Abstract
Background
Physalis alkekengi var. franchetii is an herb that possesses various ethnopharmacological applications. Herein, our current study focuses on the antitumor effect of a combination of physalins, which are regarded as the most representative secondary metabolites from calyces of Physalis alkekengi var. franchetii.
Materials and Methods
We mainly investigated the antitumor activity of the physalins extracted from Physalis alkekengi var. franchetii on both solid and hematologic cancers. The main cells used in this study were NCI-H1975 and U266 cells. The major assays used were the CCK-8 assay, Western blot analyses, immunofluorescence assay and Annexin V assay, and a xenograft mouse model was used.
Results
The results showed that physalins exhibited a strong antitumoural effect on both non-small cell lung cancer (NSCLC) and multiple myeloma (MM) cells by suppressing constitutive STAT3 activity and further inhibiting the downstream target gene expression induced by STAT3 signaling, which resulted in the enhanced apoptosis of tumor cells. Moreover, physalins significantly reduced tumor growth in xenograft models of lung cancer.
Conclusion
Collectively, these findings demonstrated that the physalins from Physalis alkekengi var. franchetii may potentially act as cancer preventive or chemotherapeutic agents for NSCLC and MM by inhibiting the STAT3 signaling pathway. The present study served as a promising guide to further explore the precise mechanism of Physalis alkekengi var. franchetii in cancer treatment.
Keywords: Physalis, apoptosis, non-small cell lung carcinoma, multiple myeloma, STAT3
Introduction
Physalis alkekengi L. (also known as “Jindenglong”) is a well-known traditional Chinese medicinal herb consisting of dried calyces or calyces-with-fruit of P. alkekengi var. franchetii (Solanaceae).1 It is widely used for the treatment and prevention of different diseases, including sore throat, cough, eczema, tonsillitis, pharyngitis, hepatitis, leishmaniasis and tumors.2 Recently, several studies show that it plays a critical role in antitumor, antioxidant, antibacterial, anti-inflammatory, immunomodulation and cytotoxic functions.3–8 The main chemical components of Physalis alkekengi L. are physalins, neophysalins, alkaloids, polysaccharides and flavonoids, among which physalins are known as the most bioactive substances.9 Physalins were evaluated for their therapeutic potential and were found to possess anti-inflammatory, immunomodulatory and antitumor properties. For example, in human melanoma cells, physalin B can induce apoptosis by increasing the protein levels of Bax and caspase-3.10 Human renal cancer cells are inhibited by physalin F by inducing reactive oxygen species (ROS)-mediated apoptosis.11 Physalin D, another active component, possesses an antioxidant activity.12 Additionally, physalin A exerts antitumor activities on NSCLC by suppressing the JAK/STAT3 signaling pathway.4 However, whether the mixture of physalins from Physalis alkekengi L. inhibits non-small cell lung cancer (NSCLC) and multiple myeloma (MM), solid and hematologic cancer, respectively, still remains unclear.
As previously reported, Michael reaction acceptors (MARs) are active molecules that may have antitumor activity. Five physalins that were investigated might be MARs and have antitumor activity.13 In the present study, we attempted to investigate the possible molecular and cellular mechanisms of physalins from P. alkekengi var. franchetii on the growth and apoptosis of NSCLC (NCI-H1975) and MM cell lines (U266). Moreover, the antitumor activity of the physalins on lung cancer was also evaluated in a xenograft mouse model to assess the efficacy and toxicity of these physalins.
Materials and Methods
Plant Material
The Physalis alkekengi var. franchetii (Lot No. 151227) used in this study was purchased from Huadong Chinese Crude Co. and passed standard tests (Report No. C151229-6) according to the Zhejiang Traditional Chinese Medicine Processing Standard (2005 Edition, QS-01-0000-03) The authentication of the herb was also performed by the chief pharmacist Min Xia Zheng of the Zhejiang Provincial Hospital of TCM, China. The Huadong Chinese Crude Co. is a designated clinical herb suppliers (Supplementary Table 1).
