Abstract
ZBP1 has been characterized as a critical innate immune sensor of not only viral RNA products but also endogenous nucleic acid ligands. ZBP1 sensing of the Z-RNA produced during influenza virus infection induces cell death in the form of pyroptosis, apoptosis, and necroptosis (PANoptosis). PANoptosis is a coordinated cell death pathway that is driven through a multiprotein complex called the PANoptosome and enables crosstalk and co-regulation among these processes. During influenza virus infection, a key step in PANoptosis and PANoptosome assembly is the formation of the ZBP1-NLRP3 inflammasome. When Z-RNA is sensed, ZBP1 recruits RIPK3 and caspase-8 to activate the ZBP1-NLRP3 inflammasome. Several other host factors have been found to be important for ZBP1-NLRP3 inflammasome assembly, including molecules involved in the type I interferon signaling pathway and caspase-6. Additionally, influenza viral proteins, such as M2, NS1, and PB1-F2, have also been shown to regulate the ZBP1-NLRP3 inflammasome. This review explains the functions of ZBP1 and the mechanistic details underlying the activation of the ZBP1-NLRP3 inflammasome and the formation of the PANoptosome. Improved understanding of the ZBP1-NLRP3 inflammasome will direct the development of therapeutic strategies to target infectious and inflammatory diseases.
Keywords: ZBP1, NLRP3, inflammasome, PANoptosis, influenza A virus, PANoptosome, pyroptosis, apoptosis, necroptosis, virus infection, innate immunity
1 |. Introduction
1. 1 |. The NLRP3 inflammasome
Inflammasomes are critical components of the innate immune system, the first line of host defense which recognizes pathogenic and sterile insults. These macromolecular complexes assemble in response to diverse stimuli, and inflammasome assembly is initiated by sensor molecules1,2. To date, there are five well-characterized inflammasome sensors, including NOD-like receptor (NLR) family pyrin domain-containing 1 (NLRP1)3, NLRP34–6, NLR family caspase recruitment domain (CARD)-containing 4 (NLRC4)7,8, absent in melanoma 2 (AIM2)9–12, and PYRIN13. Some other innate sensors have also been reported to initiate inflammasome assembly under specific conditions, such as NLRP614, NLRP915, NLRP1216, interferon-γ-inducible protein 16 (IFI16)17, retinoic acid-inducible gene I (RIG-I)18, and myxovirus resistance protein A (MxA)19. Upon activation, inflammasome sensors interact with the adaptor molecule apoptosis-associated speck-like protein containing a CARD (ASC) in the cytosol, which then recruits and activates caspase-1. Activated caspase-1 can proteolytically cleave the cytokines pro–IL-1β and pro–IL-18 into their bioactive forms to induce proinflammatory responses20,21. Simultaneously, active caspase-1 can process the pore-forming protein gasdermin D (GSDMD) to free its N-terminus, which targets the cell membrane to assemble pore structures and induce a form of inflammatory cell death termed pyroptosis22–24.
The NLRP3 inflammasome has been extensively studied in the past two decades. Several stimuli have been reported to trigger NLRP3 inflammasome activation, including endogenous danger signals like ATP4–6, ionophores like nigericin5, particulate matter such as silica and uric acid crystals6, viral infection like influenza A virus (IAV)25–28, Gram-positive bacterial infection such as Bacillus cereus29, Gram-negative bacterial infection like Citrobacter rodentium and Escherichia coli30–32, and fungal infection like Candida albicans and Aspergillus fumigatus33–35 (Figure 1). Given the diverse stimuli that can activate the NLRP3 inflammasome, it is unlikely that NLRP3 directly binds to these diverse ligands. The exact mechanism for NLRP3 inflammasome activation is still an active area of investigation.
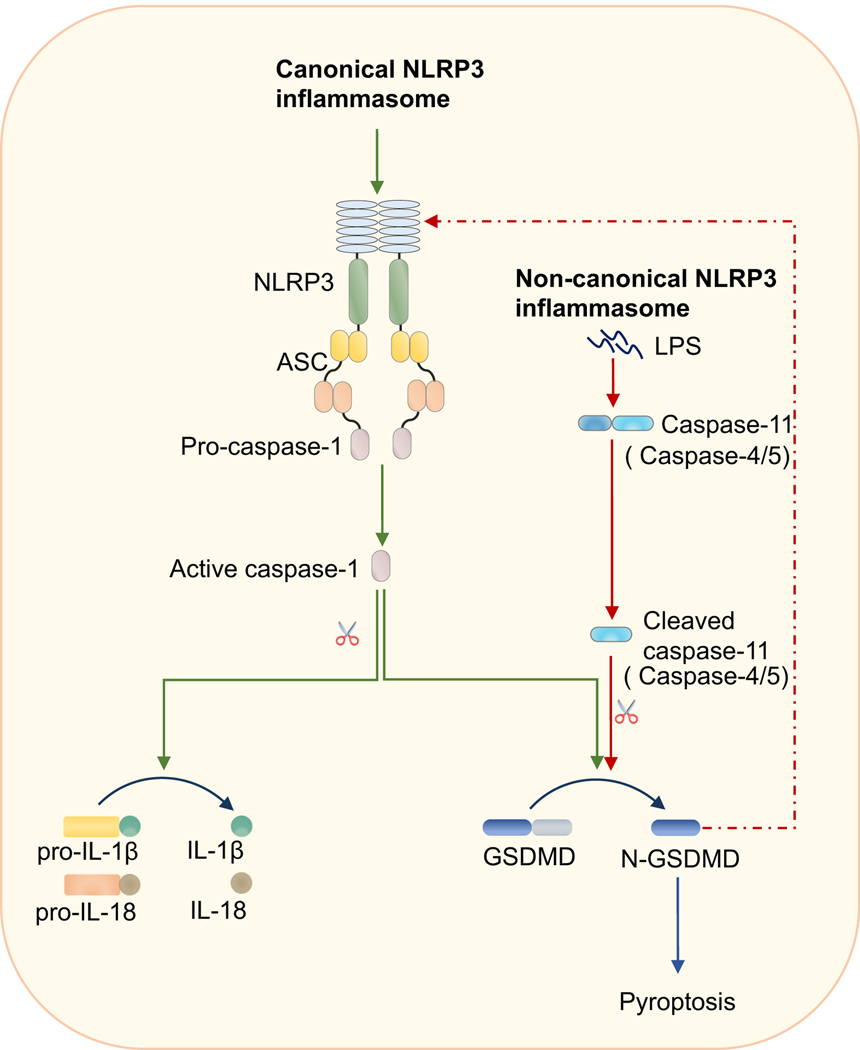
FIGURE 1.
NLRP3 inflammasome activation through canonical and non-canonical routes.
Activation of the NLRP3 inflammasome has been shown through two different pathways referred to as canonical and non-canonical NLRP3 inflammasome activation. Activation of the canonical NLRP3 inflammasome can be triggered by ATP, pore-forming toxins such as nigericin, Ca2+ signaling, lysosome disruption, particulate matter such as alum or silica, or viral, bacterial, or fungal infection. Non-canonical NLRP3 inflammasome activation is dependent on caspase-11 (or human counterparts caspase-4/5) sensing cytosolic lipopolysaccharide (LPS). After binding to the cytosolic LPS, caspase-11 will undergo auto-activation and cleave gasdermin D (GSDMD) to drive NLRP3 inflammasome activation.
Two signals are required for canonical NLRP3 inflammasome activation: a priming and an activating signal. The priming signal comes from ligands that can activate NF-κB and ERK pathways, such as lipopolysaccharide (LPS), Pam3CSK4, poly (I:C), and TNF. Two major functions have been proposed for priming36. One is to upregulate the gene expression of inflammasome components such as NLRP3 and IL-1β. The other is to provide post-translational modifications (PTMs) for NLRP3. Activation of NLRP3 is tightly regulated, and NLRP3 has been reported to undergo several PTMs, such as ubiquitination37, phosphorylation38,39, and sumoylation40, before inflammasome assembly. PTMs of NLRP3 before its activation allow NLRP3 to be maintained in an auto-suppressed but signal-competent state. Following priming, the activation step can occur. Though NLRP3 can be activated by the many different stimuli listed above, it has been suggested that all these triggers induce specific cellular stress patterns which are sensed by NLRP3. Several models, such as K+ efflux41,42, mitochondria dysfunction43,44 and trans-Golgi disassembly45, have been proposed to explain how NLRP3 is activated. But none of them have proven to be applicable to all the known NLRP3 triggers. More studies are required to fully understand the mechanism for NLRP3 inflammasome activation.
There is also an alternative route for activation of the NLRP3 inflammasome referred to as non-canonical NLRP3 inflammasome activation. During Gram-negative bacteria infection, mouse caspase-11, or the human analogues caspase-4 or −5, sense the cytosolic bacterial cell wall component LPS and subsequently cleave GSDMD to drive pyroptosis and NLRP3 inflammasome initiation22,24,30,31. Therefore, in this process, sensing by caspase-11 (or caspase-4/5) acts as the upstream step to trigger NLRP3 inflammasome assembly.
