Abstract
Background:
Human immunodeficiency virus (HIV)-associated neurocognitive disorders persist in the era of antiretroviral therapy. One factor that is elevated among persons with HIV (PWH) and independently associated with neurocognitive impairment is methamphetamine dependence (METH). Such dependence may further increase cognitive impairment among PWH, by delaying HIV diagnosis (and thus, antiretroviral therapy initiation), which has been posited to account for persistent cognitive impairment among PWH, despite subsequent treatment-related viral load suppression (VLS; ≤50 copies of the virus per milliliter in plasma or cerebrospinal fluid). This study examined the independent and combined (additive versus synergistic) effects of HIV and history of METH on the sustained attention and vigilance cognitive domain, while controlling for VLS.
Methods:
Participants included 205 (median age=44 years; 77% males; HIV-/METH- n=67; HIV+/METH - n=49; HIV-/METH+ n=36; HIV+/METH+ n=53) individuals enrolled in the Translational Methamphetamine AIDS Research Center, who completed Conners’ and the 5-Choice continuous performance tests (CPTs).
Results:
METH participants exhibited deficits in sustained attention and vigilance; however, these effects were not significant after excluding participants who had a positive urine toxicology screen for methamphetamine. Controlling for VLS, PWH did not have worse sustained attention and vigilance, but consistently displayed slower reaction times across blocks, relative to HIV- participants. There was no HIV x METH interaction on sustained attention and vigilance.
Conclusions:
Recent methamphetamine use among METH people and detectable viral loads are detrimental to sustained attention and vigilance. These findings highlight the need for prompt diagnosis of HIV and initiation of antiretroviral therapy, and METH use interventions.
Keywords: human immunodeficiency virus, methamphetamine, sustained attention, vigilance, continuous performance test
1. Introduction
Approximately 1.2 million people in the United States have human immunodeficiency virus (HIV), three quarters of which are male (Centers for Disease Control and Prevention, 2018). Methamphetamine use and methamphetamine dependence (METH) are prevalent among people with HIV (PWH) and in particular, males with HIV (Cohen et al., 2016; Dawson-Rose et al., 2017; Hartzler et al., 2017). METH was the third most commonly reported substance dependence in a sample of PWH across seven US clinics (Hartzler et al., 2017). Given the prevalence of METH among PWH, research should identify how METH affects factors integral to daily functioning among this group, including cognition.
While antiretroviral therapy has decreased incidence of HIV-associated dementia (Heaton et al., 2011), HIV-associated neurocognitive disorders persist (Antinori et al., 2007; Walker & Brown, 2018), with up to half of PWH presenting with milder neurocognitive impairment (Heaton et al., 2010). While amphetamines improve cognition among amphetamine-naïve (MacQueen, et al., 2018a; Smith & Farah, 2011) and some clinical samples (e.g., attention-deficit/ hyperactivity disorder; ADHD; Baroni & Castellanos, 2015; Sagvolden & Xu, 2008), METH is linked to neurocognitive impairment (Potvin et al., 2018). Methamphetamine use is also linked to poorer antiretroviral therapy adherence (Moore et al., 2012; Parsons et al., 2013; Passaro et al., 2015), leading to detectable viral loads and cognitive decline (Heaton et al., 2015). Conversely, viral load suppression (VLS; ≤50 copies of the virus per milliliter of plasma or cerebrospinal fluid) is associated with stable cognition over time (Sanford et al., 2018).
METH is also associated with delayed HIV diagnosis and antiretroviral therapy initiation (Kuchinad et al., 2016; Passaro et al., 2015), leading to early and more severe immunosuppression, potentially driving irreversible injury to frontal brain systems, resulting in enduring cognitive impairments that persist despite subsequent VLS (Heaton et al., 2010; Heaton et al., 2011; Muñoz-Moreno et al., 2008). Thus, HIV+/METH persons may be at greatest risk of cognitive impairment. Research underscores the combined, deleterious effects of HIV and METH on cognition, with HIV+/METH persons presenting with deficits including impaired memory, processing speed, and verbal fluency (Chana et al., 2006; Gupta et al., 2011; Soontornniyomkij et al., 2016). However, it is unclear whether the deleterious effects of HIV and METH on cognition are additive or synergistic in nature (Norman & Basso, 2015). Differentiating between the additive versus synergistic HIV and METH effects on cognition would aid clinicians in selecting appropriate treatments (Brew & McArthur, 2019).
One cognitive function disrupted in HIV+/METH persons is sustained attention and vigilance (i.e., the ability to maintain concentration and consistently respond to stimuli; Levine et al., 2008; Moran et al., 2014; Moran et al., 2019; Morgan et al., 2014; Potvin et al., 2018). Sustained attention and vigilance underlie other cognitive functions including memory and learning (Fortenbaugh et al., 2017), and are integral to everyday functioning (e.g., driving; Tabibi et al., 2015) and retention in care (Anderson et al., 2019; Hinkin et al., 2002). Thus, understanding sustained attention and vigilance deficits among HIV+/METH persons may lead to the development of strategies to increase daily functioning and healthcare engagement.
Research has yet to determine how METH affects sustained attention and vigilance in PWH. Rippeth et al., (2004) found HIV+/METH persons performed worse on a combined attention/ working memory task, relative to healthy controls. This study provides important insights into the effects of METH on cognition among PWH; however, given attention/ working memory was assessed as a single domain, it is not possible to determine whether observed deficits were attributable to impairments in attention, working memory, or both cognitive domains. Using Conners’ continuous performance test (CPT; Conners et al., 2000), Levine et al. (2006) found that cocaine and amphetamine were associated with poorer sustained attention and vigilance, among PWH. The absence of an HIV- group in Levine et al. (2006) precludes inferences regarding the additive versus synergistic effects of HIV and stimulants. Further, although a valid and reliable measure (Egeland & Kovalik-Gran, 2010), Conners’ CPT has not been adapted for non-human use, precluding the utilization of preclinical studies to determine mechanistic underpinnings of observed deficits (Young et al., 2009).
Research is needed to determine the nature of HIV and METH effects on sustained attention, using a validated, cross-species measure such as the 5-choice CPT (5C-CPT; Barnes et al., 2012; MacQueen et al., 2018b; Young et al., 2009; Young et al., 2013; Young et al., 2017; Young et al., 2019; Young et al., 2020). Thus, this study evaluated the additive versus synergistic effects of HIV and METH on sustained attention and vigilance, controlling for VLS. It was hypothesized that HIV and METH would have a synergistic deleterious effect on sustained attention and vigilance in PWH.
2. Material and methods
2.1. Participants
Participants were drawn from the Translational Methamphetamine AIDS Research Center (TMARC), an interdisciplinary research program examining HIV and METH effects on neurocognition. This study used data from 205 participants who completed Conners’ and 5C-CPT (18–87 years; see Table 1 for participant characteristics). Most PWH were male (n=97, 95%), consistent with the gender distribution of HIV infection in California (California Department of Public Health, 2019).
Table 1.
Sample characteristics
| HIV-/METH-(n = 67; A) | HIV+/METH-(n = 49; B) | HIV-/METH+(n = 36; C) | HIV+/METH+(n = 53; D) | Group Differences | |
|---|---|---|---|---|---|
| Age | 44.60 (16.27) | 47.27 (14.70) | 43.28 (12.26) | 40.85 (8.45) | A B > C D |
| Gender (% males; ref) | 41 (61%) | 45 (92%) | 20 (56%) | 52 (98%) | A C < B D |
| Education | 14.69 (1.80) | 14.61(2.33) | 12.75(3.07) | 13.98 (2.27) | A B > C D |
| Ethnicity | |||||
| % White (ref) | 37 (55%) | 27 (55%) | 19 (53%) | 31 (59%) | - |
| % Black | 8 (12%) | 9 (18%) | 6 (17%) | 5 (9%) | - |
| % Hispanic | 18 (27%) | 9 (18%) | 8 (22%) | 15 (28%) | - |
| % Othera | 4 (6%) | 4 (8%) | 3 (8%) | 2 (4%) | - |
| Mental Healthb | |||||
| ASPD | 3 (5%) | 2 (4%) | 11 (31%) | 9 (17%) | AB<CD |
| ADHD (Current) | 1 (2%) | 0 | 1 (3%) | 1 (2%) | - |
| ADHD(Lifetime) | 1 (2%) | 3 (6%) | 4 (11%) | 3 (6%) | - |
| Current nicotine dependence | 2 (3%) | 0 | 3 (8%) | 6 (11%) | AB<CD |
| Previous dependence history (>12 months ago)c | |||||
| Alcohol | 3 (5%) | 6 (12%) | 12 (33%) | 12 (23%) | AB<CD |
| Cannabis | 2 (3%) | 2 (4%) | 3 (8%) | 7 (13%) | - |
| Cocaine | 1 (2%) | 3 (6%) | 6 (17%) | 7 (13%) | AB<CD |
| Opioid | 1 (2%) | 0 | 5 (14%) | 2 (4%) | A B < C D |
| Positive urine toxicology | |||||
| Meth | 0 | 0 | 5 (14%) | 11 (21%) | - |
| THC | 2 (3%) | 7 (14%) | 2 (6%) | 5 (9%) | B D > A C |
| Amphetamine | 0 | 1 (2%) | 4 (11%) | 11 (20%) | A B < C D |
| Opiates | 0 | 5 (10%) | 0 | 0 | B > A C D |
| Benzodiazepines | 0 | 2 (4%) | 2 (6%) | 3 (6%) | - |
| Methamphetamine | |||||
| Characteristics | |||||
| Current METH+ | - | - | 7 (19%) | 8 (15%) | - |
| Ever used Meth | 2 | 6 | 36 | 53 | A B<C D |
| Age at first use | 32.50 (3.54) | 28.67 (7.29) | 26.08 (13.23) | 24.92 (7.89) | - |
| Days since use | 5387.29 (7360.52) | 1327.04 (1966.95) | 162.97 (366.73) | 139.40 (160.67) | A B > C D |
| Cumulative duration (days) | 176.00 (120.21) | 182.80 (188.30) | 2980.64 (2753.166) | 2073.61 (1885.19) | A B < C D |
| Cumulative quantity (grams) | 916.29 (1286.81) | 75.02 (141.99) | 4568.91 (8041.65) | 2668.86 (4406.01) | A B < C D |
| Current IV use | - | - | 3 (8.3%) | 7 (13%) | - |
| HIV characteristics | |||||
| Currently on ART | - | 44 (90%) | - | 46 (87%) | - |
| Virally suppressed | - | 43 (88%) | - | 35 (66%) | B > D |
| Nadir CD4 | - | 319.84 (220.809) | - | 311.60 (190.539) | - |
Note. Unless otherwise stated, binary variables are coded 0 = no, 1 = yes. HIV = Human Immunodeficiency Virus. METH = History of methamphetamine dependence. ASPD = Antisocial personality disorder. ADHD = Attention-deficit/hyperactivity disorder. THC = THC = Δ−9 Tetrahydrocannabinol. IV = intravenous.
