Abstract
Rationale
Klebsiella pneumoniae (K. pneumoniae, KP) are divided into two types: classic K. pneumoniae (cKP) and hypervirulent K. pneumoniae (hvKP). hvKP causes liver abscess and metastatic infection. Here, we report one case with pyogenic liver abscess (PLA) and endogenous endophthalmitis (EE) due to a relatively rarely reported serotype of K. pneumoniae in China.
Patient Concerns
An 80-year old man presented with nausea, vomiting, and epigastric discomfort for 2 weeks.
Diagnoses
PLA was identified by CT scan and abdominal ultrasound. Urgent ophthalmologic consultation was performed. B-scan ocular ultrasound was done and he was diagnosed as EE.
Interventions
Antibiotic treatment, intravitreal injection of eyes and eye drops were given. Percutaneous needle aspiration, evisceration, and drainage of the right eye were performed.
Outcomes
Cultures of the blood, the aspirated pus from the liver abscess, and the contents of the eyeball all yielded K. pneumoniae with a positive string test. The capsular serotype was K64. According to the existence of multiple virulence genes and the severe invasive clinical manifestation, this strain is regarded as a hvKp strain. Multilocus sequence typing (MLST) revealed the sequence type (ST) of this strain was K64-ST1764. Antimicrobial resistance genes, blaNDM-1 and blaKPC-2, were not detected in the genome. The patient lost his eyesight but his symptoms subsided. During 15 months follow-up, the result was satisfactory.
Lessons
Here, we report one case with PLA due to a relatively rarely reported serotype of K. pneumoniae in China. This K64 K. pneumoniae strain is confirmed as hvKp by multiple methods. It is noteworthy that the sequence type is K64-ST1764 instead of the commonest ST11. Moreover, this strain is not considered a K. pneumoniae carbapenemase-producing K. pneumoniae (KPC-Kp) or a carbapenem-resistant K. pneumoniae (CRKP) as it is usually. Further follow-up and research are required to investigate this strain.
Keywords: case report, hypervirulent Klebsiella pneumoniae, pyogenic liver abscess, antibiotic resistance, ultrasonography guided puncture aspiration
Introduction
PLA is a potentially life-threatening disease which is mainly caused by bacteria. Along with the increasing prevalence of hvKp, PLA due to K. pneumoniae has been steadily increasing. K. pneumoniae are divided into two types: cKP and hvKP.1 Ckp is an opportunistic pathogen which mainly infects immunodeficient patients or the immunocompromised population. HvKP can infect young healthy people without underlying diseases.2 Increased virulence of hvkp is related to its hypermucoviscous phenotype and stronger ability of iron acquisition.3,4 HvKP often causes primary liver abscess and metastatic infections like endophthalmitis, meningitis, and so on.5 HvKP infections are more common in the Asian Pacific but now are occurring globally.
Here we report one case who presented in China with PLA and EE due to a relatively rarely reported serotype of hvKP. Different from previous reports, the ST of this strain was K64-ST1764 instead of the commonest ST11. It is especially noteworthy that antimicrobial susceptibility testing showed that this strain was sensitive to all antibiotics, which means it is not a KPC-Kp or a CRKP.
Case Presentation
An 80-year old Chinese male patient presented to the emergency department with nausea, vomiting, and epigastric discomfort for 2 weeks without treatment. This patient had a history of Type II diabetes mellitus, hypertension, and coronary heart disease. There was no relevant family history or history of genetic disease. Laboratory investigations revealed a white cell count 14.42×109 cells/L (normal range=3.5–9.5×109 cells/L), 85% neutrophils (normal range=40–75%), aspartate transaminase (AST) 109 U/L (normal range<50 U/L), lactate dehydrogenase (LDH) 349 U/L (normal range=109–245 U/L), random blood glucose 19.96 mmol/L (normal range=3.8–6.2 mmol/L), blood urea 11.3 mmol/L (normal range=2.1–8.6 mmol/L), creatinine 141.7 µmol/L (normal range=35.2–97.5 μmol/L). Severe infection and ketoacidosis were suspected so emergency doctors gave him antibiotics (imipenem combined with ornidazole), rabeprazole, antiemetic drug, and fluid infusion, but his condition worsened. Fever appeared and the highest armpit temperature was 39°C.
