Abstract
Background
Coronary artery fistula complicated with giant coronary artery ectasia (CAE) is a rare cardiac malformation, and its surgical indications and treatment strategies still need further discussion.
Case summary
In this case, a 41-year-old man had complained of occasional dizziness for 2 years, but he did not seek medical attention until he started to feel palpitations. A right coronary artery (RCA)–left ventricular (LV) fistula with giant RCA of diffuse ectasia was firstly revealed by transthoracic echocardiography. A widened left ventricle and significantly constricted right atrium and right ventricle were also detected by three-dimensional coronary artery computed tomography. Surgical treatment, including the repair of the RCA-LV fistula, the resection and reconstruction of the dilated RCA and coronary artery bypass grafting (CABG) under hypothermic cardiopulmonary bypass, were performed to correct the malformation. The patient presented a favourable health condition without any discomfort at the 1-year follow-up.
Discussion
CAE can be caused by various congenital or acquired factors. Surgical treatment, such as transcatheter embolization excision, surgical ligation or resection for symptomatic patients with CAE three times or larger than the reference diameter, has been reported to have satisfactory results. Additionally, CABG can be selected if myocardial perfusion is compromised and the distal branch is of reasonable size. In this case, the giant ectasia of the RCA may have been a consequence of the congenital RCA-LV fistula. Atherosclerosis, with calcified plaques in the RCA, and the patient’s long-term history of smoking may have contributed to the development of giant ectasia of the RCA.
Keywords: Coronary artery–ventricular fistula, Giant coronary artery ectasia, Surgical treatment, Case report
Learning points
Coronary artery fistula complicated with giant coronary artery ectasia (CAE) is a rare cardiac malformation, whose treatment strategies remain controversial, especially for asymptomatic patients or patients with a few symptoms.
Surgical treatment may be of benefit for the patient with symptoms of compression cardiac chambers and risks of thrombosis or vessel rupture caused by the giant CAE.
Introduction
Coronary artery fistula is a rare anomaly of abnormal connection between coronary artery and other vessels or cardiac chambers, seen in 0.002% of the general population and 0.4% of all cardiac malformations.1,2 Coronary artery fistula may induce aneurysmal coronary artery disease (ACAD), such as coronary artery aneurysm and coronary artery ectasia (CAE).3 Although surgical treatments for coronary artery fistula with ACAD have been proven to be effective,4 not all patients meet the surgical indications. Strategies for such patients still need further discussion.
In this article, we report the case of a right coronary artery (RCA)–left ventricular (LV) fistula with diffuse ectasia of the giant RCA, and we attempt to provide a feasible strategy of treatment for patients with similar conditions.
Timeline
| Day0 (admission) | A 41-year-old man complained occasional dizziness for 2 years, palpitations for 2 months. |
| Day 1 |
Chest X-ray: enlarged cardiac silhouette with local protrusion of the right margin. Transthoracic echocardiography: preliminarily revealed the dilated right coronary artery (RCA), which connected to the left ventricle near the posterior mitral valve root through a fistula. |
| Day 2 | Three-dimensional coronary artery computed tomography: visualized the giant tortuous RCA and the position of the fistula orifice. |
| Day 5 | Coronary angiography: further confirmed the abnormal structures and excluded any stenosis or occlusion in other coronary arteries. |
| Day 15 | The patient received surgical treatment, including repairment of the RCA-left ventricular fistula, resection and reconstruction of the dilated RCA and coronary artery bypass grafting under hypothermic cardiopulmonary bypass. |
| Day 22 | Hospital discharge. |
| 1-year follow-up | No adverse clinical events were reported. |
Case presentation
A 41-year-old man, who had smoked for 30 years but had no medical or family history of hypertension, coronary heart disease, trauma or infectious diseases, complained of occasional dizziness for 2 years, and palpitation for 2 months.