Asian merged with “other” for multinominal logistic regression due to sparsity of category
Assessed using the Diagnostic Interview Schedule for DSM-IV
Participants with past 12-month substance dependence were excluded from participating in the current study
2.2. Procedures
Participants were recruited from the community, HIV clinics, and substance treatment programs in San Diego if they: (1) were aged ≥18 years; (2) met DSM-IV criteria for METH: (i) in the past 18 months, or (ii) in their lifetime, and met one DSM-IV abuse criterion in the past 18 months; or (3) had no history of METH use disorder. Participants who had a head injury with loss of consciousness >30 minutes, or a medical, serious psychiatric, or neurological condition not associated with HIV and linked to neurocognitive deficits (e.g., Hepatitis C infection), or who met past 12-month DSM-IV dependence criteria for another substance (excluding nicotine), were excluded.
Participants were assessed between 2014 and 2019. Participants provided informed consent, and demographic and substance use information. The Composite International Diagnostic Interview (CIDI) assessed substance use, while the Diagnostic Interview Schedule for DSM-IV (DIS) assessed mental health disorders. A blood test confirmed HIV status and plasma viral loads. Antiretroviral therapy use and nadir CD4 count information were also collected. Although participants were not instructed to abstain from substances, they completed a urine toxicology screen prior to cognitive testing. Participants were reimbursed for their time. Procedures were approved by the university Institutional Review Board.
2.3. Cognitive Tasks
Cognitive tasks were presented on a 56cm CRT Dell PC computer screen, using E-Prime2 software for stimulus presentation and data acquisition (Psychology Software Tools, 2012).
2.3.1. 5C-CPT
Participants responded using an arcade joystick. Trials comprised target (single circle) or non-target (five circles) stimuli that appeared for 100ms, behind an arc of 5 lines. Participants could respond for ≤1s after the stimuli disappeared (limited hold). Trials were separated by 0.5, 1, or 1.5s inter-trial interval (ITI), programmed in a quasi-random manner so the same ITI did not appear in >3 consecutive trials. Participants completed 12 practice trials (10 target, 2 non-target) to demonstrate understanding of instructions, before completing the task (225 target and 45 non-target trials).
Responding to target stimuli in the indicated direction was recorded as a hit. Inhibiting responses to non-target stimuli was a correct rejection. Failure to respond to a target was an omission, while responding to a location other than the circle was registered as incorrect. Failure to inhibit responding during non-target trials resulted in a false alarm (FA). Responding before stimuli appeared was a premature response (PR). Outcomes were calculated from these measures using signal detection theory based on hit rates (HR), FA rates (FAR), accordingly:
Responsivity index (RI) measured ‘tendency to respond’, where low numbers indicated a conservative and high numbers indicated a liberal response strategy (Frey & Colliver, 1973; Sahgal, 1987). d prime (primary outcome) measured sensitivity to appropriate responding. Accuracy (moving the joystick in the direction of the target), RI, HR, omissions, FA, reaction time (RT), variability in RT, and PR were examined as underlying drivers for performance (secondary outcomes).
2.3.2. Conners’ CPT-2
Participants were presented with letters (separated by an Inter-Stimulus Interval of 1, 2, or 4 seconds) and instructed to use the keyboard to respond to all letters except “X”, where they were instructed to withhold responses. Participants completed 18 blocks of 20 trials. The non-target stimulus (“X”) appeared on 10% of trials. Target trials were a hit (response) or omission (no response). Non-target trials were a false alarm (response) or correct rejection (non-response). Response latencies were recorded. d prime measured sensitivity to appropriate responding (primary outcome), while FA, omissions, and RT were examined as drivers of performance (secondary outcomes). ADHD confidence index, a composite measure that prospectively predicts ADHD diagnosis was examined (Breaux et al., 2016).
2.4. Statistical Analyses
Demographic, mental health, and substance use differences across all groups, antiretroviral therapy use, nadir CD4 count, and VLS across PWH, and convergence between the 5C-CPT and Conners’ CPT on d prime, omissions, and false alarms, were evaluated via regressions. Concurrent main (i.e., additive) and interactive (i.e., synergistic) HIV (HIV-/HIV+) and METH (METH-/METH+) effects on ove all sustained attention (d prime) and drivers of performance (e.g., omissions; examined regardless of d prime effects), were examined via forced entry regressions. Significant interactions were followed-up via ANOVA to examine group differences. Mixed effects linear regressions examined main and interactive effects of HIV and METH on vigilance, with performance split across three (5C-CPT) and six (Conners’ CPT) blocks. A random intercept modelled repeated measures, with trial block, HIV, and METH modelled as fixed effects. Changes in −2 log likelihood assessed model fit. The absence of a significant change in model fit resulted in the retention of the more parsimonious, lower-order model.
Continuous predictors were z-standardized and bootstrapping corrected any non-normality. Adjusted regressions controlled for age, sex, education, and VLS, given their effects on cognition (Heaton et al., 2015; Maki et al., 2018; Samson & Barnes, 2013; Satz et al., 2011). Separate regressions also controlled for current nicotine dependence, given the known effects of smoking on cognition (Conti et al., 2019). A Bonferroni alpha correction (i.e., eight comparisons in the secondary outcomes for the 5C-CPT, α = .05/8 = 0.006), was applied to all but d prime analyses, given this was the primary outcome. Data were analyzed using SPSS (v26.0).
3. Results
3.1. Data screening and group differences
Group differences are reported in Table 1. 5C-CPT d prime, omissions, and false alarms were positively associated with Conners’ CPT d prime (B=0.062, SE=0.029, 95%CI[0.003, 0.113]), omissions (B=8.891, SE=2.046, 95%CI[4.981, 12.601]), and false alarms (B=1.404, SE=3.805, 95%CI[1.062, 16.352]), respectively. Two participants (<1%) had missing data on VLS and were excluded from analyses, resulting in a final analytical sample of N=203. See supplemental tables 1 to 6 for unadjusted regressions. Given the inclusion of current nicotine dependence as a covariate did not substantially alter regression outcomes (see supplemental tables 7–12), the parsimonious model without current nicotine dependence is reported herein. Finally, 16 METH+ participants had a positive urine screen for methamphetamine, indicating recent methamphetamine use. Thus, analyses without these participants are also reported.
3.2. Overall effects of HIV and METH on the 5C-CPT
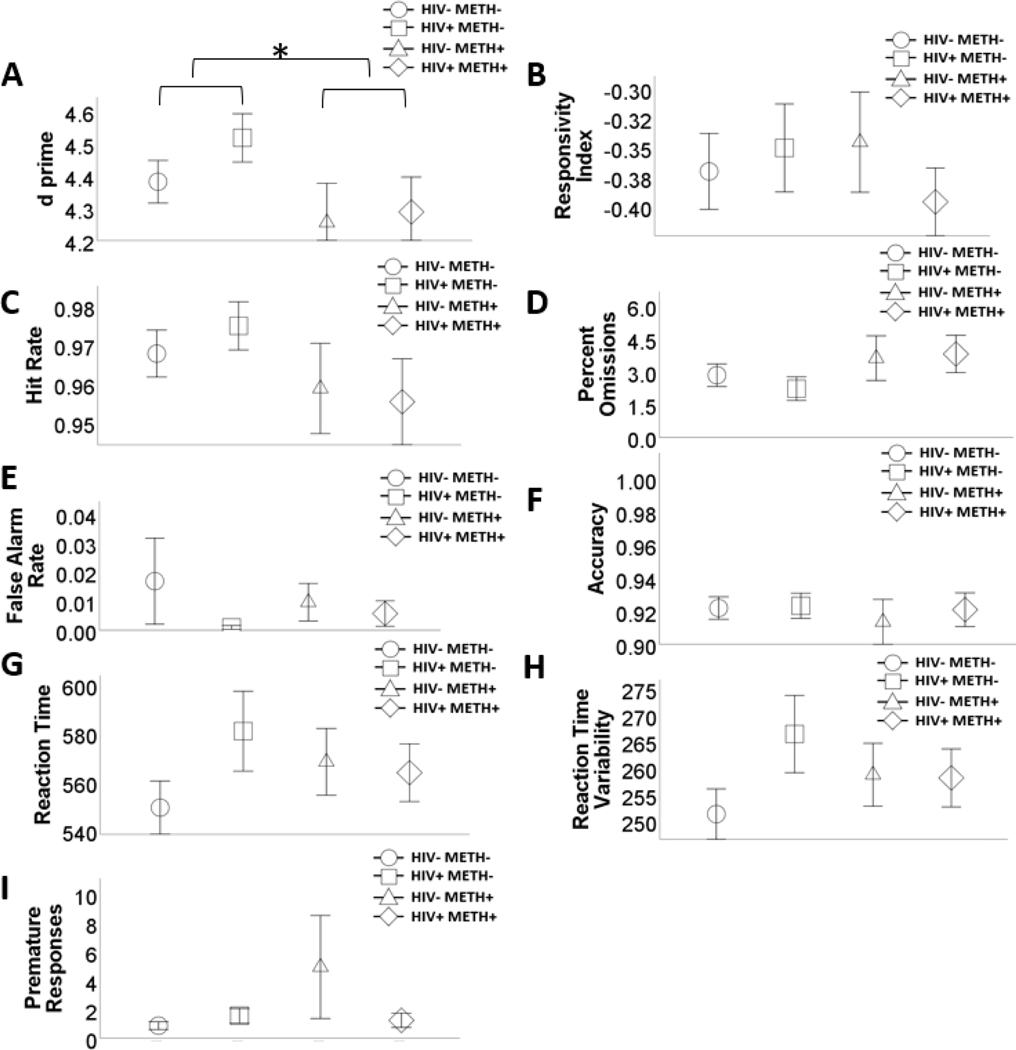
There were no HIV effects on any outcome (Table 2; Figure 1). METH+ was associated with lower d prime. There were no other METH or HIVxMETH effects.