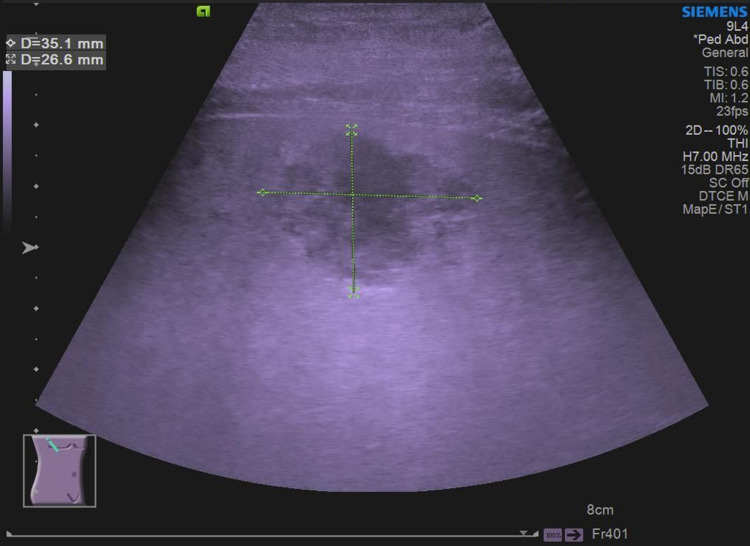
He was admitted to the Department of Gastroenterology for further treatment. Laboratory investigations revealed C-reactive protein 439.43 mg/L (normal range<10 mg/L), serum sodium (Na) 133.9 mmol/L (normal range=136–145 mmol/L), serum potassium (K) 3.71 mmol/L (normal range=3.5–5.3 mmol/L), total bilirubin 21 μmol/L (normal range=3.4–20.6 μmol/L), and alanine aminotransferase (ALT) 88.6 U/L (normal range<50 U/L). Physical examination revealed mild epigastric tenderness. A right lobe liver abscess was identified on CT scan (Figure 1). Abdominal ultrasound showed a right lobe liver abscess (mixed echoes) with the size of 3.5×2.7 cm in the right liver lobe (Figure 2).
Figure 1.
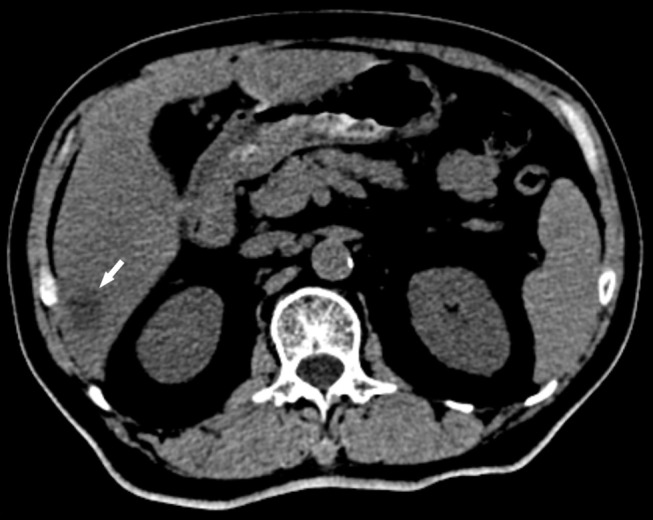
Abdominal CT scan showed a right lobe liver abscess (rounded, low density areas).
Figure 2.
Abdominal ultrasound showed a right lobe liver abscess (mixed echoes, with a size of 3.5cm×2.7 cm).