Extraction
The extraction methods were previously described.13 Eight-fold 95% EtOH under reflux was used to extract dried calyces (10 kg) from Physalis alkekengi var. franchetii three times. After vacuum drying, approximately 830 g of extract was obtained. The extract was redissolved in water (1 L) and then extracted three times with petroleum ether (1 L) and dichloromethane (1 L). The dichloromethane fraction (160 g) was finally obtained. The extract of physalins was dissolved in DMSO at a stock concentration of 20 mg/mL and aliquoted for storage at −20°C.
Quality Control of Physalis alkekengi var. franchetii
Five physalins were separated and identified from the calyces of Physalis alkekengi var. franchetii in our previous study.13 The extract contents of Physalis alkekengi var. franchetii were determined by chromatographic analyses performed with an Acquity UPLC system (Waters, Milford, MS, USA) and a BEH C18 column (2.1 mm×100 mm×1.7 μm). MS and MS-MS analyses were performed on a Micromass Quattro Premier tandem quadrupole mass spectrometer (Waters, Manchester, UK) using an electrospray (ESI) source in positive mode. The method was previously reported with minor modifications.13 One hundred milligrams of pulverized sample was accurately weighed and was then transferred to a 50 mL volumetric flask and diluted with methanol to a volume of 50 mL. Finally, both of the test samples were filtered through a 0.45 μM membrane filter prior to UPLC analysis. The UPLC parameters were used as previously reported.13
Cell Lines
Nine human cancer cell lines, including NCI-H1975, H292, H358, U266, RPMI-8226, MM1R, MKN45, MCF-7, and SW620 and a bronchial epithelium cell line (16HBE), were purchased from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All these cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA).
Cell Viability
Cancer cell growth was quantified by Cell Counting Kit-8 (CCK-8; DOJINDO) assay according to the manufacturer’s instructions.14 Briefly, the cells were plated in a 96-well plate (0.6 × 105 cells/well) and incubated overnight with 100 μL of medium. The cells were treated with different concentrations of physalins (0, 2.5, 5, 10, 15, 20 or 30 μg/mL) for 24 or 48 h. DMSO was added to reach sufficient volume for analysis. The absorbance was measured at 450 nm by a microplate spectrophotometer (Varioskan Flash, Thermo Fisher Scientific) after the cells was cultured with 10 μL of CCK-8 reagent for about 2 h. The IC50 value was used to evaluate the cytotoxicity of the drug which is needed to achieve 50% growth inhibition in vitro. The IC50 value was calculated by the fitted line (Y = aX + b) in GraphPad Prism 5.
Western Blot Analysis and the Relevant Reagents
Cold lysis buffer (150 mM NaCl, 1% NP-40, 50 mM Tris–HCl, pH 8.0, supplemented with Complete Protease Inhibitor, Roche) was added to the plates. After centrifugation and concentration qualification, the whole protein extracts were mixed with loading buffer and then boiled for 10 min. After separation with SDS-PAGE, the proteins were transferred to PVDF membranes. 5% BSA was used for blocking. Primary antibodies against STAT3, p-STAT3-Tyr, p-STAT3-Ser, Mcl-1, Bcl-2, Bcl-xL, survivin, cleaved caspase-3, cleaved caspase-9, PARP (1:1000, Cell Signaling Technology, Beverly, MA, USA), and β-Actin (1:3000 dilution, Sigma-Aldrich, Merck KGaA, St Louis, MO, USA). The membranes were then incubated with secondary antibodies (1:8000 dilution, Lianke Bio, P.R. China). The immunoreactive proteins were detected using a chemiluminescent immunodetection system (ChemiDocTM XRS). The semiquantification of protein levels was performed with ImageJ software. The quantities of the relative gray values are presented as a ratio of each protein band relative to the lane’s loading control.