Table 2.
Main and Interactive Effects of HIV and History of Methamphetamine Dependence on the 5C-CPT
| d prime | Responsivity Index | Hit Rate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | B | SE | 95% CI | B | SE | 95% CI | B | SE | 95% CI |
| Age | −0.081 | 0.048 | [−0.173, 0.017] | −0.02 | 0.018 | [−0.055, 0.017] | −0.007 | 0.005 | [−0.016, 0.002] |
| Gender | −0.315 | 0.111 | [−0.556, −0.109] | −0.029 | 0.050 | [−0.128, 0.070] | −0.008 | 0.010 | [−0.027, 0.010] |
| Education | −0.051 | 0.061 | [−0.173, 0.070] | −0.036 | 0.018 | [−0.073, 0.001] | −0.004 | 0.006 | [−0.017, 0.007] |
| VLS | 0.151 | 0.160 | [−0.165, 0.458] | 0.028 | 0.054 | [−0.082, 0.129] | 0.014 | 0.017 | [−0.017, 0.052] |
| HIV | 0.023 | 0.095 | [−0.169, 0.202] | −0.007 | 0.042 | [−0.090, 0.076] | 0.003 | 0.010 | [−0.016, 0.024] |
| METH | −0.219 | 0.100 | [−0.43, −0.025] | −0.034 | 0.038 | [−0.107, 0.041] | −0.018 | 0.010 | [−0.040, 0.002] |
| Model 2 | |||||||||
| HIVxMETH | −0.125 | 0.166 | [−0.456, 0.181] | −0.061 | 0.069 | [−0.199, 0.070] | −0.010 | 0.017 | [−0.046, 0.024] |
| Model 1 | Percent Omissions Overall | False Alarm Rate | Accuracy | ||||||
| Age | 0.625 | 0.380 | [−0.148, 1.383] | −0.003 | 0.004 | [−0.012, 0.004] | −0.003 | 0.005 | [−0.013, 0.007] |
| Gender | 0.623 | 0.827 | [−1.038, 2.215] | −0.008 | 0.015 | [−0.044, 0.014] | −0.037 | 0.012 | [−0.062, −0.013]† |
| Education | 0.310 | 0.464 | [−0.554, 1.281] | −0.003 | 0.005 | [−0.013, 0.006] | 0.002 | 0.007 | [−0.012, 0.014] |
| VLS | −1.381 | 1.427 | [−4.492, 1.138] | 0.003 | 0.005 | [−0.004, 0.014] | −0.011 | 0.012 | [−0.035, 0.013] |
| HIV | −0.407 | 0.858 | [−2.202, 1.194] | −0.012 | 0.013 | [−0.044, 0.005] | −0.012 | 0.009 | [−0.032, 0.005] |
| METH | 1.417 | 0.786 | [−0.103, 3.021] | −0.003 | 0.011 | [−0.029, 0.013] | −0.007 | 0.011 | [−0.028, 0.014] |
| Model 2 | |||||||||
| HIVxMETH | 0.751 | 1.488 | [−2.561, 3.354] | 0.013 | 0.015 | [−0.011, 0.047] | −0.003 | 0.018 | [−0.039, 0.034] |
| Model 1 | Reaction Time | Variability in RT | Premature Responses | ||||||
| Age | 32.348 | 6.821 | [18.367, 45.442] | 14.058 | 2.919 | [8.079, 19.78] | 0.913 | 0.862 | [−0.323, 2.915] |
| Gender | 27.172 | 16.094 | [−4.503, 59.139] | 9.380 | 7.205 | [−4.522, 23.772] | 2.678 | 2.121 | [−0.247, 7.428] |
| Education | −5.017 | 6.094 | [−17.027, 6.569] | −2.548 | 2.921 | [−8.681, 3.107] | −1.229 | 1.225 | [−4.124, 0.414] |
| VLS | −6.574 | 25.107 | [−55.877, 40.452] | −2.188 | 10.849 | [−24.827, 17.636] | 1.613 | 0.709 | [0.293, 3.052]† |
| HIV | 24.917 | 15.06 | [−5.390, 54.275] | 11.557 | 6.499 | [−0.807, 24.223] | 0.328 | 0.783 | [−1.542, 1.665] |
| METH | 7.168 | 12.91 | [−18.252, 33.525] | 2.173 | 5.957 | [−9.214, 14.164] | 1.623 | 1.398 | [−0.528, 4.927] |
| Model 2 | |||||||||
| HIVxMETH | −19.096 | 26.171 | [−71.094, 31.514] | −8.317 | 11.634 | [−31.052, 13.541] | −2.892 | 2.642 | [−9.162, 0.685] |
Note. HIV = human immunodeficiency virus. METH = methamphetamine dependence. VLS = Viral load suppression. Significant effects following bonferroni adjustment (p=.006) are bolded.
Significant at p<.05
Figure 1.
Effects of human immunodeficiency virus (HIV) and history of methamphetamine dependence (METH) on 5C-CPT performance.
N = 203. METH+ had lower d prime, relative to METH- people (A). There were no main or interactive effects of HIV and METH on any of the other outcome variables (B – I). Data are presented as means, with error bars representing standard error of the mean. Significant differences are denoted with an asterisk. Corresponding regression estimates, standard errors, and bootstrapped 95% confidence intervals presented in Table 2.
3.2.1. 5C-CPT Analyses excluding participants with a positive urine screen for methamphetamine
There were no significant main or interactive effects of METH or HIV on any outcomes (Supplemental Table 13).
3.3. Effects of HIV and METH, aggregated across trial blocks on the 5C-CPT
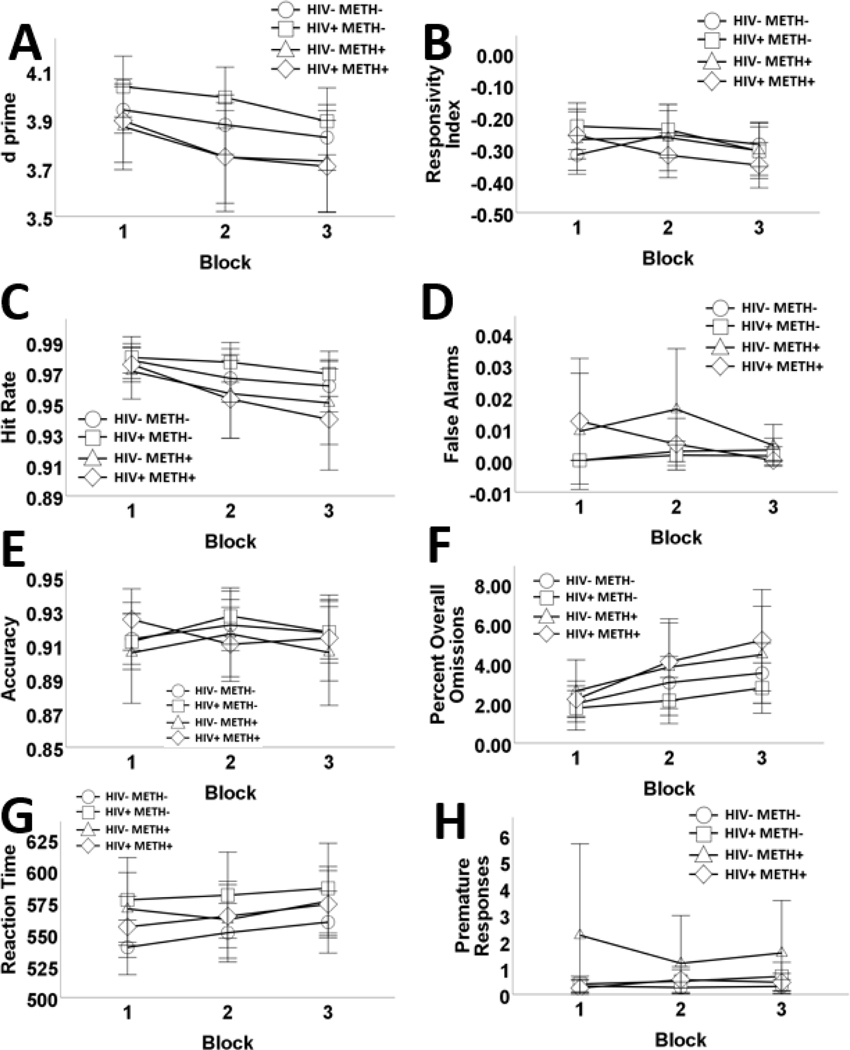
The main effects model (Table 3 and Figure 2) had better fit, relative to a two-way interaction model (Table 3). There was a significant effect of trial block on d prime, driven by reduced HR, and higher omissions. Responses slowed across trials. There were no other significant block effects. VLS was associated with higher d prime and premature responses. PWH exhibited less accuracy and slower RT, compared to HIV- participants. There were no other HIV effects. METH+ was associated with worse d prime, driven by lower HR and more omissions. METH was not associated with accuracy or RT.
Table 3.
−2LL for the Main Effects and Interaction Models Tested for the 5C-CPT
| Model 1 | Model 2 | χ2 | p | |
|---|---|---|---|---|
| d prime | 652.333 | 650.851 | 1.482 | .686 |
| Responsivity Index | 64.725 | 58.413 | 6.312 | .097 |
| Hit Rate | −1884.082 | −1889.028 | 4.946 | .176 |
| False Alarm Rate | −2626.580 | −2633.939 | 7.359 | .061 |
| Accuracy | −1912.428 | −1915.171 | 2.743 | .433 |
| Percent overall | 3515.696 | 3511.669 | 4.027 | .259 |
| Omissions | ||||
| Reaction Time | 6675.166 | 6674.505 | 0.661 | .882 |
| Premature Responses | 2735.521 | 2730.309 | 5.212 | .157 |
Note: Model 1 = Main effects of block, HIV, and history of methamphetamine dependence; Model 2 = 2-way interactions between block, HIV, and history of methamphetamine dependence. Degrees of freedom for model 1 = 10, model 2 = 13.