The patient was treated by anti-infection treatment (biapenem and morinidazole by intravenous injection), liver protection (polyene phosphatidylcholine), decreasing blood sugar, controlling blood pressure, and fluid infusion. Ultrasonography guided puncture aspiration for hepatic abscess was proposed but he refused. Both his white cell count and C-reactive protein were dropped after treatment but he was still feverish. Bacteria culturing and antimicrobial susceptibility testing were performed by using the VITEK-II Compact automated microbiological system and confirmed by using the broth microdilution method. Blood cultures, from both aerobic and anaerobic bottles, showed K. pneumoniae which was sensitive to all antibiotics.
The vision of this patient became blurred so urgent ophthalmologic consultation was performed. A B-scan ocular ultrasound was done and he was diagnosed as EE with bad prognosis. Intravitreal injection (vancomycin and cefoperazone) of eyes and eye drops were given. Finally, after obtaining the patient’s consent, ultrasonography guided puncture aspiration was performed, which resulted in lowering the temperature and improving the inflammatory marker levels. Liver aspirate culture was also positive for K. pneumoniae and sensitive to all antibiotics (Table 1). The patient’s eyes were swollen and empyema and the light perception of both eyes was basically lost. The ophthalmologist performed evisceration and drainage of the right eye, as well as repeated intravitreal injection of the left eye. Abdominal ultrasonography was performed 6 days after the first therapeutic puncture. The abscess cavity decreased conspicuously and the second percutaneous needle aspiration was given. After treatment, the patient’s eye swelling and the empyema was significantly improved and he became afebrile. He was given ceftriaxon 2 g by intravenous injection once a day for 4 weeks. Repeated blood cultures were negative and ultrosound reexamination showed the abscess cavity had disappeared. He was discharged and referred to the gastroenterology clinic for follow-up. During 15 months follow-up, the result was satisfactory.
Table 1.
Antimicrobial Susceptibility Testing Results for K. Pneumoniae Isolate, Using Reference Broth Microdilution According to Clinical and Laboratory Standards Institute (CLSI) Guidelines
| Antimicrobial Agent | Minimum Inhibitory Concentration (µg/mL) | Interpretation* |
|---|---|---|
| Amikacin | ≤2 | S |
| Ampicillin | >32 | S |
| Aztreonam | ≤1 | S |
| Ceftriaxone | ≤1 | S |
| Ceftazidime | ≤1 | S |
| Cefepime | ≤1 | S |
| Ciprofloxacin | ≤0.25 | S |
| Gentamicin | ≤1 | S |
| Imipenem | ≤1 | S |
| Levofloxacin | ≤0.25 | S |
| Ertapenem | ≤0.5 | S |
| Piperacillin-Tazobactam | ≤4 | S |
| Tigecycline | 0.5 | S |
Notes: *Interpretative criteria were applied according to CLSI document M100 (Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. 2018) except for tigecycline where criteria established by the US Food and Drug Administration were used.
Abbreviations: R, resistant; S, susceptible.
Isolates were retested by 16S rRNA gene sequences as previously reported6 and K. pneumoniae was identified and reconfirmed.
String test was performed as previously reported.2 The colony was grown at 37°C overnight on a sheep blood agar plate and then stretched by a bacteriology inoculation loop. A positive result is defined as the formation of a string >5 mm in length. Cultures of the blood, the aspirated pus from the liver abscess, and the contents of the eyeball all yielded K. pneumoniae with a positive string test (Figure 3).
Figure 3.
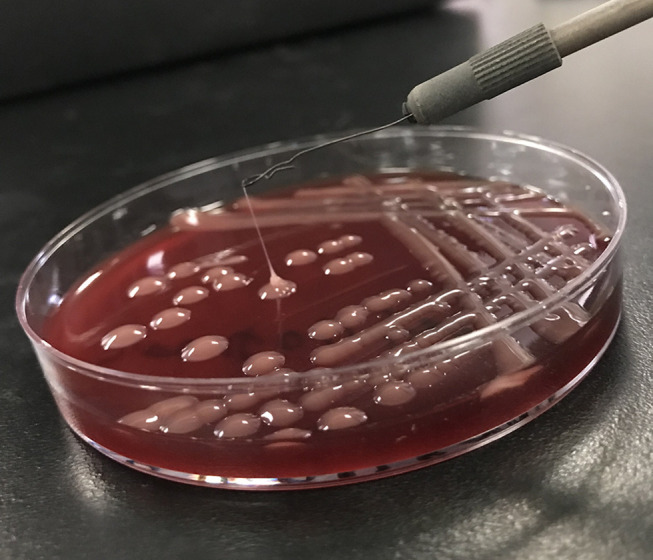
All cultures yielded K. pneumoniae with a positive string test (>5mm in string length).