STAT3 small interfering RNA (sense/antisense: 5′-GGGACCUGGUGUGAAUU AUTT-3′, 5′-AUAAUUCACACCAGGUCCCTT-3′) and scrambled control siRNA (sense/antisense 5′-UUCUCCGAACGUGUCACGUTT-3′, 5′-ACGUGACACGUUCGGAGAATT-3′) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
Apoptosis Detection Assay
Different cells (1×105 per well) were seeded overnight on 12-well plates until reaching 70% confluence, and then were treated with various concentrations of physalins (0, 5, 10, and 15 μg/mL) for 24 h. DMSO was added to reach a sufficient volume for each group. After trypsinization, the cells were washed twice with cold PBS and then mixed with binding buffer with anti-Annexin V-FITC/PI for 15 min according to the manufacturer’s instructions. Then, the apoptotic cells were evaluated by a FACSCanto II flow cytometer (BD Pharmingen) and FlowJo software (TreeStar Inc).
Immunofluorescence
H1975 cells were treated with 15 μg/mL physalins for 6 h followed by treatment with recombinant IL-6 protein (25 ng/mL) to induce pYSTAT3 levels, fixed in 4% paraformaldehyde for 15 min on ice and permeabilized with precooled methanol for another 10 min on ice. Next, the cells were blocked with blocking solution (5% normal goat serum, 0.3% Triton X-100 in PBS) for 60 mins at room temperature. After aspirating the blocking solution, the diluted primary antibody p-STAT3-Tyr (1:500, Cell Signaling Technology) was applied and incubated overnight at 4°C. The next day, the slides were rinsed in PBS 3 times and incubated with a fluorochrome-conjugated secondary antibody diluted in antibody dilution buffer (1% BSA and 0.3% Triton X-100 in PBS) for 1–2 h away from the light. The slides were rinsed with PBS 3 times and coverslipped with Prolong® Gold Anti-Fade Reagent (#9071; Cell Signaling) with DAPI (#8961; Cell Signaling).
Xenograft Mouse Model
A total of 20 male BALB/c mice were assigned to 4 groups (5 mice/group) and maintained under pathogen-free conditions at room temperature (21 to 25°C), with 12 h–12 h light–dark cycle. Food and water were offered ad libitum. A total of 5 × 106 human NCI-H1975 cells suspended in PBS were subcutaneously injected into the right front leg of 6-week-old male BALB/c mice. When the tumors reached 100–150 mm3, 100 or 200 mg/kg/day (calculated according to clinical condition and converted using formula, ie, mouse dosage = X mg/kg×70 kg×0.0026/20 g = 9.1 ×X mg/kg) of the extract of the physalins was mixed with 0.5% CMC-Na as a suspension and administered orally once a day for 12 days in the physalin-treated groups, and 0.5% CMC-Na was administered orally to the control group. In the cisplatin (DDP) treated group, 5 mg/kg/day DDP was intraperitoneally injected for 12 days. The tumor volume and body weight were assessed every two days. The tumor volume was calculated as V = length × width2/2.15 The mice were sacrificed quickly by cervical dislocation, and then, the tumors were obtained and measured.
Statistical Analysis
The statistical calculation of differences between the means was conducted by a Student’s t-tests and is shown as the mean ± SD in each group. An analysis of variance (ANOVA), including the F value, was carried out with Tukey’s post hoc test for multiple comparisons. Statistical significance was considered at P<0.05 or P<0.01. All experiments were replicated three times.