Figure 2.
Effects of human immunodeficiency virus (HIV) and history of methamphetamine dependence (METH) on 5C-CPT performance across trial blocks. N = 203. Overall performance on the 5C-CPT decreased across blocks. METH+ participants exhibited poorer 5C-CPT performance relative to METH- participants, irrespective of HIV status, as measured by d prime (A). METH+ participants had significantly poorer responsivity index, compared to METH- participants, however, this was not significant following Bonferroni correction (B). Impaired responding to targets was more exaggerated as time progressed and among METH+ participants, compared to METH- participants (C). False alarm rates did not differ across blocks, or according to HIV. METH+ participants had more false alarms compared to METH- participants, however, this effect was not significant follow Bonferroni correction (D). HIV+ had lower accuracy, compared to HIV-negative people (E). The number of omissions significantly increased across trial blocks. METH+ participants had significantly elevated misses to targets (% omission; F). Reaction time slowed over trial blocks and HIV+ had significantly slower reaction time, relative to HIV- subjects (G). Finally, premature responses did not differ across blocks, or according to HIV. METH+ participants had significantly more premature responses, however, this effect was not significant following Bonferroni correction (H). Data are presented as means, with error bars representing standard error of the mean. Corresponding regression estimates, standard errors, and bootstrapped 95% confidence intervals presented in Table 4.
3.3.1. 5C-CPT by block analyses excluding participants with a positive urine screen for methamphetamine
The main effects model had better fit, relative to a two-way interaction model (Supplemental Table 14). METH+ was not significantly associated with any outcomes, while PWH had slower reaction time, compared to HIV- participants (Supplemental Table 15). HIV was not significantly associated with accuracy.
3.4. Overall effects of HIV and METH on Conners’ CPT
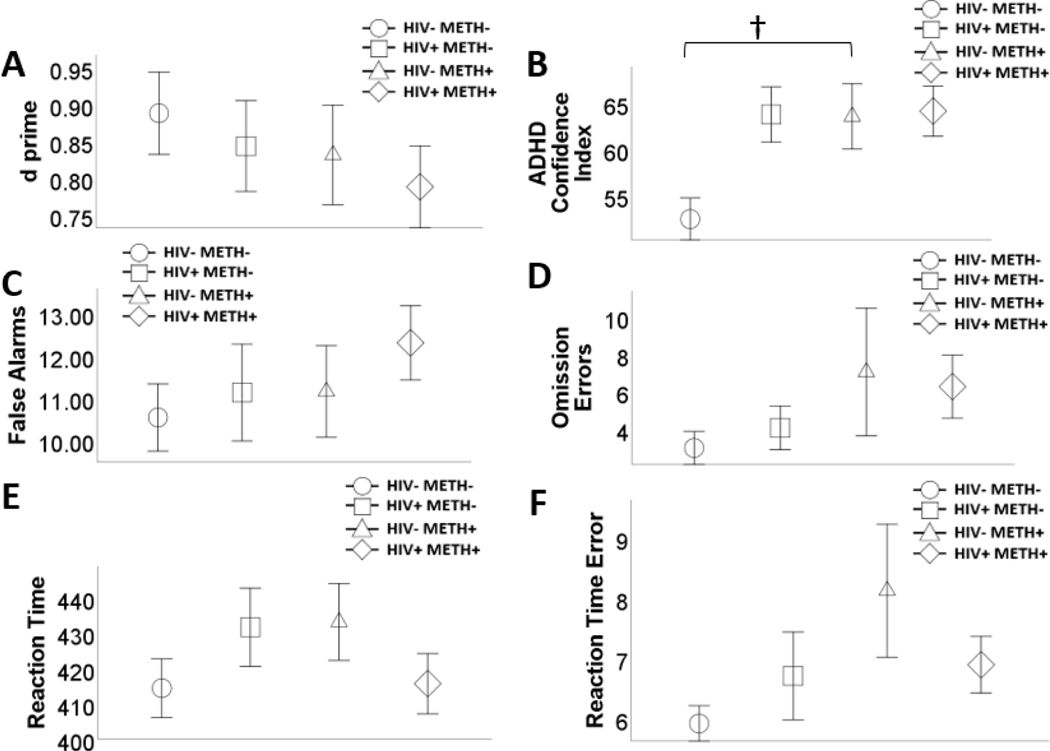
There were no significant effects of HIV or METH on any outcomes (see Table 5 and Figure 3). There was a significant HIVxMETH interaction on ADHD confidence index (prior to Bonferroni adjustment). HIV-/METH+ (M=67.750, SE=3.205) participants had a higher ADHD confidence index than HIV-/METH- (M=54.142, SE=2.335) participants (Meandiff=13.608, SE=3.879, 95%CI[6.044, 22.167]). There was no significant difference between HIV+/METH+ (M=62.938, SE=2.780) and HIV+/METH- (M=59.939, SE=2.647) on ADHD confidence index (Meandiff=2.998, SE=3.555, 95%CI[−4.410, 9.749]). There were no other significant interactions.
Table 4.
Main Effects of Block, HIV, and History of Methamphetamine Dependence on the 5C-CPT
| d prime | Responsivity Index | Hit Rate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE | 95% CI | B | SE | 95% CI | B | SE | 95% CI | |
| Age | −0.069 | 0.016 | [−0.099, −0.039] | −0.009 | 0.010 | [−0.028, 0.010] | −0.007 | 0.002 | [−0.011, −0.003] |
| Gender | −0204 | 0.036 | [−0.274, −0.129] | −0.064 | 0.025 | [−0.110, −0.016] | −0.009 | 0.004 | [−0.017, −0.001]† |
| Education | −0.033 | 0.018 | [−0.065, 0.003] | −0.031 | 0.009 | [−0.05, −0.012] | −0.004 | 0.003 | [−0.009, 0.001] |
| VLS | 0.136 | 0.050 | [0.027, 0.240] | 0.039 | 0.028 | [−0.015, 0.093] | 0.014 | 0.007 | [0.001, 0.027]† |
| Block | −0.072 | 0.017 | [−0.106, −0.037] | −0.019 | 0.012 | [−0.041, 0.002] | −0.010 | 0.02 | [−0.015, −0.006] |
| HIV | 0.016 | 0.032 | [−0.047, 0.079] | −0.005 | 0.020 | [−0.042, 0.029] | 0.003 | 0.004 | [−0.004, 0.011] |
| METH | −0.173 | 0.03 | [−0.228, −0.112] | −0.039 | 0.019 | [−0.076, −0.001]† | −0.018 | 0.004 | [−0.025, −0.009] |
| False Alarms | Accuracy | Percent overall Omissions | |||||||
| Age | 0.001 | 0.001 | [−0.001, 0.002] | −0.003 | 0.002 | [−0.007, 0.001] | 0.626 | 0.159 | [0.317, 0.924] |
| Gender | 0.007 | 0.002 | [0.002, 0.011]† | −0.037 | 0.005 | [−0.046, −0.028] | 0.615 | 0.373 | [−0.107, 1.379] |
| Education | 0.000 | 0.002 | [−0.004,0.004] | 0.002 | 0.002 | [−0.003, 0.007] | 0.310 | 0.199 | [−0.124, 0.671] |
| VLS | 0.003 | 0.003 | [−0.002, 0.009] | −0.011 | 0.006 | [−0.022, 0.001] | −1.385 | 0.616 | [−2.583, −0.088]† |
| Block | −0.001 | 0.001 | [−0.005, 0.002] | 0.000 | 0.002 | [−0.004, 0.004] | 0.907 | 0.190 | [0.568, 1.281] |
| HIV | 0.001 | 0.002 | [−0.003, 0.005] | −0.012 | 0.004 | [−0.018, −0.005] | −0.416 | 0.345 | [−1.016, 0.175] |
| METH | 0.007 | 0.003 | [0.001, 0.013]† | −0.007 | 0.004 | [−0.014, 0.001] | 1.422 | 0.327 | [0.688, 2.053] |
| Reaction Time | Premature Responses | ||||||||
| Age | 32.351 | 2.544 | [26.975, 37.558] | 0.304 | 0.108 | [−0.017, 0.52] | |||
| Gender | 27.126 | 5.125 | [16.443, 37.596] | 0.893 | 0.243 | [0.112, 1.369]† | |||
| Education | −4.909 | 2.067 | [−9.134, −0.432]† | −0.410 | 0.143 | [−0.625, −0.096]† | |||
| VLS | −6.140 | 7.700 | [−19.527, 9.748] | 0.538 | 0.099 | [0.315, 0.726] | |||
| Block | 7.191 | 2.318 | [2.351, 11.694] | 0.000 | 0.091 | [−0.188, 0.168] | |||
| HIV | 24.765 | 4.891 | [15.298, 34.334] | 0.109 | 0.109 | [−0.097, 0.313] | |||
| METH | 7.505 | 4.242 | [−1.243, 16.52] | 0.541 | 0.171 | [0.047, 0.868]† | |||
Note. HIV = human immunodeficiency virus. METH = methamphetamine dependence. VLS = Viral load suppression. Significant effects following bonferroni adjustment (p=.006) are bolded.
Significant at p=.05
Figure 3.
Effects of human immunodeficiency virus (HIV) and history of methamphetamine dependence (METH) on Conners’ CPT performance. N = 203. There was a significant HIV x METH interaction on attention-deficit/ hyperactivity disorder (ADHD) confidence index (prior to Bonferroni correction), such that among HIV-people, METH+ was associated with greater ADHD confidence index. METH+ was not associated with ADHD confidence index among people with HIV (A). There were no main or interactive effects of HIV and METH on any of the other outcome variables (A – F). Data are presented as means, with error bars representing standard error of the mean. Significant difference prior to Bonferroni correction (i.e., p<.05) is denoted with a †. Corresponding regression estimates, standard errors, and bootstrapped 95% confidence intervals presented in Table 5.
3.4.1. Conners’ CPT Analyses excluding participants with a positive urine screen for methamphetamine
There were no significant effects of METH+ or HIV on any outcome variables. There was a significant HIVxMETH interaction on ADHD confidence index (prior to Bonferroni adjustment). HIV-/METH+ (M=65.472, SE=1.309) participants had a higher ADHD confidence index than HIV-/METH- (M=54.459, SE=0.899) participants (Meandiff=11.013, SE=1.693, 95%CI[7.634, 14.340]). There was no significant difference between HIV+/METH+ (M=59.390, SE=1.198) and HIV+/METH- (M=60.575, SE=1.034) on ADHD confidence index (Meandiff=−1.185, SE=1.425, 95%CI[−3.945, 1.681]; Supplemental Table 16).
