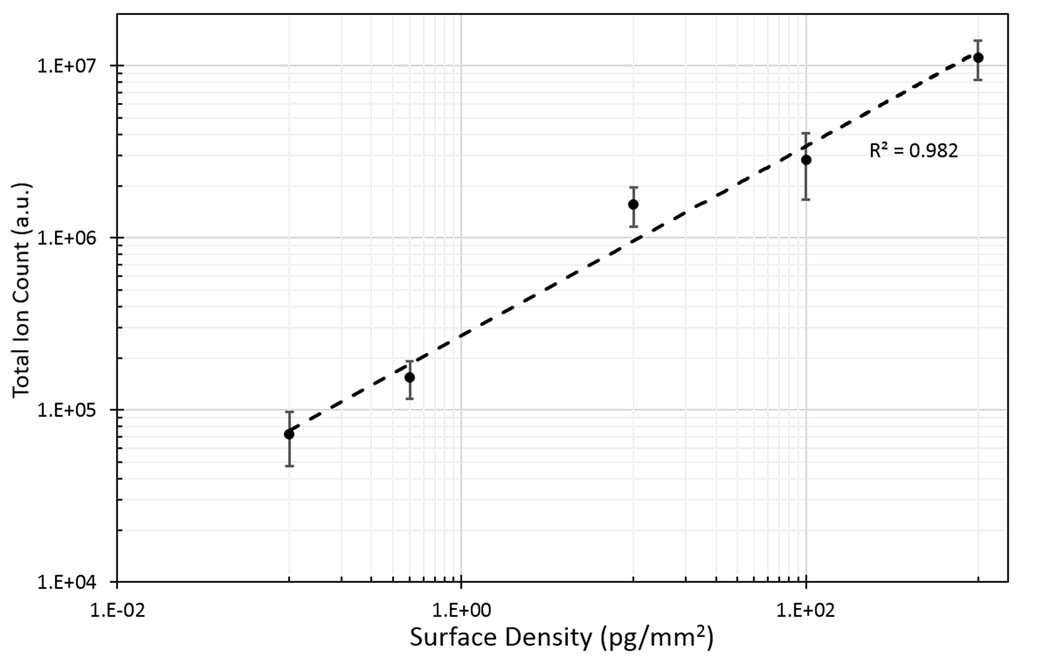
Linear curve of glucose deposited with different surface densities on glass slides from 0.1 to 1000 pg/mm2
Testing of Real Samples.
Finally, we tested whether or not the VSSI-based direct-surface analysis could be used to detect pesticides on real fruit or vegetable surfaces. The simplicity of this VSSI nebulization method allows it to be applied to chemically characterize any surface that is compatible with extraction solvents. Different surface textures and roughness have little impact on the VSSI nebulization process; we tested the VSSI nebulization on a series of fruits and vegetables including blueberries, tomato, romaine lettuce, bok choy and spinach (See Video 1, Supporting Information, for direct nebulization on the surface of a tomato). We further tested its quantification performance by spotting a pesticide, ametryn, onto the surface of supermarket, organic spinach. It was important to deposit the same surface density of pesticide on spinach leaves in order to quantify the pesticide. 10-mm diameter spinach leaves were double-side taped to a glass slide to ensure flatness, and a 3-μL solution of pesticide was spotted and stirred using a Teflon brush to ensure uniformity while the spot dried. The pesticide solution used methanol as a solvent to promote quick drying and even deposition. A micropipette was used to deposit 3 μL of methanol solution containing 1 μM of caffeine as an internal standard, for extraction and ionization of the dried pesticide (see plume in Figure 5A). The calibration curve plotted used the total ion count normalized with respect to the internal standard to reduce signal variation (see Figure 5B). This curve shows good linearity from 5 pg/mm2 to 100 pg/mm2 (R2= 0.998). By also testing blank spinach samples, we determine the LOD of ametryn on a spinach leaf surface to be 5 pg/mm2. The inset in Figure 5B shows the calibration curve for low surface densities. The maximum allowable detectable amount of ametryn on a perishable surface37 is estimated to be in the range of ~3 μg/mm2. Therefore, the current sensitivity and linear range of the VSSI method make it a useful tool for detection of pesticides on the surface of a pineapple, corn, and sugarcane for which ametryn is used as a pesticide.