1.2 |. PANoptosis
In addition to pyroptosis activated downstream of inflammasome activation, there are several other programmed cell death pathways. PANoptosis is a newly emerging concept that highlights the crosstalk and coordination that occurs between three of these pathways, i.e. pyroptosis, apoptosis, and necroptosis46,47. Our team has been working on this concept for a decade to build on our initial findings that overlapping regulation occurs between the inflammasome/pyroptotic and apoptotic and necroptotic components. We first demonstrated the crosstalk between pyroptosis and apoptosis48,49. Subsequent studies in our lab showed redundant roles for caspase-8 and caspase-1/NLRP3, suggesting intersecting functions for pyroptotic, apoptotic, and necroptotic molecules32,50,51. Our more recent studies have demonstrated the roles of ZBP128, TAK152,53, and caspase-654 in the process of PANoptosis activation, further solidifying the basis of the concept. Most recently, we found that this concept can be applied to various pathogens including IAV, vesicular stomatitis virus (VSV), Listeria monocytogenes, and Salmonella enterica serovar Typhimurium, and we characterized a single cell death complex, the PANoptosome, that initiates this process47,55. While our initial work found that NLRP3 is a key molecule in this complex53,54, our discovery of PANoptosis in the context of pathogens that are recognized by other inflammasome sensors suggests that these molecules may also play a role in PANoptosome formation47,55.
PANoptosis is also observed during development. Loss of the caspase activity of caspase-8 will lead to the activation of PANoptosis, which results in large amounts of cell death and inflammation in the intestine and embryonic lethality56,57. In addition to caspase-8, RIPK1 is also a key regulator of PANoptosis during development; both loss of RIPK1 and mutation of the caspase-8 cleavage site in RIPK1 have been shown to be embryonically lethal58,59. PANoptosis also has implications in cancer, as the regulation of PANoptosis by interferon regulatory factor 1 (IRF1) prevents the development of colorectal cancer in a mouse model60. Overall, the process of PANoptosis has diverse implications throughout infectious and inflammatory diseases, development, and cancer.
2 |. ZBP1 senses Z-RNA produced by both DNA and RNA viruses or from endogenous transcripts
Z-DNA-binding protein 1 (ZBP1), also known as DNA-dependent activator of interferon-regulatory factors (DAI) or DLM-1, is known to be a key mediator of NLRP3 inflammasome activation and PANoptosis under certain conditions. ZBP1 contains two Z-nucleic acid binding domains (Zα1 and Zα2) in the N-terminus and two RIP homotypic interaction motifs (RHIM1 and RHIM2) in the middle of the protein sequence (Figure 2A). Initially, ZBP1 was identified as a DNA sensor which could enhance the expression of type I interferons (IFNs) and inflammatory cytokines after binding to B-DNA or purified viral or bacterial DNA61. Later, however, it was demonstrated that loss of ZBP1 has no effect on DNA-induced immune responses62,63.
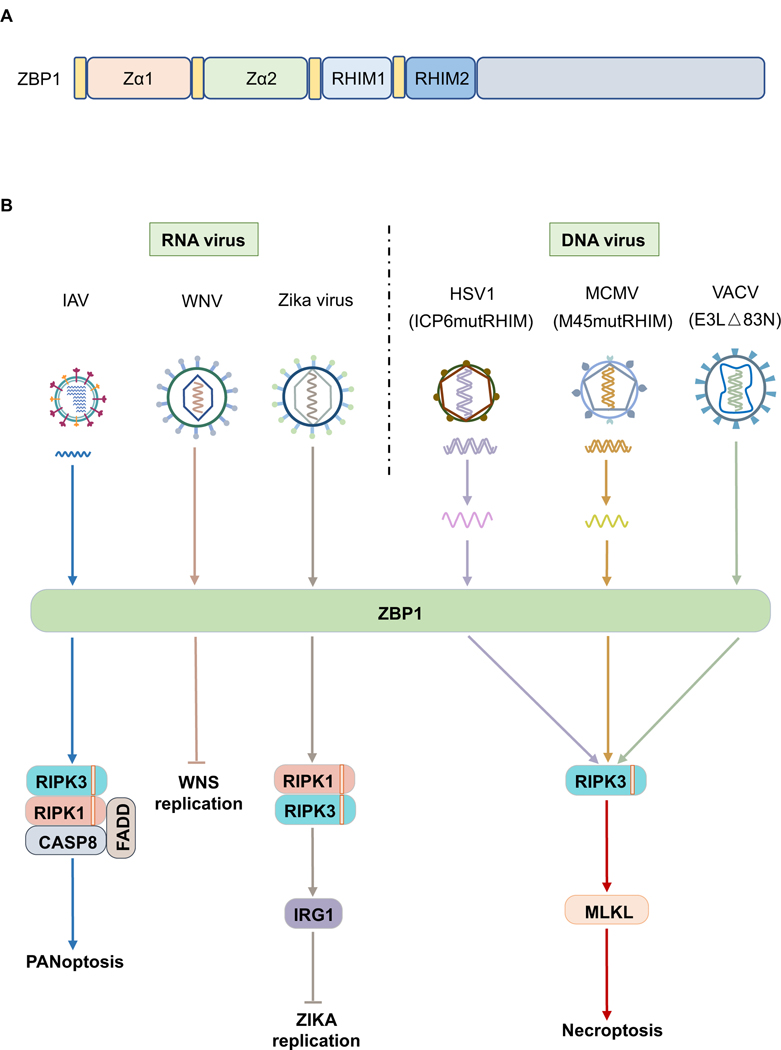
FIGURE 2.
ZBP1 senses both DNA and RNA virus infection.
A) Schematic structure of the ZBP1 protein and its domains. B) RNA viruses such as influenza A virus (IAV) can be sensed by ZBP1, following which the PANoptosome will be assembled to induce PANoptosis. ZBP1 is also able to sense West Nile virus (WNV) infection and then inhibit WNV replication. Zika virus can be sensed by ZBP1 to induce a RIPK1- and RIPK3-dependent signaling pathway to suppress viral replication in neurons. DNA viruses including mutated herpes simplex virus 1 (HSV1-ICP6mutRHIM), mutated murine cytomegalovirus (MCMV-M45mutRHIM), and mutated vaccinia virus (VACV-E3L △83N) can be sensed by ZBP1 to activate necroptosis.
2.1 |. Role of ZBP1 during virus infection
The biological role of ZBP1 was characterized in 2012, when ZBP1 was found to induce necroptosis via a RHIM-mediated homotypic interaction with RIPK3 in response to fibroblast infection with the M45 RHIM-mutated murine cytomegalovirus (MCMV-M45mutRHIM), a DNA virus64 (Figure 2B). These findings suggested that ZBP1 may be a sensor that induces cell death. To escape necroptosis-mediated inhibitory effects on viral replication, MCMV has evolved to express a RHIM-containing protein named viral inhibitor of RIP activation (vIRA) encoded by the M45 gene. This protein is a potent inhibitor of necroptosis due to its capacity to counteract the interaction between ZBP1 and RIPK365. To date, two other DNA viruses, herpes simplex virus 1 (HSV1) and vaccinia virus (VACV), have also been shown to be sensed by ZBP166,67 (Figure 2B). Similar to MCMV, both of these viruses have evolved to suppress ZBP1-initiated necroptosis. HSV1 encodes the RHIM-containing protein ICP6, while VACV encodes the Zα-containing protein E3L66–69. ICP6 of HSV1 inhibits ZBP1-mediated necroptosis via the RHIM domain in human cells66. However, this process is much more complicated in mouse cells during HSV1 infection, where the RHIM-mediated interaction between ICP6 and RIPK3 promotes cell death68,69. Interestingly, in mouse embryonic fibroblasts (MEF), HSV1-induced cell death can occur in both ICP6-dependent and -independent manners, and ICP6-independent cell death relies on ZBP166. In VACV infection, the E3L protein has been proposed to compete with ZBP1 to bind the ligand, thus blocking the activation of ZBP167.
Although ZBP1 was shown to be essential for DNA virus-induced cell death, subsequent studies from our lab and others have found that ZBP1 is also able to sense RNA virus infection. IAV infection induces multiple programmed cell death pathways concomitantly, including pyroptosis, apoptosis, and necroptosis28, through the process of PANoptosis in murine macrophages46. ZBP1 is the sensor responsible for triggering PANoptosis in response to IAV28,70,71 (Figure 2B). In addition to IAV, ZBP1 senses flavivirus infection like Zika virus and West Nile virus (WNV) and induces cell death-independent antiviral effects72,73. Notably, these antiviral effects are unlikely to be due to the induction of type I IFNs. Conversely, loss of ZBP1 results in higher type I IFN production in the WNV-infected mouse brains73. Taken together, these studies indicate that ZBP1 plays critical roles during both DNA and RNA virus infection, mediating cell death-dependent or -independent antiviral effects. Whether ZBP1 functions during other pathogenic infections like bacterial and fungal infection requires further study.