Capsular serotype of each isolate was performed by polymerase chain reaction (PCR) as previously reported.7 PCR analysis indicated that the capsular serotype of the isolated K. pneumoniae was K64, which was validated by wzi sequencing as described.8
MLST was performed by PCR amplification as previously reported9 and this strain belongs to ST1764.
Multiple biomarkers, including peg-344 (+), iroB (+), iucA (+), prmpA (+), prmpA2 (+), crmpA (-), ureA (+), uge (+), wabG (+), allS (+), mrkD (+), and fimH (+) were detected by PCR as described10 and multiple virulence genes were found in all the above isolates.
Screening for carbapenemase genes was performed by PCR as described11 and blaNDM-1 and blaKPC-2 were not found in this K64 strain.
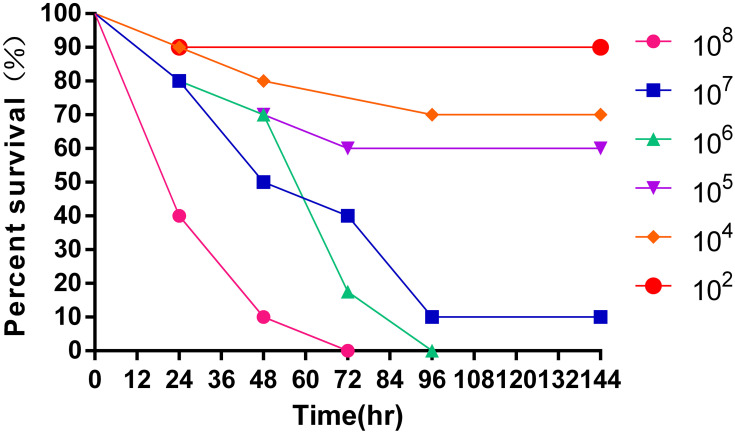
We evaluated the virulence of this K. pneumoniae strain in a Galleria mellonella infection model.12 We infected the Galleria mellonella larvae with different concentrations (102~108 colony-forming units/mL) of K. pneumoniae and recorded the survival rate (Figure 4). This strain showed a strong concentration-dependent lethality to the larvae.
Figure 4.
Survival curves for Galleria mellonella larvae inoculated with different concentrations (102~108 colony-forming units/mL) of the K. pneumoniae strain.
Discussion and Conclusions
The first clinical report about hvKp was in Taiwan in 1986.13 Although it is more common in the Asia-Pacific region, infections due to hvKp have gradually become global.14,15 Because hvKP was considered to have a hypermucoviscosity phenotype, the string test has been used to defining hvKP.3 However, more and more studies have shown that hypermucoviscosity is not pathognomonic for hvKp since this phenotype also can be observed in cKp strains.16 Recently, several virulence genes, like iuc, peg-344, rmpA, and rmpA2, have been shown to most accurately predict hvKp strains.16
Since the 1980s, PLA due to K. pneumoniae has been steadily increasing. Especially in the Asia-Pacific region, hvKP has already become the predominant pathogen of PLA.17–19 Furthermore, these infections are occurring globally. A study in China has reported that among all PLA cases, K pneumonia was the most common pathogen and a higher incidence of diabetes mellitus was shown in patients with K. pneumoniae-induced PLA.20 Another study has reported that most of the liver abscesses were caused by K. pneumoniae and mostly occurred in patients with diabetes mellitus.21 The major clinical features of KP-PLA are fever, nausea, vomiting, abdominal discomfort, and laboratory inspection shows elevated white blood cell count and liver dysfunction. In this patient, all the above features can be found.