FIGURE 5.
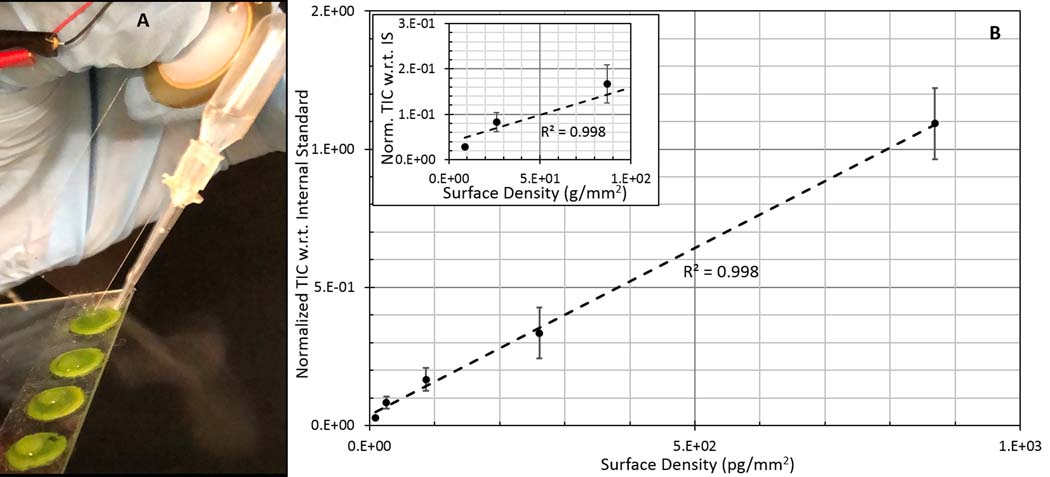
A, Direct extraction and ionization of surface chemicals using vibrating sharp‐edge spray ionization and B, linear response of ametryn spotted on spinach leaves (the inset shows data for lower surface densities)
Conclusions
We have developed a solvent-extraction MS surface analysis method based on VSSI. We systematically characterized the performance of this method and demonstrate the performance of this method as being comparable with that of existing direct-analysis ionization methods. This VSSI-based method provides a convenient means of probing surfaces for chemical characterization by MS without the need for sample preparation. The simplicity, flexibility, small footprint and low power consumption of VSSI make it an attractive ionization strategy for MS. We expect that it will be especially useful for analyzing fragile molecules that are sensitive to electrochemical oxidation or for studying biomolecules directly in their native state and environment. Future studies will include exploring the imaging capabilities of this VSSI-based method to allow for mapping the concentration of analytes on surfaces.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (R01GM135432) and the West Virginia University Deans’ Instrumentation Seed Program for Innovative Research (InSPIRe). We acknowledge use of the WVU Shared Research Facilities for mass spectrometry analysis. We thank Dr Callee Walsh in the WVU-BNRF research facility for assisting in the MS spectrometry experiments. We are also grateful for helpful discussion of the work with Professor Stephen Valentine at WVU.