2.2 |. Role of ZBP1 in development and inflammatory bowel disease
Two groups independently reported that ZBP1 plays critical roles during development and inflammatory disease by sensing endogenous transcripts74,75. Mutations in the RHIM of RIPK1 (Ripk1mR/mR) cause perinatal lethality in mice, and conditional knock-out of RIPK1 in the epidermis (Ripk1E-KO) results in skin inflammation due to the activation of necroptosis; both these detrimental effects are rescued by deleting ZBP1 in the mice76,77. But the exact functions of ZBP1 in these processes was not fully understood. Further genetic studies have indicated that loss of the Zα2 domain of ZBP1 (Zbp1ΔZα2ΔZα2) can rescue Ripk1mR/mR-induced perinatal lethality and suppress Ripk1E-KO-induced skin inflammation74,78, suggesting Z-nucleic acid sensing plays a role in the activation of ZBP1 in these mice. Additionally, the RHIM1 of ZBP1 is required to mediate Ripk1mR/mR-induced perinatal lethality and suppress Ripk1E-KO -induced skin inflammation74. These data suggest that ZBP1 can directly sense endogenous transcripts during development to induce programmed cell death and inflammation63. Interestingly, while around 85% of Zbp1–/–Ripk1E-KO and Zbp1mR1/mR1Ripk1E-KO mice remain healthy for more than 40 weeks, approximately 75% of the Zbp1mZα2/mZα2Ripk1E-KO mice develop mild skin inflammation at the age of 40 weeks, suggesting that ZBP1 may function as a adaptor protein downstream of some factors74.
In addition to its functions in infection and organismal development, ZBP1 is also involved in inflammatory bowel diseases (IBD). Gene expression of SET domain bifurcated histone lysine methyltransferase 1 (SETDB1) is reduced by about 50% in patients with IBD75,79. When this was modeled with conditional deletion of Setdb1 in the intestine of mice, tissue sample analysis unveiled that ZBP1-dependent necroptosis was activated. These mice develop spontaneous terminal ileitis and colitis, suggesting that SETDB1 participates in the pathogenesis of IBD in a ZBP1-dependent manner75. Ex vivo studies found that deleting the Zα2 or RHIM1 of ZBP1 could block SETDB1 deficiency-induced necroptosis75, suggesting that ZBP1-mediated necroptosis after sensing endogenous ligands is involved in the pathogenesis of IBD.
2.3 |. The natural ligand for ZBP1
In spite of the critical function of ZBP1 in host defense, the natural ligand for ZBP1 has not been fully established63. Inhibiting RNA synthesis but not translation can block MCMV-M45mutRHIM-induced necroptosis80. Likewise, suppressing transcription but not viral DNA replication is able to abolish the ICP6-RHIM mutated HSV1-induced cell death66. These data suggest that the natural ligands for ZBP1 during DNA virus infection should be RNA but not viral DNA. However, detailed studies are needed to differentiate the role of newly synthesized viral RNA and endogenous RNA transcripts in activating ZBP1 in response to DNA virus infection.
During IAV infection, ZBP1 has been shown by stochastic optical reconstruction microscopy (STORM) to bind the viral RNP complex70. In addition, another study using RNAseq confirmed that ZBP1 can bind to viral genomic RNA following IAV infection when ZBP1 is overexpressed in HEK293T cells71. However, the genomic RNA alone cannot mimic the cell death observed in IFN-β–primed fibroblasts after delivery to the cells70, suggesting that viral RNA alone may not be recognized by ZBP1. It is known that the Zα domain only shows high affinity for left-handed nucleic acids, i.e. Z-DNA or RNA81–85. It is possible that viral RNA alone cannot form a conformation that is able to be recognized by ZBP1. Work from our group has demonstrated that ZBP1 is able to co-immunoprecipitate viral RNP components PB1 and NP following IAV infection28. Therefore, ZBP1 may sense the viral RNP complex or replication intermediates which may take on the Z-RNA conformation to induce PANoptosis. Very recently, a study has confirmed that Z-RNA is produced in the nucleus during IAV infection, which could trigger MLKL activation there86. Interestingly, it was also noted that abolishing the nuclear transport of ZBP1 could not inhibit the cell death induced by IAV86, suggesting that ZBP1 is able to sense its ligand both in the nucleus and cytosol. However, whether the ligand for ZBP1 is the newly generated viral RNP complex or the replication intermediates remains unclear86.
While there is no doubt that ZBP1 can bind an IAV RNA product during viral infection70,71,86, it is unknown whether endogenous Z-RNAs are induced and also contribute to ZBP1-mediated cell death during IAV infection or development. It has been suggested that RNAs expressed from endogenous retrovirus-like elements can adopt the Z-RNA conformation and be sensed by ZBP1 to induce cell death in Ripk1mR/mR or Ripk1E-KO cells or in cells lacking Setdb174,75,87,88. More studies are required to differentiate the contribution of viral RNA and endogenous RNA in activating ZBP1 during virus infection63. Moreover, it is worth noting that even though ZBP1 contains two Zα domains and two RHIMs, only one of each have been found to be functional66,71,78,80. So far, only Zα2 is known to be essential for recognizing viral infection and RHIM1 is known to mediate signal transduction by interacting with RIPK3 and/or RIPK166,71,78,80. Whether Zα1 and RHIM2 function in other biological processes remains enigmatic.
3 |. Influenza A virus induces the ZBP1-NLRP3 inflammasome
Activation of the NLRP3 inflammasome by IAV was initially described in 200625. The significance of inflammasome activation in host defense against IAV infection was highlighted later by in vivo studies27. NLRP3-deficient mice are more susceptible to mouse-adapted IAV (A/PR/8/34, H1N1) infection than are wild type (WT) mice26,27. Similarly, caspase-1–deficient mice have higher mortality after IAV infection26,27, further suggesting that the inflammasome is required for host defense against IAV infection. Loss of NLRP3 leads to extensive collagen deposition in the lungs of mice following IAV infection27, indicating that inflammasome activation contributes to tissue healing after IAV infection. Additionally, elderly mice are more susceptible to IAV (A/HKx31, H3N2) infection than are younger mice due to defective NLRP3 inflammasome activation. Treatment with the NLRP3-activating compound nigericin can dramatically increase IL-1β processing in the IAV-infected lungs and reduce IAV-induced mortality in elderly mice89. In vitro, supplementing IAV-infected cells from elderly mice with nigericin can rescue NLRP3 inflammasome activation89. These results further highlight the critical role of the NLRP3 inflammasome in defending against IAV infection. Additionally, mice receiving the NLRP3 inflammasome-specific inhibitor MCC950 in the early infection phase become susceptible to IAV (A/HKx31, H3N2; A/PR/8/34, H1N1) infection90. Interestingly, it was noted that if the NLRP3 inhibitor was given in the late infection phase, mice showed some resistance to the infection, suggesting that the NLRP3 inflammasome may play a detrimental role in the late infection phase of IAV90. Moreover, the effect of the NLRP3 inflammasome on host defense may be viral strain specific. For example, while NLRP3-deficient mice are more susceptible to the A/PR/8/34, H1N1 strain of IAV compared with WT mice26,27, mice with loss of NLRP3 or caspase-1 have decreased pulmonary inflammation and are less susceptible to the A/shanghai/4664T/2013, H7N9 strain of IAV91. Furthermore, IAV-induced inflammasome activation also contributes to the development of adaptive immune responses92. Both CD4+ and CD8+ T-cell responses are impaired in the caspase-1– or ASC–deficient mice following IAV (A/PR/8/34, H1N1) infection. Unexpectedly, this report found that NLRP3 but not caspase-1 was dispensable for host defense against infection with the PR8 strain82. This inconsistency may come from mutations that occur in the virus during lab passages.
Despite the critical role of the NLRP3 inflammasome in IAV-induced pathogenicity, other host factors that contribute to this process were largely unknown until recently. In 2014, RIPK3 was found to be required for RNA virus-induced NLRP3 inflammasome activation, including IAV-induced inflammasome assembly93. Mechanistically, in response to RNA virus infection, RIPK3 interacts with RIPK1, forming the RIPK3-RIPK1 complex to phosphorylate dynamin-related protein 1 (DRP1). DRP1 then translocates to the mitochondria and promotes mitochondrial damage, which has been reported to be essential for NLRP3 inflammasome activation94. The kinase activity of RIPK1 was shown to be essential for this process93. RIPK3 is known to function as an adaptor protein and has been shown to act downstream of several receptors or sensors, including TNF receptor 1 (TNFR1), Toll-like receptors (TLRs), and ZBP164,65,95–99. However, the specific upstream factor that recruits RIPK3 to activate the RNA virus-induced inflammasome was not resolved in this report.
Our lab then showed that ZBP1, together with another known innate sensor RIG-I, was among the most up-regulated genes following IAV infection in WT bone marrow derived-macrophages (BMDMs) compared to those from type I IFN receptor I (IFNAR1)-deficient mice28. These results were consistent with the original finding that ZBP1 can be an IFN-inducible gene100. It was surprising to find that deleting ZBP1 completely abolished IAV-induced NLRP3 inflammasome activation, while loss of RIG-I only partially affected NLRP3 inflammasome assembly in response to IAV infection28,70. Given the role of RIG-I in activating type I IFN expression following IAV infection, it is not unexpected that loss of RIG-I would dampen the expression of ZBP1, which can account for the impaired NLRP3 inflammasome activation in Rig-i–/– cells70. Subsequently, another group also noted that ZBP1 was required for IL-1β release after IAV infection71, providing additional evidence for the requirement of ZBP1 in NLRP3 inflammasome activation. Collectively, these findings indicate that NLRP3 inflammasome activation during IAV infection is totally dependent on ZBP1, leading to the designation of the ZBP1-NLRP3 inflammasome. While ZBP1 is indispensable for IAV-induced NLRP3 inflammasome activation, it is dispensable for NLRP3 inflammasome activation in response to other RNA viruses such as VSV28. Detailed studies are needed to clarify this discrepancy, particularly because both IAV and VSV infection involve the ZBP1 interacting partner RIPK3 for NLRP3 inflammasome activation93.