There are mainly three treatment methods: appropriate early antibiotic treatment, ultrasonography guided puncture aspiration or draining treatment, and open surgical drainage or surgical resection of the liver abscesses. We initially treated the patient with carbapenems and Morinidazole. After the condition stabilized, the antibiotics were changed to ceftriaxone for another 4 weeks. Some studies have suggested that the majority of PLA cases require drainage.22 A report showed that antimicrobial therapy alone was effective for small abscesses, while percutaneous needle aspiration or draining should be chosen for larger abscesses.23 A study reported that liver abscesses due to K. pneumoniae that were >5 cm were likely to have a delayed response to therapy.24 In the present study, the liver abscess was <5 cm (35×27 mm) and this hvKP strain was sensitive to all antibiotics. Even so, early antimicrobial therapy alone was ineffective. Once aspiration was performed, clinical improvement was observed. This suggested that, even for small abscesses, puncture or drainage should be performed as early as possible when the effect of drug therapy is unsatisfactory.
In recent years, the incidence of EE has gradually increased. Gram-positive bacteria and fungal organisms account for the majority of the cases in the developed world, whereas gram-negative organisms like K. pneumoniae are more common in the Asia-Pacific region.25 Now it has become a frequent complication: approximately 5% of individuals with hvKp bacteremia ultimately develop EE.26
EE is a common metastatic complication of K. pneumoniae liver abscess.27 Diabetes mellitus was considered to be a significant risk factor. The prognosis of EE is generally poor even with prompt diagnosis and aggressive treatment given. The final visual outcome was only light perception or worse in 89% of patients with EE associated with K. pneumoniae-induced PLA.27 Diabetes mellitus and poor initial visual acuity were associated with poor visual outcome.28 Reports showed that the main prognostic factor in Klebsiella EE is the presence of hypopyon which can be found in our patient.29 This patient lost his eyesight.
It has long been recognized that almost all patients with severe infection with liver abscess and extrahepatic infections are infected exclusively with K. pneumoniae serotypes K1 or K2.2 However, it is clear now that non-K1/K2 hvKp strains can express virulence genes and are capable of causing liver abscess and metastatic spread.16,30 The serotype of the isolated K. pneumoniae strain in our patient was K64, a relatively rarely reported serotype. This serotype has always been considered a cKP. But it is recently recognized that K64 is predicted to be observed for hvKp strains.31,32 Recent research finds that some cKp strains can acquire the hvKp virulence plasmid and evolve into hvKp strains.12,32 Our K64 K. pneumoniae strain showed strong virulence in the Galleria mellonella infection model. According to positive string test, the existence of multiple virulence genes, the strong lethality to the larvae and the severe invasive clinical manifestation of its infection, we can regard this K64 strain as a hvKp strain. Compared to the classification based on string test alone, our method is more reasonable.
In particular, ST was determined by MLST and the result was K64-ST1764 instead of the commonest ST11, which was considered to be predominant in Extended-Spectrum β-lactamases (ESBL)-producing strains and was prevalent in CRKP strains.33,34 However, previous reports showed that ST11 was predominant in K64 capsular type, hence K64 was considered as a prevalent type of CRKP.35–38 Furthermore, blaNDM-1 and blaKPC-2, two important carbapenemase genes, were not found in this K64 strain and antimicrobial susceptibility testing showed that it was sensitive to all antibiotics. Contrary to previous studies,31,32,36,39 these findings indicated that this K64 strain is not a KPC-Kp or a CRKP strain.
In conclusion, here we report one patient with PLA and EE due to a relatively rarely reported serotype of K. pneumoniae: K64. As the existence of multiple virulence genes, the strong lethality to the larvae and the severe invasive clinical manifestation and positive string test, this K64 K. pneumoniae can be regarded as a hvKp strain. Different from previous reports, the ST of this strain was K64-ST1764 instead of the commonest ST11. It is not a KPC-Kp or a CRKP, either. Further follow-up and research are required to investigate this strain.