3.5. Effects of HIV and METH, aggregated across trial blocks on Conners’ CPT
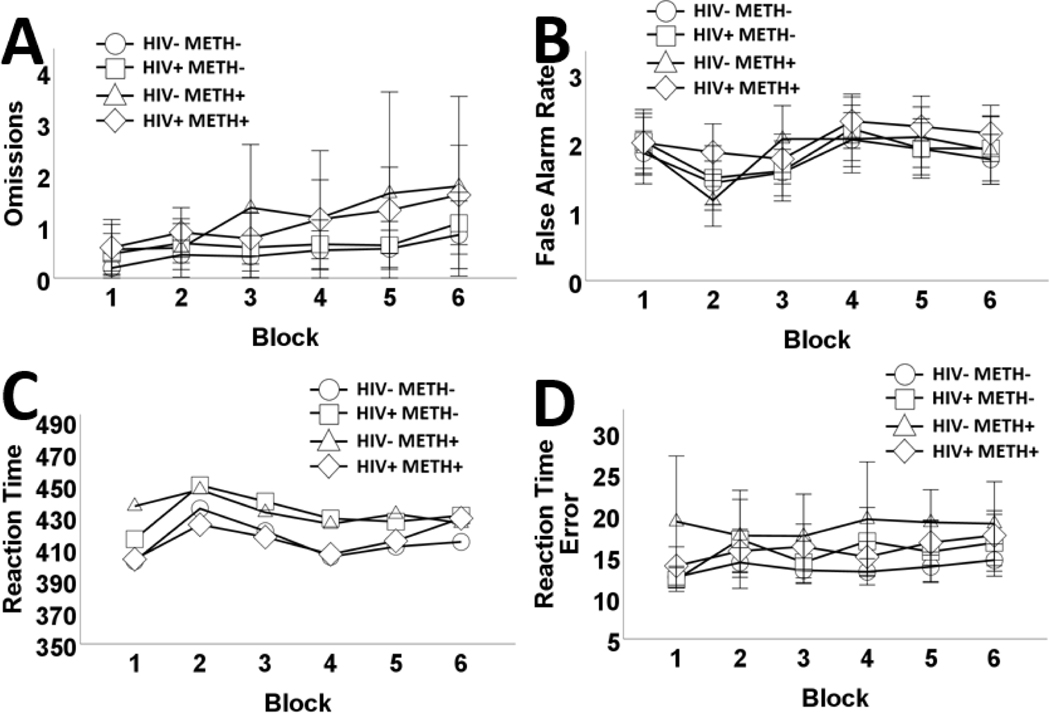
The main effects model (Table 7; Figure 4) had better fit relative to a two-way interaction model (see Table 6). Omissions, FAs, and RT error significantly increased across blocks. RT remained stable. VLS was associated with less omissions. HIV was only associated with slower RT. METH+ participants had more omissions, RT error, and slower reaction time, relative to METH- participants. METH+ was not associated with FA.
Table 6.
−2LL for the Main Effects and Interaction Models Tested for Conners’ CPT
| Model 1 | Model 2 | χ2 | p | |
|---|---|---|---|---|
| Omissions | 4976.467 | 4969.703 | 6.764 | .080 |
| False Alarm Rate | 3926.818 | 3926.126 | 0.692 | .875 |
| Reaction Time | 12839.189 | 12832.874 | 6.315 | .097 |
| Reaction Time Error | 8555.174 | 8549.930 | 5.244 | .155 |
Note: Model 1 = Main effects of block, HIV, and history of methamphetamine dependence; Model 2 = 2-way interactions between block, HIV, and history of methamphetamine dependence. Degrees of freedom for model 1 = 10, model 2 = 13.
Figure 4.
Effects of human immunodeficiency virus (HIV) and history of methamphetamine dependence (METH) on Conners’ CPT performance across trial blocks..
N = 203. Omissions increased across blocks. METH+ participants exhibited greater omissions, relative to METH- participants (A). False alarm rates also increased across blocks; however, false alarm rates did not differ according to HIV or METH status (B). HIV+ had significantly slower reaction time compared to HIV- subjects. Similarly, METH+ had significantly slower reaction time compared to METH- participants (C). Reaction time error increased across blocks. METH+ participants exhibited greater RT error, than METH- participants (D). Data are presented as means, with error bars representing standard error of the mean. Corresponding regression estimates, standard errors, and bootstrapped 95% confidence intervals presented in Table 7.
Table 5.
Main and Interactive Effects of HIV and History of Methamphetamine Dependence on Conners’ CPT
| d prime | ADHD Confidence Index | False Alarms | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | B | SE | 95% CI | B | SE | 95% CI | B | SE | 95% CI |
| Age | 0.055 | 0.030 | [−0.006, 0.114] | 6.871 | 1.33 | [4.274, 9.350] | −1.245 | 0.495 | [−2.267, −0.316] |
| Gender | 0.112 | 0.076 | [−0.043, 0.255] | −13.126 | 3.396 | [−20.108, −6.178] | −2.190 | 1.124 | [−4.382, 0.089] |
| Education | 0.020 | 0.031 | [−0.044, 0.079] | 0.928 | 1.411 | [−2.037, 3.487] | −0.323 | 0.535 | [−1.282, 0.812] |
| VLS | −0.026 | 0.102 | [−0.242, 0.164] | 0.092 | 4.675 | [−9.946, 8.915] | 0.680 | 1.524 | [−2.425, 3.552] |
| HIV | −0.015 | 0.070 | [−0.147, 0.126] | 1.741 | 2.988 | [−4.526, 7.371] | 0.276 | 1.128 | [−2.039, 2.398] |
| METH | −0.030 | 0.062 | [−0.147, 0.091] | 8.133 | 2.712 | [2.878, 13.529]† | 0.404 | 1.074 | [−1.654, 2.524] |
| Model 2 | |||||||||
| HIVxMETH | 0.022 | 0.123 | [−0.224, 0.259] | −10.610 | 5.286 | [−21.542, −1.050]† | 0.098 | 2.001 | [−3.802, 4.083] |
| Model 1 | Omission Errors | Reaction Time | Reaction Time Error | ||||||
| Age | 0.892 | 0.738 | [−0.515, 2.444] | 25.215 | 4.351 | [16.680, 33.620] | 0.473 | 0.232 | [0.003, 0.903]† |
| Gender | −2.398 | 2.170 | [−7.168, 1.152] | 12.836 | 11.829 | [−11.074, 37.625] | −0325 | 0.693 | [−1.785, 0.916] |
| Education | −0.448 | 1.086 | [−2.614, 1.589] | −3.702 | 4.965 | [−14.067, 5.185] | −0.038 | 0.315 | [−0.613, 0.630] |
| VLS | −3.255 | 3.102 | [−9.429, 2.421] | 3.105 | 15.591 | [−26.314, 33.667] | 0.033 | 0.987 | [−1.951, 1.876] |
| HIV | −1.164 | 2.263 | [−6.118, 2.595] | 7.919 | 10.467 | [−12.425, 29.801] | −0.184 | 0.767 | [−1.741, 1.263] |
| METH | 2.827 | 1.971 | [−0.569, 7.193] | 7.087 | 9.928 | [−12.372, 26.677] | 1.293 | 0.632 | [0.127, 2.570]† |
| Model 2 | |||||||||
| HIVxMETH | −2.304 | 3.952 | [−10.777, 4.530] | −21.667 | 19.296 | [−59.462, 18.401] | −1.962 | 1.501 | [−5.218, .760] |
Note. HIV = Human immunodeficiency virus. METH = methamphetamine dependence. VLS = Viral load suppression. Significant effects following bonferroni adjustment (p=.006) are bolded.
Significant at p=.05
3.5.1. Conners’ CPT by block analyses excluding participants with a positive urine screen for methamphetamine
The main effects model had better fit than a two-way interaction model (Supplemental Table 17). METH+ was not significantly associated with any outcomes. HIV+ was only associated with slower RT (Supplemental Table 18).
4. Discussion
This study examined the additive versus synergistic effects of HIV and METH on sustained attention and vigilance. METH, but not HIV, was associated with sustained attention and vigilance deficits. Contrary to hypotheses, there were no synergistic HIV and METH effects. METH was associated with poorer sustained attention and vigilance, driven by lower hit rate and more target omissions. METH was associated with slower reaction time and more reaction time error. METH effects disappeared after removing participants with a positive urine screen for METH, highlighting the role of recent methamphetamine use in this relationship.
Contrary to hypotheses, PWH did not have worse sustained attention and vigilance relative to HIV- participants, displaying only a decrement in accuracy which disappeared after removing recent methamphetamine users. PWH displayed reaction time slowing. HIV-/METH participants had a higher ADHD confidence index, relative to HIV- participants without METH (prior to Bonferroni correction). Both reaction time slowing and ADHD confidence index effects persisted after removing recent methamphetamine users.
Recent methamphetamine use among METH people, rather than METH alone, was associated with sustained attention and vigilance deficits. This result extends Levine et al. (2006), highlighting sustained attention and vigilance deficits with recent stimulant use, irrespective of HIV. Results also extend Basterfield et al. (2019), who found rebound effects for certain cognitive domains among recently abstinent METH people. Further research should examine what aspect of recent use (acute intoxication, dosage, residual effects, or withdrawal) is associated with impairment and the direction of this relationship. For instance, METH people with greater sustained attention and vigilance deficits may have used methamphetamine recently (e.g., to self-medicate); alternatively, recent methamphetamine use among METH people may confer sustained attention and vigilance deficits. These studies are important given amphetamine administration improves 5C-CPT performance in healthy humans, mice (MacQueen et al, 2018b), and rats (Young et al, 2020). Chronicity of methamphetamine use may obviate the benefits of acute effects.