Genetic evidence has shown that in addition to RIPK3, caspase-8 also acts downstream of ZBP1 to regulate ZBP1-NLRP3 inflammasome activation (Figure 3). Loss of RIPK3 leads to a dramatic decrease in NLRP3 inflammasome activation, while deletion of both RIPK3 and caspase-8 mimics the phenotype of ZBP1 deletion during IAV infection28. Since caspase-8 has no proposed domains that are involved in homotypic or heterotypic interactions with ZBP1, it is possible that RIPK1, another RHIM-containing protein, acts as the adaptor downstream of ZBP1 to recruit caspase-8 via Fas-associated protein with death domains (FADD). Indeed, loss of both FADD and RIPK3 blocks cell death similarly to loss of caspase-8 and RIPK3 in BMDMs following IAV infection, suggesting that this deletion can abolish IAV-induced ZBP1-NLRP3 inflammasome activation28. However, using mice with a kinase-dead mutant form of RIPK1, we found that the kinase activity of RIPK1 does not contribute to ZBP1-NLRP3 inflammasome activation, which is inconsistent with the results observed with the RIPK1 inhibitor Nec-128,93. It is worth noting that the study using Nec-1 only tested the requirement of this inhibitor for VSV-induced NLRP3 inflammasome93; therefore, it is possible that the kinase activity of RIPK1 only contributes to VSV-induced NLRP3 inflammasome activation.
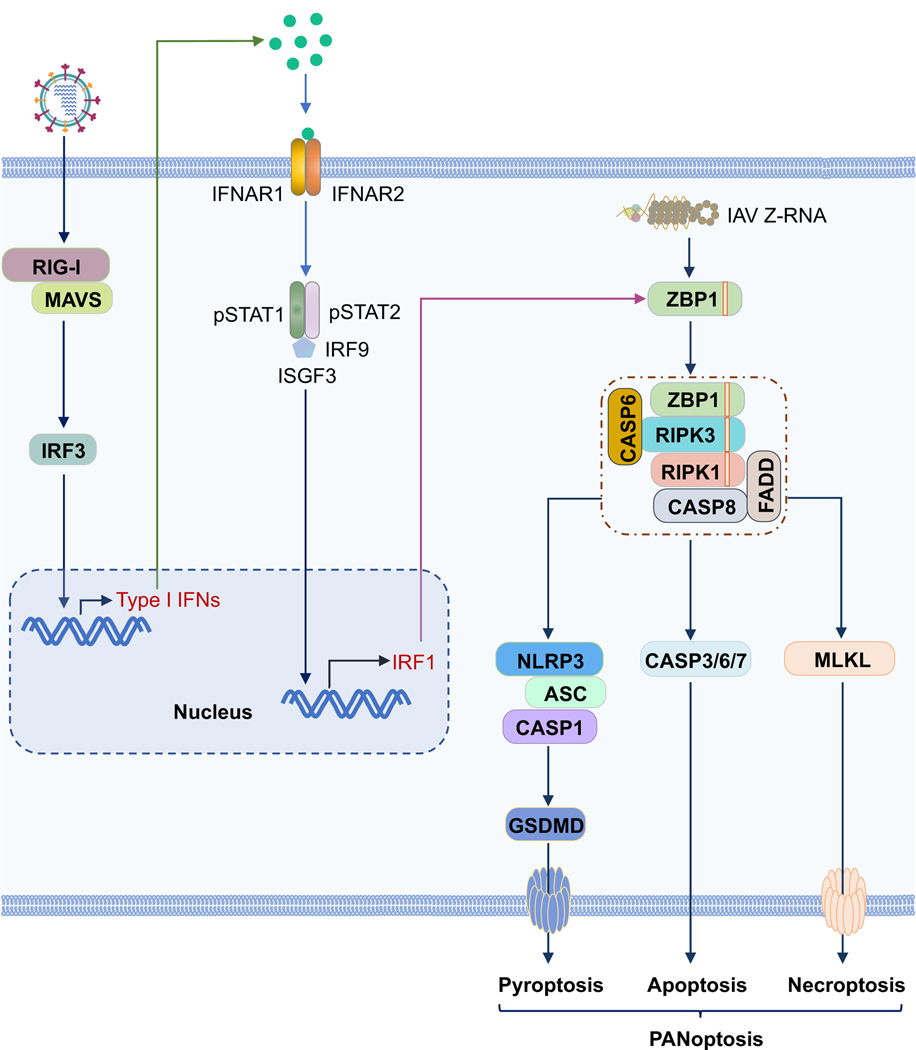
FIGURE 3.
ZBP1 can be induced by type I interferons (IFNs) and induce PANoptosis in macrophages.
Influenza virus (IAV) infection can be sensed by RIG-I, which will upregulate the expression of type I IFNs. The activated type I IFN signaling pathway can induce IRF1 expression, which acts as a transcription factor to control the expression of ZBP1. Influenza virus-produced Z-RNA can be sensed by ZBP1 in the nucleus and cytosol to induce the activation of PANoptosis.
4 |. Regulation of the ZBP1-NLRP3 inflammasome
ZBP1-NLRP3 inflammasome assembly can be regulated by both host and viral proteins. Given the requirement for a priming signal for NLRP3 inflammasome activation, factors that are able to affect the priming of the ZBP1-NLRP3 inflammasome can mediate its activation. Additionally, proteins that are required for ZBP1 upregulation are also regulators of the ZBP1-NLRP3 inflammasome.
4.1 |. Regulation by host proteins
As a single-stranded RNA virus, IAV can be sensed by several innate immune sensors, such as TLR3, TLR7, and RIG-I, to prime the ZBP1-NLRP3 inflammasome by activating inflammatory signaling and type I IFN expression101–104. All these sensors function in a cell type-specific manner; TLR3 plays a role in sensing IAV in epithelial cells104, RIG-I principally functions in fibroblasts and conventional dendritic cells (cDCs)105, and TLR7 senses IAV in plasmacytoid dendritic cells (pDCs)101,102. However, they also have redundant roles in some cells. For example, both TLR3 and RIG-I are involved in IAV sensing in lung epithelial cells to control IFN expression106,107. It has been shown that TLR7 is required for pro–IL-1β expression following influenza virus infection in BMDMs108, suggesting that TLR7 can regulate the priming of the ZBP1-NLRP3 inflammasome. In line with this, BMDMs lacking MyD88 and TRIF, adaptor proteins for TLRs, have substantially reduced ZBP1-NLRP3 inflammasome activation during IAV infection70.
Additionally, type I IFN signaling has been shown to be important for regulating the ZBP1-NLRP3 inflammasome. Type I IFN signaling is essential for ZBP1 expression in BMDMs and fibroblasts. Cells without IFNAR1 or signal factors downstream of IFNAR1, such as IRF9 and STAT1, do not have detectable ZBP1 expression28,70, which suggests that type I IFNs are not only required for up-regulating the expression of ZBP1 but are also required for maintaining the basal level of ZBP1 expression. RIG-I deficiency in BMDMs counteracts the increased expression of type I IFNs but only partially reduces the expression of ZBP1 following IAV infection, resulting in decreased ZBP1-NLRP3 inflammasome activation compared to that in WT BMDMs (Figure 3)70. These results indicate that RIG-I is the primary sensor for inducing type I IFN expression in response to IAV infection in BMDMs, but that it is dispensable for maintaining the basal expression of ZBP1. It is also possible that some other innate sensors play a role in activating the IAV-induced type I IFN response in BMDMs and could regulate the expression of ZBP1. More studies are needed to clarify this. In addition to the impact of ZBP1 expression on the regulation of the ZBP1-NLRP3 inflammasome, post-translational modifications of ZBP1 may also be critical for ZBP1 activation70. We observed that ubiquitination of ZBP1 is dramatically increased following IAV infection but not IFN-β treatment70, suggesting that ubiquitination may play some role in regulating the assembly of the ZBP1-NLRP3 inflammasome. The specific function of ZBP1 ubiquitination requires further investigation.
While it was known that type I IFNs were critical for ZBP1 expression, how type I IFN signaling mechanistically regulates the expression of ZBP1 was not clear. In the context of Francisella infection, we reported that type I IFNs are required for Francisella-induced AIM2 inflammasome activation due to the expression of IRGB10 and GBPs downstream of IRF1 expression109,110. A similar scenario was observed during IAV infection. IRF1 expression during IAV infection is largely dependent on type I IFN signaling (Figure 3)111. Without IRF1, ZBP1 expression is impaired during IAV infection, suggesting that IRF1 could be the transcription factor for ZBP1. Consistent with the role of ZBP1 in NLRP3 inflammasome activation, BMDMs without IRF1 showed impaired ZBP1-NLRP3 inflammasome activation following IAV infection111.