Acknowledgments
The key discipline of Wuxi No.ZDXK002 Workstation of academician Fan Daiming No.CYR1705
Funding Statement
This work was supported by the National Natural Science Foundation of China No.81500467, Foundation for Key Medical Talents in Jiangsu Province No.QNRC2016151, Six Talent Peaks Project of Jiangsu Province No.WSN-220, Six One Top-notch Talent Project of Jiangsu Province No.LGY201701. The funders had no role in the study design, conduct, analysis, interpretation of study results, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Abbreviations
CRKP, carbapenem-resistant K. pneumoniae; cKP, classic K. pneumoniae; ESBL, extended-spectrum β-lactamases; hvKP, hypervirulent K. pneumoniae; KPC-Kp, K. pneumoniae carbapenemase (KPC)-producing ; MLST, multilocus sequence typing; PLA, pyogenic liver abscess; ST, sequence type.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This case report was approved by the ethics committee of The Affiliated Wuxi Second People’s Hospital of Nanjing Medical University.
Consent for Publication
Written informed consent for publication of his clinical details and clinical images was obtained from the patient.
Author Contributions
Bo Zhao and Renjing Hu have contributed equally to this work and they are co-first authors. Conceived the study: Xiaoyun Wang, Bo Zhao; Acquired data, analyzed data: Bo Zhao, Renjing Hu; Performed the microbiological analysis: Renjing Hu; Wrote the manuscript: Bo Zhao; Contributed reagents/materials/analysis tools: Lei Gong, Xiaoyun Wang; and Revised the manuscript: Xiaoyun Wang, Yingwei Zhu, Gaojue Wu.
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest to this work.
References
- 1.Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107–118. doi: 10.4161/viru.22718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siu LK, Yeh K-M, Lin J-C, Fung C-P, Chang F-Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12(11):881–887. doi: 10.1016/S1473-3099(12)70205-0 [DOI] [PubMed] [Google Scholar]
- 3.Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med. 2004;199(5):697–705. doi: 10.1084/jem.20030857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang YH, Chou SH, Liang SW, et al. Emergence of an XDR and carbapenemase-producing hypervirulent Klebsiella pneumoniae strain in Taiwan. J Antimicrob Chemother. 2018;73(8):2039–2046. doi: 10.1093/jac/dky164 [DOI] [PubMed] [Google Scholar]
- 5.Shon AS, Russo TA. Hypervirulent Klebsiella pneumoniae: the next superbug? Future Microbiol. 2012;7(6):669–671. doi: 10.2217/fmb.12.43 [DOI] [PubMed] [Google Scholar]
- 6.He Y, Guo X, Xiang S, et al. Comparative analyses of phenotypic methods and 16S rRNA, khe, rpoB genes sequencing for identification of clinical isolates of Klebsiella pneumoniae. Antonie Van Leeuwenhoek. 2016;109(7):1029–1040. doi: 10.1007/s10482-016-0702-9 [DOI] [PubMed] [Google Scholar]
- 7.Turton J, Perry C, Elgohari S, Hampton C. PCR characterization and typing of Klebsiella pneumoniae using capsular type-specific, variable number tandem repeat and virulence gene targets. J Med Microbiol. 2010;59:541–547. doi: 10.1099/jmm.0.015198-0 [DOI] [PubMed] [Google Scholar]
- 8.Brisse S, Passet V, Haugaard A, et al. wzi Gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol. 2013;51(12):4073–4078. doi: 10.1128/JCM.01924-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diancourt L, Passet V, Verhoef J, Grimont P, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43(8):4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Q, Zhou M, Zou M, Liu W. Hypervirulent Klebsiella pneumoniae induced ventilator-associated pneumonia in mechanically ventilated patients in China. European J Clin Microbiol Infectious Diseases. 2016;35(3):387–396. doi: 10.1007/s10096-015-2551-2 [DOI] [PubMed] [Google Scholar]