Although the lack of METH effects following the removal of recent methamphetamine users are at odds with Potvin et al., (2018), it should be noted that the studies included in that meta-analysis differed on methamphetamine use recency. Some studies required participants to have a positive urine screen for methamphetamine during cognitive testing, whereas others included recently abstinent METH participants. Since Potvin et al., (2018) did not control for methamphetamine use recency, it is difficult to disentangle whether METH alone, or recent methamphetamine use among METH people (as found in this study), affected cognition.
Several reasons may account for the lack of synergistic HIV and METH effects on sustained attention and vigilance. METH may not have pre-dated HIV seroconversion. Thus, HIV diagnosis (and antiretroviral therapy initiation) may not have been delayed, sparing this group from enduring brain injury and cognitive impairment. This notion is supported by Montoya et al. (2016) who found 20% of HIV+/METH persons initiated methamphetamine use after HIV diagnosis. Alternatively, the lack of synergistic HIVxMETH effects may indicate that HIV+/METH persons are no more susceptible than HIV+/METH- persons, to early immunosuppression and enduring cognitive impairment. Indeed, our results found no difference in nadir CD4 count between the two HIV groups. Further research should explore risk factors associated with early immunosuppression in PWH.
Importantly, HIV-/METH participants had a higher ADHD confidence index than HIV-/METH- persons, a group difference not found using the DIS for DSM-IV, which relies on self-report of symptoms. This finding may underscore the sensitivity of Conners’ CPT ADHD confidence index in capturing elevated rates of ADHD among methamphetamine users, noted in previous studies (Bordoloi et al., 2019). Further research should aim to replicate these results, which were not significant following Bonferroni correction and thus, should be interpreted with caution.
HIV was only consistently associated with psychomotor slowing. This finding may indicate that the current sample of PWH had less cognitive impairment relative to other virally suppressed PWH (Sanford et al., 2018). This discrepancy in findings may be attributable to the high nadir CD4 counts among PWH in the current study, reflecting on average, the absence of previous immunosuppression. Alternatively, these results may indicate that while untreated HIV has an irreversible, detrimental impact on psychomotor and some cognitive functions (Muñoz-Moreno et al., 2008), sustained attention and vigilance may be spared from enduring deficits. Results may also indicate that while antiretroviral therapy has pro-cognitive effects for some domains, it has little effect on psychomotor function. Both notions are supported by Eggers et al. (2017) who posited psychomotor slowing to be a salient feature of HIV-associated neurocognitive disorders. Alternatively, psychomotor slowing may be indicative of a speed/ accuracy trade-off, whereby PWH compensate for cognitive impairment by slowing responding to prioritize accuracy, as found previously (Kronemer et al., 2017). Animal models are well-positioned to test these hypotheses.
4.1. Implications
Current findings highlight the importance of VLS on cognition among PWH, reiterating the need for prompt HIV diagnosis and antiretroviral therapy initiation/maintenance. Routine opt-out screening may be a cost-effective means of increasing prompt HIV diagnosis (Krueger et al., 2019; Sanders et al., 2005). Peer-to-peer social support increases antiretroviral therapy adherence among substance-using and non-using PWH (Horvath et al., 2013; Hosek et al., 2018; Kerrigan et al., 2018). Clinicians should also treat METH to mitigate cognitive impairments associated with recurrent use, which may indirectly lead to improvements to daily functioning and increase retention in HIV care, given the known link between sustained attention and vigilance and these outcomes (Anderson et al., 2019; Hinkin et al., 2002; Tabibi et al., 2015). Cognitive behavioral therapy and contingency management have some efficacy in treating METH (Lee & Rawson, 2008; Rawson et al., 2006; Roll, 2007). Such suggestions, while not novel, are strongly supported by the current data.
Significant associations were observed between the 5-Choice and Conners’ CPTs. Both CPTs captured vigilance deficits among METH persons and slowed reaction time in PWH. These findings reiterate the validity and provide further psychometric support for 5C-CPT as a measure of sustained attention and vigilance. Using 5C-CPT allows for comparisons with future animal research aimed at delineating mechanisms underpinning the deficits seen in this study.
4.2. Strengths and limitations
We controlled for age, sex, education, and VLS, which are associated with cognitive function. We excluded people with current substance dependence (excluding nicotine, which was controlled for in supplemental regressions reported in tables 7–12), a history of head injuries, medical, psychotic, or neurological conditions known to impact cognition (Cunha et al., 2013; Perry et al., 2008; Senathi-Raja et al., 2010; Stavro et al., 2013). Thus, participant performance was unlikely confounded by these factors. Another strength was the use of a urine screen which allowed us to examine the effects of METH on sustained attention and vigilance, with and without recent methamphetamine users.
This study has some limitations. Although we controlled for education, this reflects only one aspect of a multifaceted cognitive reserve construct, which buffers against HIV-associated neurocognitive disorders in PWH (Morgan et al., 2012). Subjects who reported past 12-month alcohol dependence were excluded from this study. However, 16% of all subjects reported having alcohol dependence at some point in their lifetime. While a history of alcohol dependence is associated with persistent impairment in some cognitive domains, research points to a rebound in attention among abstinent (≥1 year) alcohol-dependent persons, to levels in line with healthy controls (Le Berre et al., 2017; Stavro et al., 2013). 15% of participants in this study had a positive urine screen for substances other than methamphetamine – in particular, tetrahydrocannabinol, benzodiazepines – which may affect cognition. Polysubstance use is prevalent among METH persons (Kelly et al., 2017; Quinn et al., 2013), as are cannabis and benzodiazepine use among PWH (Dawson-Rose et al., 2017). Future research should examine how polysubstance use/dependence may affect sustained attention and vigilance among HIV+/METH persons.
Our sample was predominantly male (95%), limiting the generalizability of results to females with HIV. Given the known sex differences in cognitive function among PWH (Heaton et al., 2015; Maki et al., 2018), future research could examine potential interactions among HIV, METH, and sex, within a more representative sample. Such studies can also be conducted in rodents. A majority of nadir CD4 values (94%) in the present study were self-reported. Given the known memory deficits associated with HIV (Walker & Brown, 2018), the reliability of self-reported nadir CD4 is questionable. Future research should obtain nadir CD4 information from other sources. Finally, given the prevalence of hepatitis C virus (HCV) co-infection among PWH (Buxton et al., 2010; Prussing et al., 2014) and given HCV exacerbates cognitive impairment among HIV+/METH persons (Cherner et al., 2005), future research should examine the effects of HCV coinfection on sustained attention and vigilance among HIV+/METH persons.
5. Conclusions
Controlling for VLS – which was associated with better vigilance – PWH did not exhibit sustained attention and vigilance deficits, but rather, exhibited only psychomotor slowing. Further research should examine whether psychomotor slowing reflects pervasive deficits despite VLS, or an adaptive, speed/ accuracy trade-off. METH was associated with deficits in sustained attention and vigilance. This effect was no longer significant after removing recent methamphetamine users, potentially suggesting a sustained attention and vigilance rebound among METH people, following abstinence. Further research should examine the direction of this relationship and identify what aspects of recent use are associated with deficits. These effects can be tested directly in animals. There were no combined HIV and METH effects on sustained attention and vigilance. Longitudinal research should examine whether onset of METH prior to HIV seroconversion is associated with worse cognitive outcomes. The 5C-CPT was found to be an appropriate measure of sustained attention and vigilance deficits in PWH and METH people. Using the 5C-CPT enables future comparisons with rodent research examining the underlying mechanisms driving deficits found in this study. Ultimately, results underscore the need for prompt HIV diagnosis and antiretroviral therapy initiation, and treatment of METH to preserve cognitive function.
Supplementary Material
Table 7.
Main Effects of Block, HIV, and History of Methamphetamine Dependence on Conners’ CPT
| Omissions | False Alarm Rate | Reaction Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | 95% CI | B | SE | 95% CI | B | SE | 95% CI | ||
| Age | 0.149 | 0.049 | [0.063, 0.246] | −0.207 | 0.032 | [−0.268, −0.141] | 25.258 | 1.076 | [23.332, 27.225] | |
| Gender | −0.400 | 0.122 | [−0.621, −0.179] | −0365 | 0.08 | [−0.525, −0.206] | 13.025 | 2.713 | [7.449, 18.507] | |
| Education | −0.075 | 0.052 | [−0.170, 0.024] | −0.054 | 0.033 | [−0.120, 0.012] | −3.500 | 1.164 | [−5.62, −1.294] | |
| VLS | −0.543 | 0.186 | [−0.903, −0.174] | 0.113 | 0.101 | [−0.086, 0.296] | 3.021 | 4.023 | [−4.895, 10.836] | |
| Block | 0.149 | 0.033 | [0.091, 0.208] | 0.054 | 0.017 | [0.019, 0.088] | −0.208 | 0.732 | [−1.661, 1.262] | |
| HIV | −0.194 | 0.104 | [−0.391, 0.011] | 0.046 | 0.069 | [−0.100, 0.193] | 7.995 | 2.568 | [3.224, 13.012] | |
| METH | 0.471 | 0.097 | [0.271, 0.669] | 0.067 | 0.064 | [−0.060, 0.199] | 7.053 | 2.339 | [2.348, 11.677] | |
| Reaction Time Error | ||||||||||
| Age | 1.15 | 0.167 | [0.822, 1.451] | |||||||
| Gender | −0.821 | 0.476 | [−1.687, 0.146] | |||||||
| Education | −0.125 | 0.210 | [−0.543, 0.272] | |||||||
| VLS | 0.199 | 0.616 | [−1.058, 1.466] | |||||||
| Block | 0.394 | 0.124 | [0.162, 0.623] | |||||||
| HIV | −0.397 | 0.456 | [−1.339, 0.580] | |||||||
| METH | 2.939 | 0.402 | [2.117, 3.731] | |||||||
Note. HIV = human immunodeficiency virus. METH = methamphetamine dependence. VLS = Viral load suppression. Significant effects following bonferroni adjustment (p=.006) are bolded.
Significant at p=.05
Highlights.
Methamphetamine dependence (METH+) impaired sustained attention and vigilance
METH+ was unassociated with impairments after removing recent methamphetamine users
Viral load suppression (VLS) was associated with better vigilance
Human immunodeficiency virus (HIV) was associated with psychomotor slowing
HIV was not associated with sustained attention and vigilance
Acknowledgements
We acknowledge Nathan Wood for his contribution to the project.