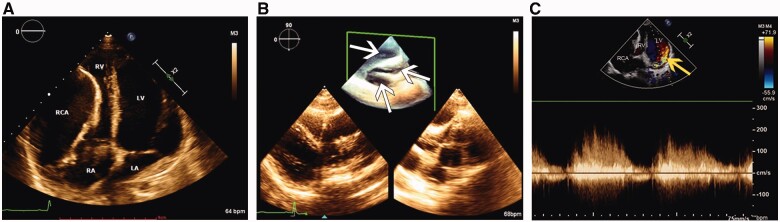
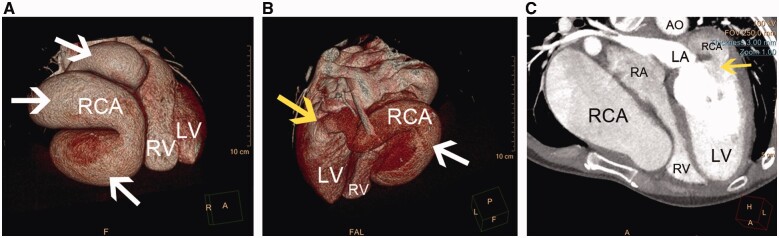
On admission, physical examination revealed arrhythmia and apical diastolic grade III murmur. Laboratory tests were unremarkable. Chest roentgenogram showed an enlarged cardiac silhouette with local protrusion of the right margin (Supplementary material online, sFigure 1). Transthoracic echocardiography revealed an RCA-LV fistula with giant RCA of diffuse ectasia, which had a maximum diameter of 50 mm (Figure 1A and B, white arrows) and drained into the LV cavity near the posterior mitral valve root through a fistula orifice with diameter of 20 mm. Colour Doppler flow imaging detected a continuous double-phase turbulent spectrum at the fistula orifice (Figure 1C, yellow arrow). The left ventricle was enlarged, and the right atrium and ventricle had significantly decreased in size because of the compression of the RCA. The peak pulmonary artery flow velocity was 56 cm/s, which was mildly reduced compared to normal values. The right ventricular ejection fraction was 41.7%, and the LV ejection fraction was 63.5%. Three-dimensional coronary artery computed tomography (3D-CT) visualized the RCA (Figure 2A and B, white arrows; Supplementary material online), and many calcified plaques were located on the RCA wall and in the fistula orifice (Figure 2B and C, yellow arrows), the widened left ventricle, and the significantly constricted right atrium and ventricle (Figure 2C; Supplementary material online, Videos S1 and S2). Coronary angiography further confirmed the abnormalities in these structures and excluded any other coronary artery lesions (Supplementary material online, Videos S3 and S4).
Figure 1.
(A) Transthoracic echocardiography revealed the enlarged left ventricle and constrictive right atrium and right ventricle compressed by the giant right coronary artery. (B) Three-dimensional echocardiography showed the cavity of the giant right coronary artery of diffuse ectasia (white arrows). (C) Colour Doppler flow imaging detected continuous double-phase turbulent spectrum at the fistula orifice (yellow arrow). LA, left atrium; LV, left ventricle; RA, right atrium; RCA, right coronary artery; RV, right ventricle (original scale: 1743*473 pixels).
Figure 2.
(A and B) Three-dimensional coronary artery computed tomography visualized the giant right coronary artery ectasia (white arrows) and the fistular orifice location (yellow arrow). (C) The widened left ventricle, significantly constrictive right atrium and ventricle and the orifice of the right coronary artery–left ventricular fistula (yellow arrow). AO, aorta; LA, left atrium; LV, left ventricle; RA, right atrium; RCA, right coronary artery; RV, right ventricle (original scale: 1608*476 pixels).
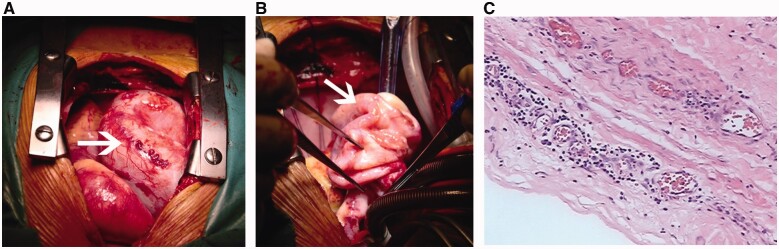
The patient had potential risks of dilated calcified RCA rupture and fistula enlargement, which could alter the haemodynamics or induce more serious consequences. Taking all these factors into consideration, to relieve the compression of his right heart, the patient was referred for surgical treatment under hypothermic cardiopulmonary bypass. After pericardiotomy, a dilated tortuous RCA on the surface of the right atrium and ventricle was found (Figure 3A and B). The dilated RCA drained into the back of the left ventricle at the beginning part of the posterior descending artery. The diameter of the distal posterior descending artery was approximately 2 mm. The RCA ostium and the intracardiac fistula located in the myocardium of the P2 region of the posterior mitral valve were exposed after opening the dilated RCA. Then, the fistula was closed by 5–0 polypropylene sutures. The RCA ostium was repaired by a bovine pericardium patch, and the dilated RCA was partially resected and finally reconstructed by 4–0 polypropylene sutures. Due to compromised distal perfusion, coronary artery bypass grafting (CABG) was performed between the aorta and the posterior descending artery by anastomosis with the autologous great saphenous vein. Histological examination of the resected RCA indicated local fibrous tissue proliferation with collagen, calcification and chronic inflammation (Figure 3C).
Figure 3.
(A and B) The giant right coronary artery of diffuse ectasia during the surgery. (C) Histological examination of the resected right coronary artery (haematoxylin and eosin stain, magnification ×200) (original scale: 1547*485 pixels).

Oral aspirin treatment (100 mg daily) was initiated after surgery. No adverse clinical events were reported at the 1-year follow-up.