References:
- 1.Castro-Puyana M, Herrero M. Metabolomics approaches based on mass spectrometry for food safety, quality and traceability. TrAC Trends in Analytical Chemistry. 2013;52:74–87. [Google Scholar]
- 2.Kaufmann A. The current role of high-resolution mass spectrometry in food analysis. Anal Bioanal Chem. 2012;403(5):1233–1249. [DOI] [PubMed] [Google Scholar]
- 3.Malik AK, Blasco C, Picó Y. Liquid chromatography–mass spectrometry in food safety. J Chromatogr A. 2010;1217(25):4018–4040. [DOI] [PubMed] [Google Scholar]
- 4.Aksenov AA, da Silva R, Knight R, Lopes NP, Dorrestein PC. Global chemical analysis of biology by mass spectrometry. Nature Reviews Chemistry. 2017;1(7):0054. [Google Scholar]
- 5.Liang Z, Zhang S, Li X, et al. Tip-enhanced ablation and ionization mass spectrometry for nanoscale chemical analysis. Science Advances. 2017;3(12):eaaq1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma X. Ambient ionization and miniature mass spectrometry system for chemical and biological analysis. TrAC Trends in Analytical Chemistry. 2016;85:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollender J, Schymanski EL, Singer HP, Ferguson PL. Nontarget Screening with High Resolution Mass Spectrometry in the Environment: Ready to Go? Environ Sci Technol. 2017;51(20):11505–11512. [DOI] [PubMed] [Google Scholar]
- 8.Lübbert C, Baars C, Dayakar A, et al. Environmental pollution with antimicrobial agents from bulk drug manufacturing industries in Hyderabad, South India, is associated with dissemination of extended-spectrum beta-lactamase and carbapenemase-producing pathogens. Infection. 2017;45(4):479–491. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Carballo E, González-Barreiro C, Scharf S, Gans O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environmental Pollution. 2007;148(2):570–579. [DOI] [PubMed] [Google Scholar]
- 10.Rager JE, Strynar MJ, Liang S, et al. Linking high resolution mass spectrometry data with exposure and toxicity forecasts to advance high-throughput environmental monitoring. Environment International. 2016;88:269–280. [DOI] [PubMed] [Google Scholar]
- 11.Yuan B, Koss AR, Warneke C, Coggon M, Sekimoto K, de Gouw JA. Proton-Transfer-Reaction Mass Spectrometry: Applications in Atmospheric Sciences. Chem Rev. 2017;117(21):13187–13229. [DOI] [PubMed] [Google Scholar]
- 12.Laskin J, Laskin A, Nizkorodov SA. Mass Spectrometry Analysis in Atmospheric Chemistry. Anal Chem. 2018;90(1):166–189. [DOI] [PubMed] [Google Scholar]
- 13.Jorge TF, Rodrigues JA, Caldana C, et al. Mass spectrometry-based plant metabolomics: Metabolite responses to abiotic stress. Mass Spectrom Rev. 2016;35(5):620–649. [DOI] [PubMed] [Google Scholar]
- 14.Jaeger RJR, Lamshöft M, Gottfried S, Spiteller M, Spiteller P. HR-MALDI-MS Imaging Assisted Screening of β-Carboline Alkaloids Discovered from Mycena metata. J Nat Prod. 2013;76(2):127–134. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Zhang W, Zhang H, et al. Polydopamine-Modified Substrates for High-Sensitivity Laser Desorption Ionization Mass Spectrometry Imaging. ACS Applied Materials & Interfaces. 2019;11(49):46140–46148. [DOI] [PubMed] [Google Scholar]
- 16.Wagner M, Tonoli D, Varesio E, Hopfgartner G. The use of mass spectrometry to analyze dried blood spots. Mass Spectrom Rev. 2016;35(3):361–438. [DOI] [PubMed] [Google Scholar]
- 17.Verbeck GF, Bierbaum VM. Focus on Harsh Environment and Field-Portable Mass Spectrometry: Editorial. J Am Soc Mass Spectrom. 2015;26(2):199–200. [DOI] [PubMed] [Google Scholar]
- 18.Wichert WRA, Dhummakupt ES, Zhang C, et al. Detection of Protein Toxin Simulants from Contaminated Surfaces by Paper Spray Mass Spectrometry. J Am Soc Mass Spectrom. 2019;30(8):1406–1415. [DOI] [PubMed] [Google Scholar]