Results
Quality Evaluation of Physalis alkekengi var. franchetii
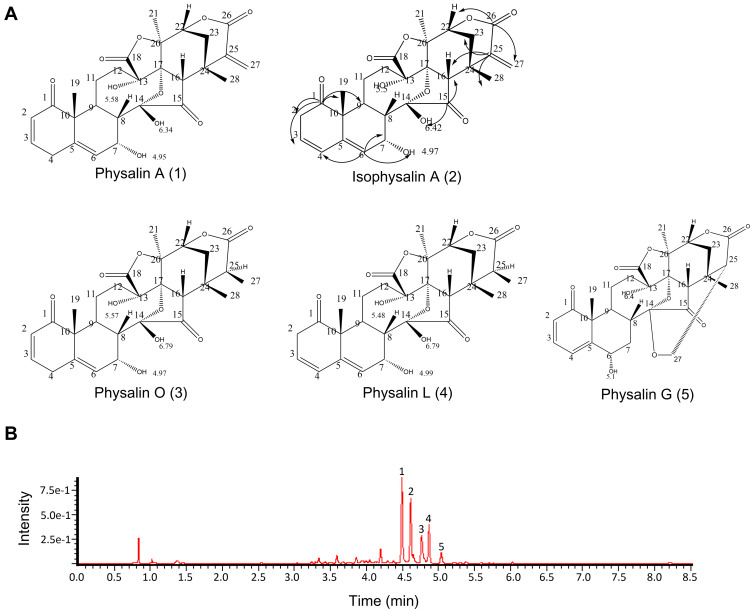
According to previous studies, physalins are identified as the main active and characteristic components of Physalis alkekengi var. franchetii.9 Five physalins were isolated and identified from the calyces of P. alkekengi var. franchetii in our previous study.13 The results from UPLC-MS also showed that the main extracts contained five physalins, physalin A, physalin G, physalin O, physalin L, and isophysalin, of which the levels were 26.03%, 2.66%, 52.06%, 12.92%, and 1.33%, respectively (Figure 1). However, based on a peak area normalization method, other components constituted only 5% of the extracts, and the extracts used in this study were endotoxin-free. Thus, the effects of the other components on the in vitro experiment could be ignored.
Figure 1.
The main extracts of Physalis alkekengi var. franchetii. (A) Chemical structures of the physalins extracted from Physalis alkekengi var. franchetii. (B) UPLC and total ion current chromatogram. Peaks: 1=physalin A, 2= isophysalin A, 3= physalin O, 4= physalin L and 5= physalin G.
Physalis alkekengi var. franchetii Extracts Inhibit the Viability of NCI-H1975 and U266 Cells
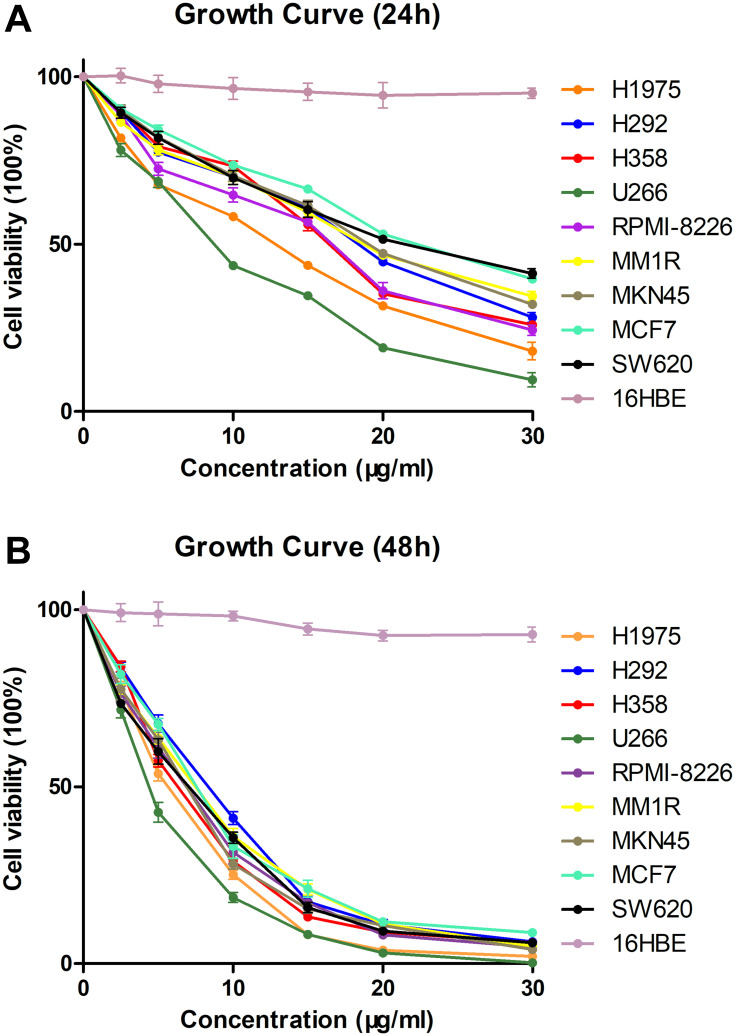
To evaluate the antitumoural activities of the physalins isolated from Physalis alkekengi var. franchetii, nine human tumor cell lines (NCI-H1975, NCI-H292, NCI-H358, U266, RPMI-8226, MM1R, MKN45, MCF-7, and SW620 cells) and a bronchial epithelium cell line (16HBE cells) were treated with different doses of the extracts and incubated for 24 h or 48 h and were then subjected to a CCK-8 assay (Cell Counting Kit-8, DOJINDO) (Figure 2A and B). The results showed that all the detected tumor cell lines were sensitive to physalins; in particular, physalins had greater inhibitory effects on NCI-H1975 and U266 cells with lower IC50 values after 24 or 48 h of incubation (Table 1). There was no significant inhibition on human normal cell line 16HBE.