In addition to controlling the expression of the inflammasome components, the assembly of the ZBP1-NLRP3 inflammasome complex can also be regulated. We very recently discovered that the host factor caspase-6, the physiological role of which has been enigmatic for decades, acts as a ZBP1-NLRP3 inflammasome regulator (Figure 3)54. We found that loss of caspase-6 in BMDMs leads to reduced ZBP1-NLRP3 inflammasome activation following IAV infection54. The reduced ZBP1-NLRP3 inflammasome assembly is not due to defective priming, since the activation of both NF-κB and pERK are not dampened54. Additionally, the induction of ZBP1 expression in Casp6–/– cells is normal following IAV infection54. Furthermore, loss of caspase-6 does not affect the replication of IAV in BMDMs54. All these data together suggest a different mechanism for caspase-6 regulation of ZBP1-NLRP3 inflammasome activation. By characterizing the intrinsic ability of caspase-6 to interact with other key signaling proteins present in the ZBP1-containing complex, we observed that caspase-6 can interact with RIPK3 but not ZBP1, RIPK1, or caspase-854. Interestingly, the interaction between caspase-6 and RIPK3 can enhance the interaction between RIPK3 and ZBP1 when RIPK1 is present, suggesting that caspase-6 regulates the ZBP1-NLRP3 inflammasome by mediating the interaction between ZBP1 and RIPK354. Previous studies of caspase-6 have been focused on its caspase activity, but our finding revealed that even when the catalytic site of caspase-6 is mutated, caspase-6 continues to enhance the interaction between ZBP1 and RIPK3. The intrinsically disorder regions (IDRs) may be critical to mediate the interaction between caspase-6 and RIPK3, suggesting a scaffold function for caspase-6 during IAV infection54. Whether this model can be applied to other viruses or endogenous ligand-induced ZBP1-RIPK3 pathways needs further investigation.
4.2 |. Regulation by viral proteins
The activation of the ZBP1-NLRP3 inflammasome can also be regulated by viral proteins (Figure 4). The matrix protein 2 (M2) of IAV was the first viral protein shown to be critical for ZBP1-NLRP3 inflammasome activation. It was shown that IAV (A/Udorn/72, H3N2) lacking the ion channel activity of M2 (referred to as M2del29–31) loses the capacity to activate the NLRP3 inflammasome108. Furthermore, overexpressing M2 using lentiviruses can induce IL-1β and IL-18 release after LPS priming, suggesting that M2 alone is able to provide the activation signal for the NLRP3 inflammasome108. Since the mechanisms by which ZBP1-RIPK3 complex formation leads to NLRP3 inflammasome activation remain unclear, it is possible that the ion channel of M2 plays some role in ZBP1-NLRP3 inflammasome assembly in the early infection phase. But this role should be carefully explored during IAV infection. It is well known that the M2 protein is essential for the IAV life cycle, and without it IAV propagates poorly112. Two major functions of M2 have been unveiled: one is to acidify the viral interior to release the viral RNP complex from the M1 coat and the other is to maintain the pH of the Golgi apparatus and stabilize the conformation of the HA protein during its transportation; both of these functions rely on the ion channel activity of M2113. To overcome the functional deficiencies caused by the loss of M2, the virus can even adapt to gain a new viral protein, which performs exactly the same function as M2, via mutations114, indicating the indispensable role of M2 for IAV. Moreover, it has been reported that the M2-mutant virus Udorn (M2del29–31) showed significantly less replication than its WT counterpart, and its viral protein production in infected cells was reduced by about 90%115. How this defective replication of IAV affects ZBP1-NLRP3 inflammasome activation needs detailed exploration. Furthermore, because recent studies have demonstrated that either pannexin-1 pore formation following apoptotic caspase-mediated cleavage or MLKL pore formation after the initiation of necroptosis can provide the activation signal for the NLRP3 inflammasome116,117 and because IAV infection leads to both apoptosis and necroptosis, it is necessary to reconsider whether M2 channel activity is essential for ZBP1-NLRP3 inflammasome activation.
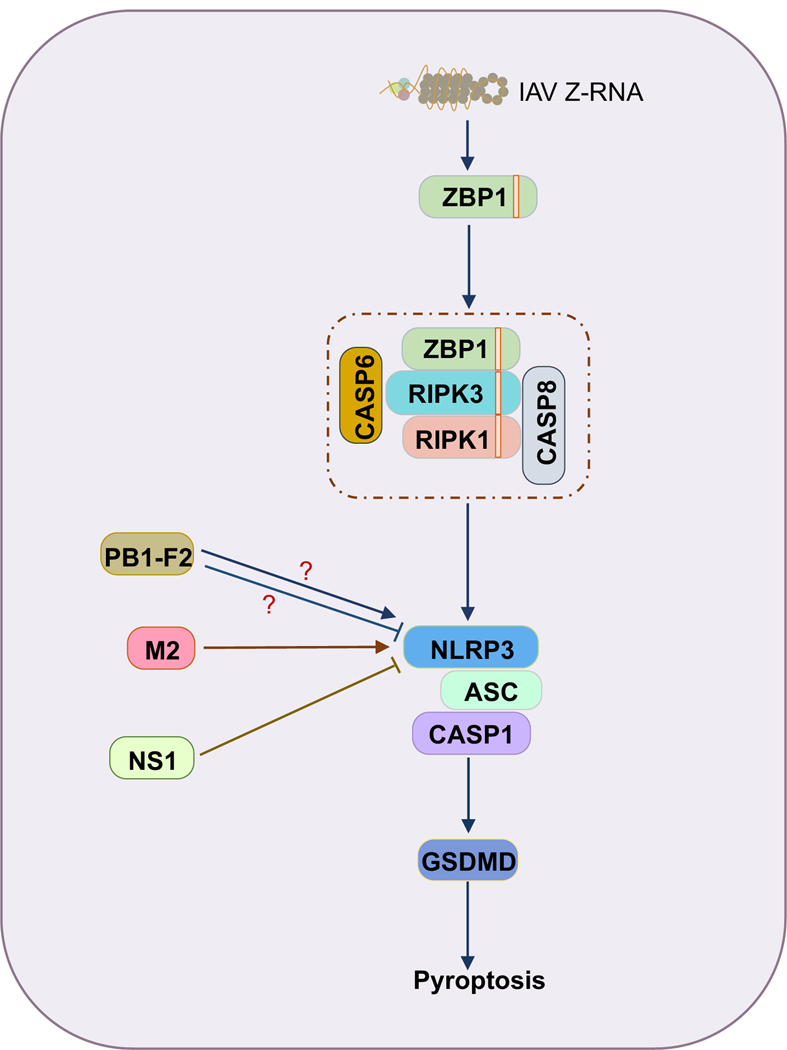
FIGURE 4.
ZBP1-NLRP3 inflammasome assembly can be regulated by influenza viral proteins.
The ion channel protein M2 contributes to ZBP1-NLRP3 inflammasome activation, while the non-structural protein 1 (NS1) has been shown to suppress ZBP1-NLRP3 inflammasome activation.
In addition to the M2 protein, two other viral proteins, PB1-F2 and non-structural protein 1 (NS1), have also been reported to regulate ZBP1-NLRP3 inflammasome activation. The peptide derived from the IAV PB1-F2 protein can induce NLRP3 inflammasome activation after priming118,119. However, another study using PB1-F2 from the same strain showed that PB1-F2 was able to inhibit NLRP3 inflammasome assembly in an overexpression system120. Additional studies are required to resolve this discrepancy. NS1 is a multifunctional protein in IAV which has been shown to regulate type I IFN expression, inflammatory responses, and cell death during IAV infection121. Several studies have shown that NS1 is also involved in ZBP1-NLRP3 inflammasome activation122–124. It seems that NS1 has a global inhibitory effect on NLRP3 inflammasome assembly, not just on IAV-induced NLRP3 inflammasome activation122,124. However, the inhibitory effect varies between different strains122,123; this is consistent with other strain-specific abilities observed with NS1121.
5 |. ZBP1-NLRP3 inflammasome: a critical component of PANoptosis
It is known that both pyroptosis and necroptosis are inflammatory forms of programmed cell death which can release large amounts of proinflammatory cytokines. While apoptosis was originally recognized as an immunologically-silent form of programmed cell death, studies of the crosstalk between apoptosis and pyroptosis indicate that in some cell types apoptotic cell death is also inflammatory48,125–129. Therefore, PANoptosis represents an inflammatory form of cell death, and it can occur in the context of infectious and inflammatory diseases, development, and cancer. The ZBP1-NLRP3 inflammasome is a critical component of IAV-induced PANoptosis. Following influenza virus infection, the ZBP1-NLRP3 inflammasome becomes activated, contributing to proinflammatory cytokine maturation and also leading to GSDMD cleavage to activate pyroptosis. Therefore, the ZBP1-NLRP3 inflammasome contributes to IAV-induced PANoptosis in two aspects: it facilitates the maturation of proinflammatory cytokines, i.e. IL-1β and IL-18, following IAV infection, and it also is directly responsible for the activation of the pyroptotic executioner of PANoptosis. Furthermore, our group has recently shown that cells undergoing PANoptosis in the context of TAK1 inhibition following TLR priming form a large puncta in the cytosol that contains RIPK1, ASC, and caspase-853. These findings suggest that the inflammasome may be a critical component of the protein complex that initiates PANoptosis, the PANoptosome. Several other studies have also reported that caspase-8 can interact with ASC56,57,130, providing further evidence for the inflammasome being part of the PANoptosome. Thus, it is possible that the ZBP1-NLRP3 inflammasome is assembled with the ZBP1-RIPK3-caspase-8 complex as a large multiprotein complex following IAV infection. We recently showed that immunoprecipitation of RIPK3 in an overexpression system could co-immunoprecipitate caspase-8, ASC, RIPK1, NLRP3 and ZBP1, providing further support for this hypothesis55. Detailed studies are needed to further understand this complex.