- 19.Triebl A, Trötzmüller M, Hartler J, Stojakovic T, Köfeler HC. Lipidomics by ultrahigh performance liquid chromatography-high resolution mass spectrometry and its application to complex biological samples. J Chromatogr B. 2017;1053:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasiljevic T, Gómez-Ríos GA, Pawliszyn J. Single-Use Poly(etheretherketone) Solid-Phase Microextraction–Transmission Mode Devices for Rapid Screening and Quantitation of Drugs of Abuse in Oral Fluid and Urine via Direct Analysis in Real-Time Tandem Mass Spectrometry. Anal Chem. 2018;90(1):952–960. [DOI] [PubMed] [Google Scholar]
- 21.Ren Y, Chiang S, Zhang W, Wang X, Lin Z, Ouyang Z. Paper-capillary spray for direct mass spectrometry analysis of biofluid samples. Anal Bioanal Chem. 2016;408(5):1385–1390. [DOI] [PubMed] [Google Scholar]
- 22.Valdez Carlos A, Leif Roald N, Hok S, Hart Bradley R. Analysis of chemical warfare agents by gas chromatography-mass spectrometry: methods for their direct detection and derivatization approaches for the analysis of their degradation products. In. Reviews in Analytical Chemistry. Vol 372018. [Google Scholar]
- 23.Takáts Z, Wiseman JM, Gologan B, Cooks RG. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science. 2004;306(5695):471. [DOI] [PubMed] [Google Scholar]
- 24.Cody RB, Laramée JA, Durst HD. Versatile New Ion Source for the Analysis of Materials in Open Air under Ambient Conditions. Anal Chem. 2005;77(8):2297–2302. [DOI] [PubMed] [Google Scholar]
- 25.Leuthold LA, Mandscheff J-F, Fathi M, et al. Desorption electrospray ionization mass spectrometry: direct toxicological screening and analysis of illicit Ecstasy tablets. Rapid Commun Mass Spectrom. 2006;20(2):103–110. [DOI] [PubMed] [Google Scholar]
- 26.Talaty N, Mulligan CC, Justes DR, Jackson AU, Noll RJ, Cooks RG. Fabric analysis by ambient mass spectrometry for explosives and drugs. Analyst. 2008;133(11):1532–1540. [DOI] [PubMed] [Google Scholar]
- 27.D’Agostino PA, Chenier CL. Desorption electrospray ionization mass spectrometric analysis of organophosphorus chemical warfare agents using ion mobility and tandem mass spectrometry. Rapid Commun Mass Spectrom. 2010;24(11):1617–1624. [DOI] [PubMed] [Google Scholar]
- 28.Ma X, Ouyang Z. Ambient ionization and miniature mass spectrometry system for chemical and biological analysis. TrAC Trends in Analytical Chemistry. 2016;85:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García-Reyes JF, Jackson AU, Molina-Díaz A, Cooks RG. Desorption Electrospray Ionization Mass Spectrometry for Trace Analysis of Agrochemicals in Food. Anal Chem. 2009;81(2):820–829. [DOI] [PubMed] [Google Scholar]
- 30.Douglass KA, Jain S, Brandt WR, Venter AR. Deconstructing Desorption Electrospray Ionization: Independent Optimization of Desorption and Ionization by Spray Desorption Collection. J Am Soc Mass Spectrom. 2012;23(11):1896–1902. [DOI] [PubMed] [Google Scholar]
- 31.Haddad R, Milagre HMS, Catharino RR, Eberlin MN. Easy Ambient Sonic-Spray Ionization Mass Spectrometry Combined with Thin-Layer Chromatography. Anal Chem. 2008;80(8):2744–2750. [DOI] [PubMed] [Google Scholar]
- 32.Haddad R, Sparrapan R, Eberlin MN. Desorption sonic spray ionization for (high) voltage-free ambient mass spectrometry. Rapid Commun Mass Spectrom. 2006;20(19):2901–2905. [DOI] [PubMed] [Google Scholar]
- 33.Román JK, Walsh CM, Oh J, et al. Spatially resolved chemical analysis of cicada wings using laser-ablation electrospray ionization (LAESI) imaging mass spectrometry (IMS). Anal Bioanal Chem. 2018;410(7):1911–1921. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Balan P, Vertes A. Molecular Imaging of Growth, Metabolism, and Antibiotic Inhibition in Bacterial Colonies by Laser Ablation Electrospray Ionization Mass Spectrometry. Angew Chem Int Ed. 2016;55(48):15035–15039. [DOI] [PubMed] [Google Scholar]