Figure 2.
Viability of human tumor cells in the presence of Physalis alkekengi var. franchetii extracts. Cells treated with various doses of physalins (0, 2.5, 5, 10, 15, 20 and 30 μg/mL) for 24 h (A) or 48 h (B). CCK-8 assays were performed to examine cell viability.
Table 1.
IC50 Values of Physalis alkekengi var. franchetii Extracts for Nine Human Tumor Cell Lines
| Cell Line | 24h | 48h | ||||
|---|---|---|---|---|---|---|
| IC50 (μg/mL) | R Square | Std. Error (LogIC50) | IC50 (μg/mL) | R Square | Std. Error (LogIC50) | |
| NCI-H1975 | 10.78 | 0.9648 | 0.01832 | 6.442 | 0.9956 | 0.02125 |
| NCI-H292 | 17.35 | 0.9532 | 0.01934 | 9.011 | 0.9937 | 0.01838 |
| NCI-H358 | 15.67 | 0.9535 | 0.01849 | 5.879 | 0.9973 | 0.02170 |
| U266 | 8.071 | 0.9727 | 0.01749 | 4.131 | 0.9970 | 0.06401 |
| RPMI-8226 | 14.32 | 0.9421 | 0.02240 | 8.125 | 0.9955 | 0.01711 |
| MM1R | 18.68 | 0.9671 | 0.01692 | 9.053 | 0.9976 | 0.01321 |
| MKN45 | 18.68 | 0.9770 | 0.01340 | 7.650 | 0.9954 | 0.01536 |
| MCF7 | 22.82 | 0.9787 | 0.01390 | 7.883 | 0.9946 | 0.01703 |
| SW620 | 21.68 | 0.9904 | 0.009706 | 8.944 | 0.9935 | 0.01825 |
Previous studies have shown that STAT3 is activated and is highly expressed in these two cell lines, which indicates a connection between the inhibitory mechanism of physalins and the STAT3 signaling pathway. Therefore, three NSCLC cell lines and three MM cell lines were treated with the physalins for 4 h, and the results showed that the p-Tyr levels of STAT3 were extremely high in NCI-H1975 and U266 cells comparing to other cell lines (Supplementary Figure S1A and B). After physalin treatment, Tyr705 phosphorylation levels of STAT3 were inhibited in all six cell lines but were greatly inhibited in NCI-H1975 and U266 cells (Supplementary Figure S1A, B). Therefore, STAT3 activation was the underlying mechanism in the present study.