When PANoptosis is activated, blocking just one arm (i.e., blocking just pyroptosis or apoptosis or necroptosis alone) cannot prevent cell death or inflammatory cytokine-induced disease. For example, blocking pyroptosis by deleting NLRP3 or GSDMD alone does not abolish IAV-induced cell death. Similarly, addition of the caspase-8 inhibitor or RIPK3 kinase inhibitor cannot block cell death during IAV infection28,131. The emergence of PANoptosis is evolutionally beneficial for the host to control pathogen invasion by eliminating the infected cells and providing functional redundancies to overcome pathogen immune evasion strategies that may allow them to evade one or two of the cell death pathways. However, in cases where cytokine storm is occurring and causing detrimental inflammation and tissue damage in the host, it is important to note that it may be necessary to target all these pathways simultaneously to adequately control the cytokine release. Overall, in-depth characterization of the molecular mechanisms of PANoptosis activation will be important to inform the development of treatments for infectious and inflammatory diseases and cancer.
6 |. Summary and perspectives
ZBP1 is the sensor for influenza virus-induced NLRP3 inflammasome activation and plays a role similar to that of caspase-11 (or caspase-4/5) in LPS-induced non-canonical NLRP3 inflammasome activation28,46,70. Additionally, it has key roles in the initiation of IAV-induced PANoptosis28,46,47,54. Even though the core complex responsible for ZBP1-NLRP3 inflammasome activation has been identified, how ZBP1 sensing of influenza virus leads to NLRP3 inflammasome activation is still not fully understood. Furthermore, host factors that can regulate ZBP1-NLRP3 inflammasome activation and PANoptosome assembly remain largely unknown, and whether the ZBP1-NLRP3 inflammasome can be triggered by the other viruses or endogenous ligands remains to be investigated in the future.
To date, all the functional studies of the ZBP1-NLRP3 inflammasome have been focused on mice or mouse cells. There is an urgent need to clarify the role of ZBP1 in human cells. A recent study has demonstrated that MxA can act as the inflammasome sensor following IAV infection in human respiratory epithelial cells19. It is known that Mx1 (the mouse counterpart of human MxA) is not functional in most strains of inbred mice132. It will be interesting to test whether ZBP1 could interact with MxA during IAV infection in respiratory epithelial cells.
Additionally, the rise of the circulating SARS-CoV-2 virus throughout the world has raised concerns about the effect of inflammasome activation on the development of COVID-19. Because SARS-CoV-2 is also an RNA virus, similar to IAV, it is worth examining whether SARS-CoV-2 can induce the ZBP1-NLRP3 inflammasome and PANoptosis after infection. If ZBP1 is the sensor for SARS-CoV-2–induced cell death and inflammasome activation, our foundation of mechanistic knowledge surrounding the ZBP1-NLRP3 inflammasome would provide important clues to direct the therapeutic advances.
Acknowledgements
We apologize to our colleagues in the field whose work could not be cited due to space limitations. We thank all the members of the Kanneganti laboratory for their comments and suggestions and Rebecca Tweedell, PhD, for scientific editing and writing support. Work from our laboratory is supported by the US National Institutes of Health (AI101935, AI124346, AR056296, and CA163507 to T.-D.K.) and the American Lebanese Syrian Associated Charities (to T.-D.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Christgen S, Place DE, Kanneganti T-D. Toward targeting inflammasomes: insights into their regulation and activation. Cell Research. 2020;30(4):315–327. doi: 10.1038/s41422-020-0295-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Place DE, Kanneganti T-D. Recent advances in inflammasome biology. Current Opinion in Immunology. 2018;50:32–38. doi: 10.1016/j.coi.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3 [DOI] [PubMed] [Google Scholar]
- 4.Kanneganti T-D, Ozören N, Body-Malapel M, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440(7081):233–236. doi: 10.1038/nature04517 [DOI] [PubMed] [Google Scholar]
- 5.Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. doi: 10.1038/nature04515 [DOI] [PubMed] [Google Scholar]
- 6.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi: 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- 7.Franchi L, Amer A, Body-Malapel M, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7(6):576–582. doi: 10.1038/ni1346 [DOI] [PubMed] [Google Scholar]
- 8.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-Deficient Legionella Mutants Evade Caspase-1- and Naip5-Mediated Macrophage Immunity. PLOS Pathogens. 2006;2(3):e18. doi: 10.1371/journal.ppat.0020018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes-Alnemri T, Yu J-W, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458(7237):509–513. doi: 10.1038/nature07710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornung V, Ablasser A, Charrel-Dennis M, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–518. doi: 10.1038/nature07725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts TL, Idris A, Dunn JA, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323(5917):1057–1060. doi: 10.1126/science.1169841 [DOI] [PubMed] [Google Scholar]
- 12.Bürckstümmer T, Baumann C, Blüml S, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10(3):266–272. doi: 10.1038/ni.1702 [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Yang J, Gao W, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513(7517):237–241. doi: 10.1038/nature13449 [DOI] [PubMed] [Google Scholar]
- 14.Elinav E, Strowig T, Kau AL, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–757. doi: 10.1016/j.cell.2011.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu S, Ding S, Wang P, et al. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature. 2017;546(7660):667–670. doi: 10.1038/nature22967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vladimer GI, Weng D, Paquette SWM, et al. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 2012;37(1):96–107. doi: 10.1016/j.immuni.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerur N, Veettil MV, Sharma-Walia N, et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9(5):363–375. doi: 10.1016/j.chom.2011.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poeck H, Bscheider M, Gross O, et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010;11(1):63–69. doi: 10.1038/ni.1824 [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Ishitsuka A, Noguchi M, et al. Influenza restriction factor MxA functions as inflammasome sensor in the respiratory epithelium. Sci Immunol. 2019;4(40). doi: 10.1126/sciimmunol.aau4643 [DOI] [PubMed] [Google Scholar]
- 20.Schroder K, Tschopp J. The Inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 21.Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16(1):7–21. doi: 10.1038/nri.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi: 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- 23.He WT, Wan H, Hu L, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25(12):1285–1298. doi: 10.1038/cr.2015.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671. doi: 10.1038/nature15541 [DOI] [PubMed] [Google Scholar]
- 25.Kanneganti TD, Body-Malapel M, Amer A, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281(48):36560–36568. doi: 10.1074/jbc.M607594200 [DOI] [PubMed] [Google Scholar]
- 26.Allen IC, Scull MA, Moore CB, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30(4):556–565. doi: 10.1016/j.immuni.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas PG, Dash P, Aldridge JR, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30(4):566–575. doi: 10.1016/j.immuni.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuriakose T, Man SM, Malireddi RK, et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 2016;1(2). doi: 10.1126/sciimmunol.aag2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathur A, Feng S, Hayward JA, et al. A multicomponent toxin from Bacillus cereus incites inflammation and shapes host outcome via the NLRP3 inflammasome. Nature Microbiology. 2019;4(2):362–374. doi: 10.1038/s41564-018-0318-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayagaki N, Warming S, Lamkanfi M, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. doi: 10.1038/nature10558 [DOI] [PubMed] [Google Scholar]
- 31.Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi: 10.1038/nature13683 [DOI] [PubMed] [Google Scholar]
- 32.Gurung P, Anand PK, Malireddi RK, et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192(4):1835–1846. doi: 10.4049/jimmunol.1302839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karki R, Man SM, Malireddi RKS, et al. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe. 2015;17(3):357–368. doi: 10.1016/j.chom.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamkanfi M, Malireddi RKS, Kanneganti T-D. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 2009;284(31):20574–20581. doi: 10.1074/jbc.M109.023689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Briard B, Karki R, Malireddi RKS, et al. Fungal ligands released by innate immune effectors promote inflammasome activation during Aspergillus fumigatus infection. Nature Microbiology. 2019;4(2):316–327. doi: 10.1038/s41564-018-0298-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Py BF, Kim M-S, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 Critically Regulates Inflammasome Activity. Molecular Cell. 2013;49(2):331–338. doi: 10.1016/j.molcel.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 38.Song N, Liu Z-S, Xue W, et al. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol Cell. 2017;68(1):185–197.e6. doi: 10.1016/j.molcel.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 39.Stutz A, Kolbe C-C, Stahl R, et al. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. The Journal of Experimental Medicine. 2017;214(6):1725–1736. doi: 10.1084/jem.20160933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barry R, John SW, Liccardi G, et al. SUMO-mediated regulation of NLRP3 modulates inflammasome activity. Nat Commun. 2018;9(1):3001. doi: 10.1038/s41467-018-05321-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14(9):1583–1589. doi: 10.1038/sj.cdd.4402195 [DOI] [PubMed] [Google Scholar]