- 11.Poirel L, Walsh T, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–123. doi: 10.1016/j.diagmicrobio.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 12.Gu D, Dong N, Zheng Z, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18(1):37–46. doi: 10.1016/S1473-3099(17)30489-9 [DOI] [PubMed] [Google Scholar]
- 13.Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146(10):1913–1916. doi: 10.1001/archinte.1986.00360220057011 [DOI] [PubMed] [Google Scholar]
- 14.Rossi B, Gasperini ML, Leflon-Guibout V, et al. Hypervirulent klebsiella pneumoniae in cryptogenic liver abscesses, Paris, France. Emerg Infect Dis. 2018;24(2):221–229. doi: 10.3201/eid2402.170957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fazili T, Sharngoe C, Endy T, Kiska D, Javaid W, Polhemus M. Klebsiella pneumoniae liver abscess: an emerging disease. Am J Med Sci. 2016;351(3):297–304. doi: 10.1016/j.amjms.2015.12.018 [DOI] [PubMed] [Google Scholar]
- 16.Russo TA, Olson R, Fang CT, et al. Identification of biomarkers for differentiation of hypervirulent klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol. 2018;56:9. doi: 10.1128/JCM.00776-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng DL, Liu YC, Yen MY, Liu CY, Shi FW, Wang LS. Causal bacteria of pyogenic liver abscess. Taiwan Yi Xue Hui Za Zhi. 1989;88(10):1008–1011. [PubMed] [Google Scholar]
- 18.Chang FY, Chou MY, Fan RL, Shaio MF. A clinical study of Klebsiella liver abscess. Taiwan Yi Xue Hui Za Zhi. 1988;87(3):282–287. [PubMed] [Google Scholar]
- 19.Lee TY, Wan YL, Tsai CC. Gas-containing liver abscess: radiological findings and clinical significance. Abdom Imaging. 1994;19(1):47–52. doi: 10.1007/BF02165861 [DOI] [PubMed] [Google Scholar]
- 20.Kong H, Yu F, Zhang W, Li X. Clinical and microbiological characteristics of pyogenic liver abscess in a tertiary hospital in East China. Medicine. 2017;96(37):e8050. doi: 10.1097/MD.0000000000008050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang WJ, Tao Z, Wu HL. Etiology and clinical manifestations of bacterial liver abscess: A study of 102 cases. Medicine. 2018;97(38):e12326. doi: 10.1097/MD.0000000000012326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waghmare M, Shah H, Tiwari C, Khedkar K, Gandhi S. Management of liver abscess in children: our experience. Euroasian J Hepatogastroenterol. 2017;7(1):23–26. doi: 10.5005/jp-journals-10018-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheema HA, Saeed A. Etiology, presentation and management of liver abscesses at the children’ s hospital lahore. Annals King Edward Medical University. 2008;14(4):148. [Google Scholar]
- 24.Chan DS, Archuleta S, Llorin RM, Lye DC, Fisher D. Standardized outpatient management of Klebsiella pneumoniae liver abscesses. Int J Infect Dis. 2013;17(3):e185–188. doi: 10.1016/j.ijid.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 25.Sadiq MA, Hassan M, Agarwal A, et al. Endogenous endophthalmitis: diagnosis, management, and prognosis. J Ophthalmic Inflamm Infect. 2015;5(1):32. doi: 10.1186/s12348-015-0063-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HC, Chuang YC, Yu WL, et al. Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community-acquired bacteraemia. J Intern Med. 2006;259(6):606–614. doi: 10.1111/j.1365-2796.2006.01641.x [DOI] [PubMed] [Google Scholar]