- 35.Laiko VV, Baldwin MA, Burlingame AL. Atmospheric Pressure Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry. Anal Chem. 2000;72(4):652–657. [DOI] [PubMed] [Google Scholar]
- 36.Keller C, Maeda J, Jayaraman D, et al. Comparison of Vacuum MALDI and AP-MALDI Platforms for the Mass Spectrometry Imaging of Metabolites Involved in Salt Stress in Medicago truncatula. Frontiers in Plant Science. 2018;9:1238–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trimpin S. “Magic” Ionization Mass Spectrometry. J Am Soc Mass Spectrom. 2016;27(1):4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trimpin S, Inutan ED. Matrix Assisted Ionization in Vacuum, a Sensitive and Widely Applicable Ionization Method for Mass Spectrometry. J Am Soc Mass Spectrom. 2013;24(5):722–732. [DOI] [PubMed] [Google Scholar]
- 39.Trimpin S, Pophristic M, Adeniji-Adele A, Tomsho JW, McEwen CN. Vacuum Matrix-Assisted Ionization Source Offering Simplicity, Sensitivity, and Exceptional Robustness in Mass Spectrometry. Anal Chemistry. 2018;90(19):11188–11192. [DOI] [PubMed] [Google Scholar]
- 40.Roach PJ, Laskin J, Laskin A. Nanospray desorption electrospray ionization: an ambient method for liquid-extraction surface sampling in mass spectrometry. Analyst. 2010;135(9):2233–2236. [DOI] [PubMed] [Google Scholar]
- 41.Chen F, Lin L, Zhang J, He Z, Uchiyama K, Lin J-M. Single-Cell Analysis Using Drop-on-Demand Inkjet Printing and Probe Electrospray Ionization Mass Spectrometry. Anal Chem. 2016;88(8):4354–4360. [DOI] [PubMed] [Google Scholar]
- 42.Issart A, Szpunar J. Potential of Liquid Extraction Surface Analysis Mass Spectrometry (LESA-MS) for the Characterization of Polymer-Based Materials. Polymers. 2019;11(5):802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagnotti VS, Chubatyi ND, McEwen CN. Solvent Assisted Inlet Ionization: An Ultrasensitive New Liquid Introduction Ionization Method for Mass Spectrometry. Anal Chem. 2011;83(11):3981–3985. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Attanayake K, Valentine SJ, Li P. Vibrating Sharp-edge Spray Ionization (VSSI) for Voltage-Free Direct Analysis of Samples using Mass Spectrometry. Rapid Commun Mass Spectrom. 2018. DOI. 10.1002/rcm.8232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ranganathan N, Li C, Suder T, et al. Capillary Vibrating Sharp-Edge Spray Ionization (cVSSI) for Voltage-Free Liquid Chromatography-Mass Spectrometry. J Am Soc Mass Spectrom. 2019;30(5):824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao Y-N, Wu L, Di D, Yuan Z- C, Hu B. Vibrating tip spray ionization mass spectrometry for direct sample analysis. J Mass Spectrom. 2019;54(9):772–779. [DOI] [PubMed] [Google Scholar]
- 47.Heron SR, Wilson R, Shaffer SA, Goodlett DR, Cooper JM. Surface Acoustic Wave Nebulization of Peptides As a Microfluidic Interface for Mass Spectrometry. Anal Chem. 2010;82(10):3985–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forbes TP, Dixon RB, Muddiman DC, Degertekin FL, Fedorov AG. Characterization of charge separation in the array of Micromachined UltraSonic Electrospray (AMUSE) ion source for mass spectrometry. J Am Soc Mass Spectrom. 2009;20(9):1684–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takats Z, Wiseman JM, Cooks RG. Ambient mass spectrometry using desorption electrospray ionization (DESI): instrumentation, mechanisms and applications in forensics, chemistry, and biology. J Mass Spectrom. 2005;40(10):1261–1275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.