- 42.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–1153. doi: 10.1016/j.immuni.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dostert C, Pétrilli V, Bruggen RV, Steele C, Mossman BT, Tschopp J. Innate Immune Activation Through Nalp3 Inflammasome Sensing of Asbestos and Silica. Science. 2008;320(5876):674–677. doi: 10.1126/science.1156995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruz CM, Rinna A, Forman HJ, Ventura ALM, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282(5):2871–2879. doi: 10.1074/jbc.M608083200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Chen ZJ. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. 2018;564(7734):71–76. doi: 10.1038/s41586-018-0761-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malireddi RKS, Kesavardhana S, Kanneganti T-D. ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis). Front Cell Infect Microbiol. 2019;9. doi: 10.3389/fcimb.2019.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samir P, Malireddi RKS, Kanneganti T-D. The PANoptosome: A deadly protein complex driving PANoptosis. Frontiers in Cellular and Infection Microbiology. Published online 2020:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamkanfi M, Kanneganti TD, Van Damme P, et al. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics. 2008;7(12):2350–2363. doi: 10.1074/mcp.M800132-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malireddi RKS, Ippagunta S, Lamkanfi M, Kanneganti T-D. Cutting edge: proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. J Immunol. 2010;185(6):3127–3130. doi: 10.4049/jimmunol.1001512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gurung P, Burton A, Kanneganti T-D. NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1β-mediated osteomyelitis. Proc Natl Acad Sci USA. 2016;113(16):4452–4457. doi: 10.1073/pnas.1601636113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lukens JR, Gurung P, Vogel P, et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature. 2014;516(7530):246–249. doi: 10.1038/nature13788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malireddi RKS, Gurung P, Mavuluri J, et al. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. Journal of Experimental Medicine. 2018;215(4):1023–1034. doi: 10.1084/jem.20171922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malireddi RKS, Gurung P, Kesavardhana S, et al. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity–independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J Exp Med. 2020;217(3). doi: 10.1084/jem.20191644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng M, Karki R, Vogel P, Kanneganti T-D. Caspase-6 Is a Key Regulator of Innate Immunity, Inflammasome Activation, and Host Defense. Cell. Published online April 15, 2020. doi: 10.1016/j.cell.2020.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christgen S, Zheng M, Kesavardana S, et al. Identification of the PANoptosome: A molecular platform triggering cell death. Frontiers in Cellular and Infection Microbiology. Published online 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fritsch M, Günther SD, Schwarzer R, et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575(7784):683–687. doi: 10.1038/s41586-019-1770-6 [DOI] [PubMed] [Google Scholar]
- 57.Newton K, Wickliffe KE, Maltzman A, et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature. 2019;575(7784):679–682. doi: 10.1038/s41586-019-1752-8 [DOI] [PubMed] [Google Scholar]
- 58.Dillon CP, Weinlich R, Rodriguez DA, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157(5):1189–1202. doi: 10.1016/j.cell.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newton K, Wickliffe KE, Dugger DL, et al. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature. 2019;574(7778):428–431. doi: 10.1038/s41586-019-1548-x [DOI] [PubMed] [Google Scholar]
- 60.Karki R, Sharma BR, Lee E, et al. Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer. JCI Insight. 2020;5(12):136720. doi: 10.1172/jci.insight.136720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takaoka A, Wang Z, Choi MK, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448(7152):501–505. doi: 10.1038/nature06013 [DOI] [PubMed] [Google Scholar]
- 62.Ishii KJ, Kawagoe T, Koyama S, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451(7179):725–729. doi: 10.1038/nature06537 [DOI] [PubMed] [Google Scholar]
- 63.Kesavardhana S, Kanneganti TD. ZBP1: A STARGᐰTE to decode the biology of Z-nucleic acids in disease. J Exp Med. 2020;217(7):e20200885. doi: 10.1084/jem.20200885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11(3):290–297. doi: 10.1016/j.chom.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rebsamen M, Heinz LX, Meylan E, et al. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 2009;10(8):916–922. doi: 10.1038/embor.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo H, Gilley RP, Fisher A, et al. Species-independent contribution of ZBP1/DAI/DLM-1-triggered necroptosis in host defense against HSV1. Cell Death Dis. 2018;9(8):816. doi: 10.1038/s41419-018-0868-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koehler H, Cotsmire S, Langland J, et al. Inhibition of DAI-dependent necroptosis by the Z-DNA binding domain of the vaccinia virus innate immune evasion protein, E3. Proc Natl Acad Sci U S A. 2017;114(43):11506–11511. doi: 10.1073/pnas.1700999114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Z, Wu S-Q, Liang Y, et al. RIP1/RIP3 Binding to HSV-1 ICP6 Initiates Necroptosis to Restrict Virus Propagation in Mice. Cell Host & Microbe. 2015;17(2):229–242. doi: 10.1016/j.chom.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Li Y, Liu S, et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci USA. 2014;111(43):15438–15443. doi: 10.1073/pnas.1412767111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kesavardhana S, Kuriakose T, Guy CS, et al. ZBP1/DAI ubiquitination and sensing of influenza vRNPs activate programmed cell death. J Exp Med. 2017;214(8):2217–2229. doi: 10.1084/jem.20170550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thapa RJ, Ingram JP, Ragan KB, et al. DAI Senses Influenza A Virus Genomic RNA and Activates RIPK3-Dependent Cell Death. Cell Host Microbe. 2016;20(5):674–681. doi: 10.1016/j.chom.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daniels BP, Kofman SB, Smith JR, et al. The Nucleotide Sensor ZBP1 and Kinase RIPK3 Induce the Enzyme IRG1 to Promote an Antiviral Metabolic State in Neurons. Immunity. 2019;50(1):64–76.e4. doi: 10.1016/j.immuni.2018.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rothan HA, Arora K, Natekar JP, Strate PG, Brinton MA, Kumar M. Z-DNA-Binding Protein 1 Is Critical for Controlling Virus Replication and Survival in West Nile Virus Encephalitis. Front Microbiol. 2019;10:2089. doi: 10.3389/fmicb.2019.02089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiao H, Wachsmuth L, Kumari S, et al. Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature. Published online March 25, 2020:1–5. doi: 10.1038/s41586-020-2129-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang R, Li H, Wu J, et al. Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature. Published online March 25, 2020:1–5. doi: 10.1038/s41586-020-2127-x [DOI] [PubMed] [Google Scholar]
- 76.Lin J, Kumari S, Kim C, et al. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature. 2016;540(7631):124–128. doi: 10.1038/nature20558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Newton K, Wickliffe KE, Maltzman A, et al. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature. 2016;540(7631):129–133. doi: 10.1038/nature20559 [DOI] [PubMed] [Google Scholar]
- 78.Kesavardhana S, Malireddi RKS, Burton AR, et al. The Zα2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. J Biol Chem. Published online April 29, 2020. doi: 10.1074/jbc.RA120.013752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.VanDussen KL, Stojmirović A, Li K, et al. Abnormal Small Intestinal Epithelial Microvilli in Patients With Crohn’s Disease. Gastroenterology. 2018;155(3):815–828. doi: 10.1053/j.gastro.2018.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maelfait J, Liverpool L, Bridgeman A, Ragan KB, Upton JW, Rehwinkel J. Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J. 2017;36(17):2529–2543. doi: 10.15252/embj.201796476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown BA, Lowenhaupt K, Wilbert CM, Hanlon EB, Rich A. The zalpha domain of the editing enzyme dsRNA adenosine deaminase binds left-handed Z-RNA as well as Z-DNA. Proc Natl Acad Sci USA. 2000;97(25):13532–13536. doi: 10.1073/pnas.240464097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herbert A, Alfken J, Kim YG, Mian IS, Nishikura K, Rich A. A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase. Proc Natl Acad Sci USA. 1997;94(16):8421–8426. doi: 10.1073/pnas.94.16.8421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim Y-G, Lowenhaupt K, Oh D-B, Kim KK, Rich A. Evidence that vaccinia virulence factor E3L binds to Z-DNA in vivo: Implications for development of a therapy for poxvirus infection. Proc Natl Acad Sci USA. 2004;101(6):1514–1518. doi: 10.1073/pnas.0308260100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwartz T, Behlke J, Lowenhaupt K, Heinemann U, Rich A. Structure of the DLM-1-Z-DNA complex reveals a conserved family of Z-DNA-binding proteins. Nat Struct Biol. 2001;8(9):761–765. doi: 10.1038/nsb0901-761 [DOI] [PubMed] [Google Scholar]