- 27.Yang CS, Tsai HY, Sung CS, Lin KH, Lee FL, Hsu WM. Endogenous Klebsiella endophthalmitis associated with pyogenic liver abscess. Ophthalmology. 2007;114(5):876–880. doi: 10.1016/j.ophtha.2006.12.035 [DOI] [PubMed] [Google Scholar]
- 28.Sheu SJ, Kung YH, Wu TT, Chang FP, Horng YH. Risk factors for endogenous endophthalmitis secondary to klebsiella pneumoniae liver abscess: 20-year experience in Southern Taiwan. Retina. 2011;31(10):2026–2031. doi: 10.1097/IAE.0b013e31820d3f9e [DOI] [PubMed] [Google Scholar]
- 29.Ang M, Jap A, Chee SP. Prognostic factors and outcomes in endogenous Klebsiella pneumoniae endophthalmitis. Am J Ophthalmol. 2011;151(2):338–344.e332. doi: 10.1016/j.ajo.2010.08.036 [DOI] [PubMed] [Google Scholar]
- 30.Gu DX, Huang YL, Ma JH, et al. Detection of colistin resistance gene mcr-1 in hypervirulent klebsiella pneumoniae and escherichia coli isolates from an infant with diarrhea in China. Antimicrob Agents Chemother. 2016;60(8):5099–5100. doi: 10.1128/AAC.00476-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Liu PP, Wang LH, Wei DD, Wan LG, Zhang W. Capsular polysaccharide types and virulence-related traits of epidemic kpc-producing klebsiella pneumoniae isolates in a Chinese University Hospital. Microb Drug Resist. 2017;23(7):901–907. doi: 10.1089/mdr.2016.0222 [DOI] [PubMed] [Google Scholar]
- 32.Yu F, Lv J, Niu S, et al. In vitro activity of ceftazidime-avibactam against carbapenem-resistant and hypervirulent klebsiella pneumoniae isolates. Antimicrob Agents Chemother. 2018;62:8. doi: 10.1128/AAC.01031-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhee JY, Park YK, Shin JY, et al. KPC-producing extreme drug-resistant Klebsiella pneumoniae isolate from a patient with diabetes mellitus and chronic renal failure on hemodialysis in South Korea. Antimicrob Agents Chemother. 2010;54(5):2278–2279. doi: 10.1128/AAC.00011-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MY, Ko KS, Kang CI, Chung DR, Peck KR, Song JH. High prevalence of CTX-M-15-producing Klebsiella pneumoniae isolates in Asian countries: diverse clones and clonal dissemination. Int J Antimicrob Agents. 2011;38(2):160–163. doi: 10.1016/j.ijantimicag.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 35.Pan YJ, Lin TL, Lin YT, et al. Identification of capsular types in carbapenem-resistant Klebsiella pneumoniae strains by wzc sequencing and implications for capsule depolymerase treatment. Antimicrob Agents Chemother. 2015;59(2):1038–1047. doi: 10.1128/AAC.03560-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu MC, Tang HL, Chiou CS, Wang YC, Chiang MK, Lai YC. Clonal dissemination of carbapenemase-producing Klebsiella pneumoniae: two distinct sub-lineages of Sequence Type 11 carrying blaKPC-2 and blaOXA-48. Int J Antimicrob Agents. 2018;52(5):658–662. doi: 10.1016/j.ijantimicag.2018.04.023 [DOI] [PubMed] [Google Scholar]
- 37.Cheng YH, Lin TL, Pan YJ, Wang YP, Lin YT, Wang JT. Colistin resistance mechanisms in Klebsiella pneumoniae strains from Taiwan. Antimicrob Agents Chemother. 2015;59(5):2909–2913. doi: 10.1128/AAC.04763-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu F, Lv J, Niu S, et al. Multiplex pcr analysis for rapid detection of klebsiella pneumoniae carbapenem-resistant (Sequence Type 258 [ST258] and ST11) and Hypervirulent (ST23, ST65, ST86, and ST375) Strains. J Clin Microbiol. 2018;56:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong N, Zhang R, Liu L, et al. Genome analysis of clinical multilocus sequence Type 11 Klebsiella pneumoniae from China. Microb Genom. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]