Funding Sources/Acknowledgments
This study was supported by P50 DA26306 and R01DA043535. Nina Pocuca is also supported by an Interdisciplinary Research Fellowship in NeuroAIDS (IRFN; R25MH081482). We acknowledge Nathan Wood for his contribution to the project.
Author Disclosures
Role of Funding Sources
This study was supported by P50 DA26306 and R01DA043535. Nina Pocuca is also supported by an Interdisciplinary Research Fellowship in NeuroAIDS (IRFN; R25MH081482). These funding sources had no role in study design, collection, analysis, or interpretation of data, writing the manuscript, and the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
No conflicts declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AN, Haardörfer R, Holstad MM, Nguyen MLT, & Waldrop-Valverde D. (2019). A Path Analysis of Patient and Social-Level Factors on Health Literacy and Retention in Care Among African Americans Living with HIV. AIDS and Behavior, 24, 1–9. doi: 10.1007/s10461-019-02699-y [DOI] [PubMed] [Google Scholar]
- Barnes SA, Young JW, & Neill JC (2012). Rats tested after a washout period from sub-chronic PCP administration exhibited impaired performance in the 5-Choice Continuous Performance Test (5C-CPT) when the attentional load was increased. Neuropharmacology, 62(3), 1432–1441. doi: 10.1016/j.neuropharm.2011.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni A, & Castellanos FX (2015). Stimulants, cognition and ADHD. Current Opinion in Behavioral Sciences, 4, 109–114. doi: 10.1016/j.cobeha.2015.04.010 [DOI] [Google Scholar]
- Basterfield C, Hester R, & Bowden SC (2019). A meta-analysis of the relationship between abstinence and neuropsychological functioning in methamphetamine use disorder. Neuropsychology, 33(5), 739–753. doi: 10.1037/neu0000552 [DOI] [PubMed] [Google Scholar]
- Bordoloi M, Chandrashekar G, & Yarasi N. (2019). ADHD in Adults and Its Relation with Methamphetamine Use: National Data. Current Developmental Disorders Reports, 6(4), 224–227. doi: 10.1007/s40474-019-00174-w [DOI] [Google Scholar]
- Breaux RP, Griffith SF, & Harvey EA (2016). Preschool neuropsychological measures as predictors of later attention deficit hyperactivity disorder. Journal of abnormal child psychology, 44(8), 1455–1471. Doi: 10.1007/s10802-016-0140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew BJ, & McArthur J. (2019). Distinguishing cognitive impairment from HIV-associated neurocognitive disorder versus substance use? Aids, 33(12), 1943–1944. doi: 10.1097/QAD.0000000000002292 [DOI] [PubMed] [Google Scholar]
- Buxton JA, Yu A, Kim PH, Spinelli JJ, Kuo M, Alvarez M, ... & Krajden M. (2010). HCV co-infection in HIV positive population in British Columbia, Canada. BMC public health, 10(1), 225 10.1186/14712458-10225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Department of Public Health (2019). The California HIV Surveillance Report. Retrieved from https://www.cdph.ca.gov/Programs/CID/DOA/CDPH%20Document%20Library/Californ
- ia%20HIV%20Surveillance%20Report%20-%202017.pdf
- Centers for Disease Control and Prevention (2018). HIV Surveillance Report (Updated), volume 31 Published May 2020. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, ... & Masliah E. (2006). Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology, 67(8), 1486–1489. [DOI] [PubMed] [Google Scholar]
- Cherner M, Letendre S, Heaton RK, Durelle J, Marquie-Beck J, Gragg B, ... & HIV Neurobehavioral Research Center Group. (2005). Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology, 64(8), 1343–1347. [DOI] [PubMed] [Google Scholar]
- Cohen JK, Santos G-M, Moss NJ, Coffin PO, Block N, & Klausner JD (2016). Regular clinic attendance in two large San Francisco HIV primary care settings. AIDS care, 28(5), 579–584. doi: 10.1080/09540121.2015.1118431 [DOI] [PubMed] [Google Scholar]
- Conners CK, Staff MHS, Connelly V, Campbell S, MacLean M, & Barnes J. (2000). Conners’ continuous performance Test II (CPT II v. 5). Multi-Health Syst Inc, 29, 175–196. [Google Scholar]
- Conti AA, McLean L, Tolomeo S, Steele JD, & Baldacchino A. (2019). Chronic tobacco smoking and neuropsychological impairments: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 96, 143–154. doi: 10.1016/j.neubiorev.2018.11.017 [DOI] [PubMed] [Google Scholar]
- Cope ZA, & Young JW (2017). The five‐ choice continuous performance task (5C‐ CPT): a cross‐ species relevant paradigm for assessment of vigilance and response inhibition in rodents. Current protocols in neuroscience, 78(1), 9–56. doi: 10.1002/cpns.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha PJ, Gonçalves PD, Ometto M, Dos Santos B, Nicastri S, Busatto GF, & de Andrade AG (2013). Executive cognitive dysfunction and ADHD in cocaine dependence: searching for a common cognitive endophenotype for addictive disorders. Frontiers in psychiatry, 4, 126. doi: 10.3389/fpsyt.2013.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Rose C, Draughon JE, Zepf R, Cuca YP, Huang E, Freeborn K, & Lum PJ (2017). Prevalence of substance use in an HIV primary care safety net clinic: a call for screening. Journal of the Association of Nurses in AIDS Care, 28(2), 238–249. doi: 10.1016/j.jana.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland J, & Kovalik-Gran I. (2010). Validity of the factor structure of Conners’ CPT. Journal of attention disorders, 13(4), 347–357. doi: 10.1177/1087054709332477 [DOI] [PubMed] [Google Scholar]
- Eggers C, Arendt G, Hahn K, Husstedt IW, Maschke M, Neuen-Jacob E, . . . Straube E. (2017). HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. Journal of neurology, 264(8), 1715–1727. doi: 10.1007/s00415-017-8503-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; Frey PW, & Colliver JA (1973). Sensitivity and responsibility measures for discrimination learning. Learning and Motivation, 4, 327–342. [Google Scholar]
- Gupta S, Bousman CA, Chana G, Cherner M, Heaton RK, Deutsch R, ... & Everall IP (2011). Dopamine receptor D3 genetic polymorphism (rs6280TC) is associated with rates of cognitive impairment in methamphetamine-dependent men with HIV: preliminary findings. Journal of Neurovirology, 17(3), 239–247. doi: 10.1007/s13365-011-0028-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; Fortenbaugh FC, DeGutis J, & Esterman M. (2017). Recent theoretical, neural, and clinical advances in sustained attention research. Annals of the New York Academy of Sciences, 1396(1), 70–91. doi: 10.1111/nyas.13318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Dombrowski JC, Crane HM, Eron JJ, Geng EH, Mathews WC, . . . Napravnik S. (2017). Prevalence and predictors of substance use disorders among HIV care enrollees in the United States. AIDS and Behavior, 21(4), 1138–1148. doi: 10.1007/s10461-016-1584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, . . . Atkinson JH (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75(23), 2087–2096. doi: 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, . . .Woods SP (2011). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology, 17(1), 3–16. doi: 10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR Jr, Deutsch R, Letendre S, Ellis RJ, Casaletto K, . . . Atkinson JH (2015). Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clinical Infectious Diseases, 60(3), 473–480. doi: 10.1093/cid/ciu862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, Thrasher D, Goetz MB, & Stefaniak M. (2002). Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology, 59(12), 1944–1950. 10.1212/01.wnl.0000038347.48137.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath KJ, Oakes JM, Rosser BRS, Danilenko G, Vezina H, Amico KR, . . . Simoni J. (2013). Feasibility, acceptability and preliminary efficacy of an online peer-to-peer social support ART adherence intervention. AIDS and Behavior, 17(6), 2031–2044. doi: 10.1007/s10461-013-0469-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosek SG, Harper GW, Lemos D, Burke-Miller J, Lee S, Friedman L, & Martinez J. (2018). Project ACCEPT: Evaluation of a group-based intervention to improve engagement in care for youth newly diagnosed with HIV. AIDS and Behavior, 22(8), 2650–2661. doi: 10.1007/s10461-018-2034-4 [DOI] [PubMed] [Google Scholar]
- Kelly PJ, Robinson LD, Baker AL, Deane FP, McKetin R, Hudson S, & Keane C. (2017). Polysubstance use in treatment seekers who inject amphetamine: Drug use profiles, injecting practices and quality of life. Addictive Behaviors, 71, 25–30. doi: 10.1016/j.addbeh.2017.02.006 [DOI] [PubMed] [Google Scholar]
- Kerrigan D, Grieb SM, Ellen J, & Sibinga E. (2018). Exploring the dynamics of ART adherence in the context of a mindfulness instruction intervention among youth living with HIV in Baltimore, Maryland. AIDS care, 30(11), 1400–1405. doi: 10.1080/09540121.2018.1492699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronemer SI, Mandel JA, Sacktor NC, & Marvel CL (2017). Impairments of motor function while multitasking in HIV. Frontiers in human neuroscience, 11, 212. doi: 10.3389/fnhum.2017.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger A, Van Handel M, Dietz PM, Williams WO, Patel D, & Johnson AS (2019). HIV Testing, Access to HIV-Related Services, and Late-Stage HIV Diagnoses Across US States, 2013–2016. American journal of public health, 109(11), 1589–1595. doi: 10.2105/AJPH.2019.305273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinad KE, Hutton HE, Monroe AK, Anderson G, Moore RD, & Chander G. (2016). A qualitative study of barriers to and facilitators of optimal engagement in care among PLWH and substance use/misuse. BMC Research Notes, 9(1), 229. doi: 10.1186/s13104-016-2032-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Fama R, & Sullivan EV (2017). Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcoholism: Clinical and Experimental Research, 41(8), 1432–1443. doi: 10.1111/acer.13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, & Rawson RA (2008). A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug and alcohol review, 27(3), 309–317. doi: 10.1080/09595230801919494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Hardy DJ, Barclay TR, Reinhard MJ, Cole MM, & Hinkin CH (2008). Elements of attention in HIV-infected adults: evaluation of an existing model. Journal of Clinical and Experimental Neuropsychology, 30(1), 53–62. doi: 10.1080/13803390601186684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Hardy DJ, Miller E, Castellon SA, Longshore D, & Hinkin CH (2006). The effect of recent stimulant use on sustained attention in HIV-infected adults. Journal of Clinical and Experimental Neuropsychology, 28(1), 29–42. doi: 10.1080/13803390490918066 [DOI] [PubMed] [Google Scholar]