- 85.Schwartz T, Rould MA, Lowenhaupt K, Herbert A, Rich A. Crystal structure of the Zalpha domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA. Science. 1999;284(5421):1841–1845. doi: 10.1126/science.284.5421.1841 [DOI] [PubMed] [Google Scholar]
- 86.Zhang T, Yin C, Boyd DF, et al. Influenza Virus Z-RNAs Induce ZBP1-Mediated Necroptosis. Cell. 2020;180(6):1115–1129.e13. doi: 10.1016/j.cell.2020.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mannion NM, Greenwood SM, Young R, et al. The RNA-Editing Enzyme ADAR1 Controls Innate Immune Responses to RNA. Cell Rep. 2014;9(4):1482–1494. doi: 10.1016/j.celrep.2014.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ahmad S, Mu X, Yang F, et al. Breaching Self-Tolerance to Alu Duplex RNA Underlies MDA5-Mediated Inflammation. Cell. 2018;172(4):797–810.e13. doi: 10.1016/j.cell.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stout-Delgado HW, Vaughan SE, Shirali AC, Jaramillo RJ, Harrod KS. Impaired NLRP3 Inflammasome Function in Elderly Mice during Influenza Infection Is Rescued by Treatment with Nigericin. JI. 2012;188(6):2815–2824. doi: 10.4049/jimmunol.1103051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tate MD, Ong JDH, Dowling JK, et al. Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Scientific Reports. 2016;6:27912. doi: 10.1038/srep27912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ren R, Wu S, Cai J, et al. The H7N9 influenza A virus infection results in lethal inflammation in the mammalian host via the NLRP3-caspase-1 inflammasome. Sci Rep. 2017;7(1):7625. doi: 10.1038/s41598-017-07384-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206(1):79–87. doi: 10.1084/jem.20081667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X, Jiang W, Yan Y, et al. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat Immunol. 2014;15(12):1126–1133. doi: 10.1038/ni.3015 [DOI] [PubMed] [Google Scholar]
- 94.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]
- 95.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A. 2011;108(50):20054–20059. doi: 10.1073/pnas.1116302108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaiser WJ, Sridharan H, Huang C, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288(43):31268–31279. doi: 10.1074/jbc.M113.462341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim SJ, Li J. Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis. 2013;4:e716. doi: 10.1038/cddis.2013.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao J, Jitkaew S, Cai Z, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. PNAS. 2012;109(14):5322–5327. doi: 10.1073/pnas.1200012109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vanlangenakker N, Bertrand MJM, Bogaert P, Vandenabeele P, Vanden Berghe T. TNF-induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis. 2011;2:e230. doi: 10.1038/cddis.2011.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fu Y, Comella N, Tognazzi K, Brown LF, Dvorak HF, Kocher O. Cloning of DLM-1, a novel gene that is up-regulated in activated macrophages, using RNA differential display. Gene. 1999;240(1):157–163. doi: 10.1016/S0378-1119(99)00419-9 [DOI] [PubMed] [Google Scholar]
- 101.Lund JM, Alexopoulou L, Sato A, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101(15):5598–5603. doi: 10.1073/pnas.0400937101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. doi: 10.1126/science.1093616 [DOI] [PubMed] [Google Scholar]
- 103.Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105. doi: 10.1038/nature04734 [DOI] [PubMed] [Google Scholar]
- 104.Guillot L, Le Goffic R, Bloch S, et al. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280(7):5571–5580. doi: 10.1074/jbc.M410592200 [DOI] [PubMed] [Google Scholar]
- 105.Kato H, Sato S, Yoneyama M, et al. Cell Type-Specific Involvement of RIG-I in Antiviral Response. Immunity. 2005;23(1):19–28. doi: 10.1016/j.immuni.2005.04.010 [DOI] [PubMed] [Google Scholar]
- 106.Goffic RL, Pothlichet J, Vitour D, et al. Cutting Edge: Influenza A Virus Activates TLR3-Dependent Inflammatory and RIG-I-Dependent Antiviral Responses in Human Lung Epithelial Cells. The Journal of Immunology. 2007;178(6):3368–3372. doi: 10.4049/jimmunol.178.6.3368 [DOI] [PubMed] [Google Scholar]
- 107.Wu W, Zhang W, Duggan ES, Booth JL, Zou M-H, Metcalf JP. RIG-I and TLR3 are both required for maximum interferon induction by influenza virus in human lung alveolar epithelial cells. Virology. 2015;482:181–188. doi: 10.1016/j.virol.2015.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes through intracellular M2 channel. Nat Immunol. 2010;11(5):404–410. doi: 10.1038/ni.1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Man SM, Karki R, Sasai M, et al. IRGB10 Liberates Bacterial Ligands for Sensing by the AIM2 and Caspase-11-NLRP3 Inflammasomes. Cell. 2016;167(2):382–396.e17. doi: 10.1016/j.cell.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Man SM, Karki R, Malireddi RKS, et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol. 2015;16(5):467–475. doi: 10.1038/ni.3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuriakose T, Zheng M, Neale G, Kanneganti TD. IRF1 Is a Transcriptional Regulator of ZBP1 Promoting NLRP3 Inflammasome Activation and Cell Death during Influenza Virus Infection. J Immunol. 2018;200(4):1489–1495. doi: 10.4049/jimmunol.1701538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hutchinson EC, Curran MD, Read EK, Gog JR, Digard P. Mutational Analysis of cis-Acting RNA Signals in Segment 7 of Influenza A Virus. Journal of Virology. 2008;82(23):11869–11879. doi: 10.1128/JVI.01634-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pielak RM, Chou JJ. Influenza M2 proton channels. Biochim Biophys Acta. 2011;1808(2):522–529. doi: 10.1016/j.bbamem.2010.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wise HM, Hutchinson EC, Jagger BW, et al. Identification of a Novel Splice Variant Form of the Influenza A Virus M2 Ion Channel with an Antigenically Distinct Ectodomain. PLOS Pathogens. 2012;8(11):e1002998. doi: 10.1371/journal.ppat.1002998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takeda M, Pekosz A, Shuck K, Pinto LH, Lamb RA. Influenza A Virus M2 Ion Channel Activity Is Essential for Efficient Replication in Tissue Culture. Journal of Virology. 2002;76(3):1391–1399. doi: 10.1128/JVI.76.3.1391-1399.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen KW, Demarco B, Heilig R, et al. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. EMBO J. 2019;38(10). doi: 10.15252/embj.2019101638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kang S, Fernandes-Alnemri T, Rogers C, et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat Commun. 2015;6(1):1–15. doi: 10.1038/ncomms8515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McAuley JL, Tate MD, MacKenzie-Kludas CJ, et al. Activation of the NLRP3 Inflammasome by IAV Virulence Protein PB1-F2 Contributes to Severe Pathophysiology and Disease. PLOS Pathogens. 2013;9(5):e1003392. doi: 10.1371/journal.ppat.1003392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pinar A, Dowling JK, Bitto NJ, et al. PB1-F2 Peptide Derived from Avian Influenza A Virus H7N9 Induces Inflammation via Activation of the NLRP3 Inflammasome. J Biol Chem. 2017;292(3):826–836. doi: 10.1074/jbc.M116.756379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoshizumi T, Ichinohe T, Sasaki O, et al. Influenza A virus protein PB1-F2 translocates into mitochondria via Tom40 channels and impairs innate immunity. Nature Communications. 2014;5(1):1–14. doi: 10.1038/ncomms5713 [DOI] [PubMed] [Google Scholar]
- 121.Hale BG, Randall RE, Ortín J, Jackson D. The multifunctional NS1 protein of influenza A viruses. J Gen Virol. 2008;89(Pt 10):2359–2376. doi: 10.1099/vir.0.2008/004606-0 [DOI] [PubMed] [Google Scholar]
- 122.Moriyama M, Chen I-Y, Kawaguchi A, et al. The RNA- and TRIM25-Binding Domains of Influenza Virus NS1 Protein Are Essential for Suppression of NLRP3 Inflammasome-Mediated Interleukin-1β Secretion. J Virol. 2016;90(8):4105–4114. doi: 10.1128/JVI.00120-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park H-S, Liu G, Thulasi Raman SN, Landreth SL, Liu Q, Zhou Y. NS1 Protein of 2009 Pandemic Influenza A Virus Inhibits Porcine NLRP3 Inflammasome-Mediated Interleukin-1 Beta Production by Suppressing ASC Ubiquitination. J Virol. 2018;92(8). doi: 10.1128/JVI.00022-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chung W-C, Kang H-R, Yoon H, Kang S-J, Ting JP-Y, Song MJ. Influenza A Virus NS1 Protein Inhibits the NLRP3 Inflammasome. PLoS ONE. 2015;10(5):e0126456. doi: 10.1371/journal.pone.0126456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi: 10.1038/nature22393 [DOI] [PubMed] [Google Scholar]
- 126.Chauhan D, Bartok E, Gaidt MM, et al. BAX/BAK-Induced Apoptosis Results in Caspase-8-Dependent IL-1beta Maturation in Macrophages. Cell Rep. 2018;25(9):2354–2368 e5. doi: 10.1016/j.celrep.2018.10.087 [DOI] [PubMed] [Google Scholar]
- 127.Sarhan J, Liu BC, Muendlein HI, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115(46):E10888–E10897. doi: 10.1073/pnas.1809548115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Orning P, Weng D, Starheim K, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362(6418):1064–1069. doi: 10.1126/science.aau2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. doi: 10.1038/ncomms14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vajjhala PR, Lu A, Brown DL, et al. The Inflammasome Adaptor ASC Induces Procaspase-8 Death Effector Domain Filaments. J Biol Chem. 2015;290(49):29217–29230. doi: 10.1074/jbc.M115.687731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nogusa S, Thapa RJ, Dillon CP, et al. RIPK3 Activates Parallel Pathways of MLKL-Driven Necroptosis and FADD-Mediated Apoptosis to Protect against Influenza A Virus. Cell Host Microbe. 2016;20(1):13–24. doi: 10.1016/j.chom.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Iwasaki A. Antiviral responses of inbred mice. Nature Reviews Immunology. 2016;16(6):339–339. doi: 10.1038/nri.2016.44 [DOI] [PubMed] [Google Scholar]