Discussion
In surgical anatomy, single coronary artery fistula is more common (74–90%) than multiple fistulas (10.7–16%).1 More than half of fistulas originate from the RCA, ranging from 50% to 60%, with 15–43% of drainage sites located in the pulmonary artery, 19–26% in the right atrium, 14–40% in the right ventricle, and only 2–19% in the left ventricle, as reported in various studies.1,2 According to previous studies, CAE, a kind of ACAD defined as diffuse dilatation that involves more than 50% of the length of the coronary artery, can be induced by congenital coronary artery fistulas or acquired from atherosclerosis, arteritis, infection, iatrogenic injury, Kawasaki disease, etc.3 CAE may increase the risk of complications such as thrombus formation, distal embolization, myocardial infarction, and rupture.5 Because there are no controlled clinical trials to evaluate the optimal therapy because of its rarity, the treatment strategy for CAE remains controversial. For symptomatic patients with CAE three times or larger than the reference diameter, medication treatment, such as antiplatelet and anticoagulant therapy to prevent thromboembolic events; immunosuppressive therapy to combat the underlying inflammatory process; and surgical treatment, such as transcatheter embolization excision, surgical ligation or resection, can be effective.3 Additionally, CABG can be selected for giant CAE if myocardial perfusion is compromised and the distal branch is of reasonable size.2,6,7 Although medication treatments have provided benefits in some cases,7 medications cannot reverse the structural changes of the heart and vessels. Furthermore, antiplatelet and anticoagulant therapy may increase the risk of bleeding and should be used cautiously in patients who have a risk of CAE rupture. Likewise, immunosuppressive therapy may raise the risk of infection. For surgical treatment, abnormal structural changes of the heart and vessels can be repaired, but patients incur the risks of cardiac operation and postoperative complications such as arrhythmia, bleeding and pericardial tamponade. In summary, individual treatment strategies should be made based on the specific conditions of patients and the severity of the lesions.
In this case, considering the patient’s medical history and imaging studies, we thought that the giant RCA with diffuse ectasia might have been caused by the congenital anomaly of the RCA-LV fistula. 3D-CT detected RCA atherosclerosis with calcified plaques, which was associated with his long-term history of smoking, and this might have contributed to the development of RCA ectasia.3,5 Because the initial fistula caused only a small extra shunt flow, the patient was asymptomatic for decades. However, over time, the shunt flow volume increased, which produced a run-off from the aorta, mimicking aortic valve regurgitation,1 and the left ventricle became overloaded and enlarged. Although the LV ejection fraction value was normal, as determined by echocardiography, the effective stroke volume of the patient was, in fact, decreased. The decreased effective stroke volume caused the recent symptoms of dizziness and palpitation. If the patient had not received surgical treatment, the gradually enlarged RCA would have further compressed the right heart and reduced the volume of the right heart chamber. Then, the pulmonary circulation blood flow would have been further reduced, and the increased ventilation/perfusion ratio might have led to hypoxaemia.
In conclusion, we report the case of an RCA-LV fistula with diffuse ectasia of the giant RCA. The patient underwent surgical repair and presented a favourable health condition without any discomfort at the 1-year follow-up.
Lead author biography
Dr Jiancheng Xiu, PhD, graduated from the First Military Medical University, received his post-doctoral fellowship at Columbia University Medical Center. After finishing his IVUS fellowship at New York-Presbyterian Hospital, Dr Xiu now works as the deputy director of Department of Cardiology, Nanfang Hospital, Southern Medical University, whose principal field of interest is diagnosis and treatment of coronary heart disease and critical cardiovascular disease, intervention therapy of complex coronary artery lesions and IVUS or FFR-guided percutaneous coronary intervention.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors appreciate the multiple contributions made by Dr Huicheng Wang, Dr Li Lei, Prof. Peng Wang, Prof. Jinguo Xie (Department of Cardiology, Nanfang Hospital, Southern Medical University, Guangzhou, China), Prof. Shaoyi Zheng, Prof. Zhenkang Wang, and Dr Shengping He (Department of Cardiovascular surgery, Nanfang Hospital, Southern Medical University, Guangzhou, China).
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Funding: This work was supported by the National Key R&D Program of China (2018YFC1312803) and National Natural Science Foundation (81470598).
References
- 1. Mangukia CV. Coronary artery fistula. Ann Thorac Surg 2012;93:2084–2092. [DOI] [PubMed] [Google Scholar]
- 2. Li R, Ni Y, Teng P, Li W.. A giant right coronary artery of diffuse ectasia induced by a right ventricular fistula. Heart Surg Forum 2015;18:E253–E254. [DOI] [PubMed] [Google Scholar]
- 3. ElGuindy MS, ElGuindy AM.. Aneurysmal coronary artery disease: an overview. Glob Cardiol Sci Pract 2018;2017:e201726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Isobe S, Hakuno D, Isoda S, Hayashi K, Adachi T.. Right coronary artery-left ventricle fistula with giant coronary artery aneurysm. Eur Heart J Cardiovasc Imaging 2015;16:231–231. [DOI] [PubMed] [Google Scholar]
- 5. Devabhaktuni S, Mercedes A, Diep J, Ahsan C.. Coronary artery ectasia—a review of current literature. CCR 2016;12:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beckmann E, Rustum S, Marquardt S, Merz C, Shrestha M, Martens A. et al. Surgical treatment of coronary artery aneurysms. J Card Surg 2017;32:674–679. [DOI] [PubMed] [Google Scholar]
- 7. Vasu N, Narayanan S, Ajit MS.. Clinical outcome of various management strategies in coronary artery ectasia. Indian Heart J 2017;69:319–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.