- MacQueen DA, Minassian A, Henry BL, Geyer MA, Young JW, & Perry W. (2018a). Amphetamine modestly improves Conners’ continuous performance test performance in healthy adults. Journal of the International Neuropsychological Society, 24(3), 283–293. doi: 10.1017/S135561771700090X [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen DA, Minassian A, Kenton JA, Geyer MA, Perry W, Brigman JL, & Young JW (2018b). Amphetamine improves mouse and human attention in the 5-choice continuous performance test. Neuropharmacology, 138, 87–96. doi: 10.1016/j.neuropharm.2018.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Springer G, Seaberg EC, Sacktor N, Miller EN, . . . Martin EM (2018). Differences in cognitive function between women and men with HIV. Journal of acquired immune deficiency syndromes (1999), 79(1), 101–107. doi: 10.1097/QAI.0000000000001764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya JL, Cattie J, Morgan E, Woods SP, Cherner M, Moore DJ, . . . Translational Methamphetamine Aids Research Center Group, T. (2016). The impact of age, HIV serostatus and seroconversion on methamphetamine use. The American Journal of Drug and Alcohol Abuse, 42(2), 168–177. doi: 10.3109/00952990.2015.1114625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, Heaton RK, . . . Group T. (2012). Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS care, 24(12), 1504–1513. doi: 10.1080/09540121.2012.672718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, & Mactutus CF (2014). Modeling deficits in attention, inhibition, and flexibility in HAND. Journal of Neuroimmune Pharmacology, 9(4), 508–521. doi: 10.1007/s11481-014-9539-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, McLaurin KA, Booze RM, & Mactutus CF (2019). Neurorestoration of Sustained Attention in a Model of HIV-1 Associated Neurocognitive Disorders. Frontiers in behavioral neuroscience, 13, 169. doi: 10.3389/fnbeh.2019.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Doyle KL, Minassian A, Henry BL, Perry W, Marcotte TD, . . . Grant I. (2014). Elevated intraindividual variability in methamphetamine dependence is associated with poorer everyday functioning. Psychiatry research, 220(1–2), 527–534. doi: 10.1016/j.psychres.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Smith C, Weber E, Scott JC, Grant I, & Group HIVNRP (2012). Lower cognitive reserve among individuals with syndromic HIV-associated neurocognitive disorders (HAND). AIDS and Behavior, 16(8), 2279–2285. doi: 10.1007/s10461-012-0229-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Moreno JA, Fumaz CR, Ferrer MJ, Prats A, Negredo E, Garolera M, . . . Clotet B. (2008). Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS research and human retroviruses, 24(10), 1301–1307. doi: 10.1089/aid.2007.0310 [DOI] [PubMed] [Google Scholar]
- Norman LR, & Basso M. (2015). An Update of the Review of Neuropsychological Consequences of HIV and Substance Abuse: A Literature Review and Implications for Treatment and Future Research. Current drug abuse reviews, 8(1), 50–71. doi: 10.2174/1874473708666150309124820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Kowalczyk WJ, Botsko M, Tomassilli J, & Golub SA (2013). Aggregate versus day level association between methamphetamine use and HIV medication non-adherence among gay and bisexual men. AIDS and Behavior, 17(4), 1478–1487. doi: 10.1007/s10461-013-0463-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaro RC, Pandhare J, Qian H-Z, & Dash C. (2015). The complex interaction between methamphetamine abuse and HIV-1 pathogenesis. Journal of Neuroimmune Pharmacology, 10(3), 477–486. doi: 10.1007/s11481-015-9604-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Hilsabeck RC, & Hassanein TI (2008). Cognitive dysfunction in chronic hepatitis C. a review. Digestive diseases and sciences, 53(2), 307–321. doi: 10.1007/s10620-007-9896-z [DOI] [PubMed] [Google Scholar]
- Potvin S, Pelletier J, Grot S, Hebert C, Barr AM, & Lecomte T. (2018). Cognitive deficits in individuals with methamphetamine use disorder: A meta-analysis. Addictive behaviors, 80, 154–160. doi: 10.1016/j.addbeh.2018.01.021 [DOI] [PubMed] [Google Scholar]
- Prussing C, Chan C, Pinchoff J, Kersanske L, Bornschlegel K, Balter S, ... & Fuld, J. (2015). HIV and viral hepatitis co-infection in New York City, 2000–2010: prevalence and case characteristics. Epidemiology & Infection, 143(7), 1408–1416. doi: 10.1017/S0950268814002209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychology, & Software Tools Inc. (2012). E-Prime 2.0. Retrieved from https://www.pstnet.com [Google Scholar]
- Quinn B, Stoové M, Papanastasiou C, & Dietze P. (2013). Methamphetamine use in Melbourne, Australia: Baseline characteristics of a prospective methamphetamine-using cohort and correlates of methamphetamine dependence. Journal of Substance Use, 18(5), 349–362. doi: 10.3109/14659891.2012.675400 [DOI] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, & Ling W. (2006). A comparison of contingency management and cognitive‐ behavioral approaches for stimulant‐ dependent individuals. Addiction, 101(2), 267–274. doi: 10.1111/j.1360-0443.2006.01312.x [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, ... & HNRC group. (2004). Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society, 10(1), 1. doi: 10.10170S1355617704101021 [DOI] [PubMed] [Google Scholar]
- Roll JM (2007). Contingency management: an evidence‐ based component of methamphetamine use disorder treatments. Addiction, 102, 114–120. doi: 10.1111/j.1360-0443.2006.01774.x [DOI] [PubMed] [Google Scholar]
- Sagvolden T, & Xu T. (2008). l-Amphetamine improves poor sustained attention while d-amphetamine reduces overactivity and impulsiveness as well as improves sustained attention in an animal model of Attention-Deficit/Hyperactivity Disorder (ADHD). Behavioral and Brain Functions, 4(3). doi: 10.1186/1744-9081-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahgal A. (1987). Some limitations of indices derived from signal detection theory: evaluation of an alternative index for measuring bias in memory tasks. Psychopharmacology (Berl), 91(4), 517–520. doi: 10.1007/bf00216022 [DOI] [PubMed] [Google Scholar]
- Samson RD, & Barnes CA (2013). Impact of aging brain circuits on cognition. European Journal of Neuroscience, 37(12), 1903–1915. doi: 10.1111/ejn.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders GD, Bayoumi AM, Sundaram V, Bilir SP, Neukermans CP, Rydzak CE, . . . Owens DK (2005). Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. New England Journal of Medicine, 352(6), 570–585. doi: 10.1056/NEJMsa042657 [DOI] [PubMed] [Google Scholar]
- Sanford R, Fellows LK, Ances BM, & Collins DL (2018). Association of brain structure changes and cognitive function with combination antiretroviral therapy in HIV-positive individuals. JAMA neurology, 75(1), 72–79. doi: 10.1001/jamaneurol.2017.3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satz P, Cole MA, Hardy DJ, & Rassovsky Y. (2011). Brain and cognitive reserve: mediator (s) and construct validity, a critique. Journal of clinical and experimental neuropsychology, 33(1), 121–130. doi: 10.1080/13803395.2010.493151 [DOI] [PubMed] [Google Scholar]
- Senathi-Raja D, Ponsford J, & Schönberger M. (2010). Impact of age on long-term cognitive function after traumatic brain injury. Neuropsychology, 24(3), 336. doi: 10.1037/a0018239 [DOI] [PubMed] [Google Scholar]
- Smith ME, & Farah MJ (2011). Are prescription stimulants “smart pills”? The epidemiology and cognitive neuroscience of prescription stimulant use by normal healthy individuals. Psychological bulletin, 137(5), 717. doi: 10.1037/a0023825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Kesby JP, Morgan EE, Bischoff-Grethe A, Minassian A, Brown GG, . . . Translational Methamphetamine, A. R. C. G. (2016). Effects of HIV and Methamphetamine on Brain and Behavior: Evidence from Human Studies and Animal Models. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology, 11(3), 495–510. doi: 10.1007/s11481-016-9699-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, & Potvin S. (2013). Widespread and sustained cognitive deficits in alcoholism: a meta‐ analysis. Addiction biology, 18(2), 203–213. doi: 10.1111/j.1369-1600.2011.00418.x [DOI] [PubMed] [Google Scholar]
- Tabibi Z, Borzabadi HH, Stavrinos D, & Mashhadi A. (2015). Predicting aberrant driving behaviour: The role of executive function. Transportation research part F: traffic psychology and behaviour, 34, 18–28. doi: 10.1016/j.trf.2015.07.015 [DOI] [Google Scholar]
- Walker KA, & Brown GG (2018). HIV-associated executive dysfunction in the era of modern antiretroviral therapy: A systematic review and meta-analysis. Journal of clinical and experimental neuropsychology, 40(4), 357–376. doi: 10.1080/13803395.2017.1349879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Bismark AW, Sun Y, Zhang W, McIlwain M, Grootendorst I, & Light GA (2017). Neurophysiological characterization of attentional performance dysfunction in schizophrenia patients in a reverse-translated task. Neuropsychopharmacology, 42(6), 1338–1348. doi: 10.1038/npp.2016.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA, Halberstadt AL, van Enkhuizen J, Minassian A, Khan A, . . . Eyler LT (2019). Convergent neural substrates of inattention in bipolar disorder patients and dopamine transporter‐ deficient mice using the 5‐ choice CPT. Bipolar disorders. doi: 10.1111/bdi.12786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA, Rissling AJ, Sharp RF, Eyler LT, Asgaard GL, & Light GA (2013). Reverse translation of the rodent 5C-CPT reveals that the impaired attention of people with schizophrenia is similar to scopolamine-induced deficits in mice. Translational psychiatry, 3(11), e324–e324. doi: 10.1038/tp.2013.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, & Geyer MA (2009). The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PloS one, 4(1). doi: 10.1371/journal.pone.0004227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Roberts BZ, Breier M, Swerdlow NR (2020). Amphetamine improves rat 5-choice continuous performance test (5C-CPT) irrespective of concurrent low-dose haloperidol treatment. Psychopharmacology. doi: 10.1007/s00213-020-05511-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.