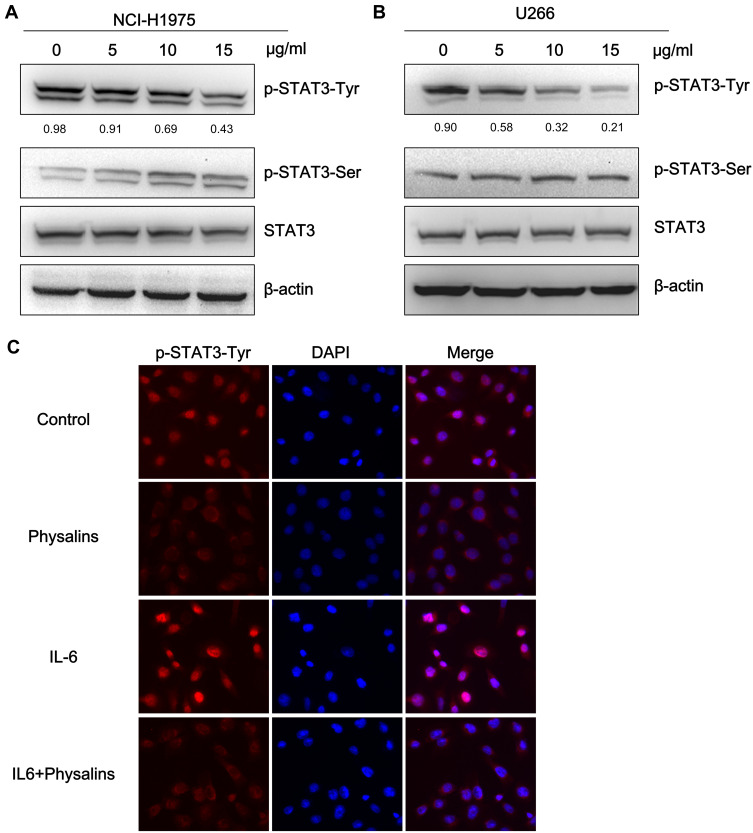
Physalis alkekengi var. franchetii Extracts Reduced the Phosphorylation (Tyr705) of STAT3 in NCI-H1975 and U266 Cells
To test the above hypothesis, we initially observed the STAT3 activity differences after physalin treatment by examining the phosphorylation levels of STAT3. NCI-H1975 and U266 cells were first administered different concentrations of physalins for 4 h. The Western blot results showed that the level of STAT3-Tyr705-phosphorylation in the physalin-treated group decreased significantly in a concentration-dependent manner compared with the level in the DMSO treated group (Figure 3A and B). In contrast, the level of p-Ser727 of STAT3 in the NCI-H1975 cells was increased after the physalins treatment, which may be attributed to the intrinsic mechanism for shortening the duration of STAT3 activity. However, in the U266 cells we did not find a change in p-Ser727 levels. The total STAT3 levels also showed no change in either cell line. Collectively, these results suggested that physalins suppress constitutive STAT3 Tyr705 phosphorylation in NCI-H1975 and U266 cells.
Figure 3.
Physalis alkekengi var. franchetii extracts suppressed constitutive STAT3 activity, and IL-6-induced STAT3 Tyr705 phosphorylation in NCI-H1975 and U266 cells. (A) NCI-H1975 cells were treated for 4 h with physalins (0, 5, 10, 15 μg/mL). The levels of p-STAT3-Tyr, p-STAT3-Ser, STAT3 and β-actin were detected by Western blot analysis. (B) U266 cells were treated for 4 h with physalins in a dose-dependent manner. The cell lysates were subjected to a Western blot analysis using antibodies specific for p-STAT3-Tyr, p-STAT3-Ser, STAT3 and β-actin. The semiquantification of the protein levels was performed with Image J software. The relative gray values of p-STAT3-Tyr are shown below. (C) Physalins suppressed p-STAT3 nuclear translocation. H1975 cells were treated with 15 μg/mL of physalins for 6 h with or without 25 ng/mL IL-6. Immunofluorescence analysis was performed with an anti-p-STAT3-Tyr primary antibody followed by an anti-rabbit IgG Fab2 Alexa Fluor 555 antibody. Coverslipped slides were covered with anti-fade reagents with DAPI. The merged images show the overlay of red Alexa Fluor 555 and blue DAPI fluorescence.
Previous reports identified that STAT3 phosphorylation at Tyr705 (pYSTAT3) leads to STAT3 nuclear translocation in response to various extracellular cytokines, such as interleukin (IL)-6.16 Therefore, we examined whether physalins can repress the nuclear translocation of pYSTAT3 in NCI-H1975 cells. Immunofluorescence assays showed that the immunoreactivity of anti-pYSTAT3 was predominantly increased in the nuclei of IL-6–stimulated or unstimulated NCI-H1975 cells but was apparently decreased by 15 μg/mL physalin treatment for 6 h (Figure 3C).
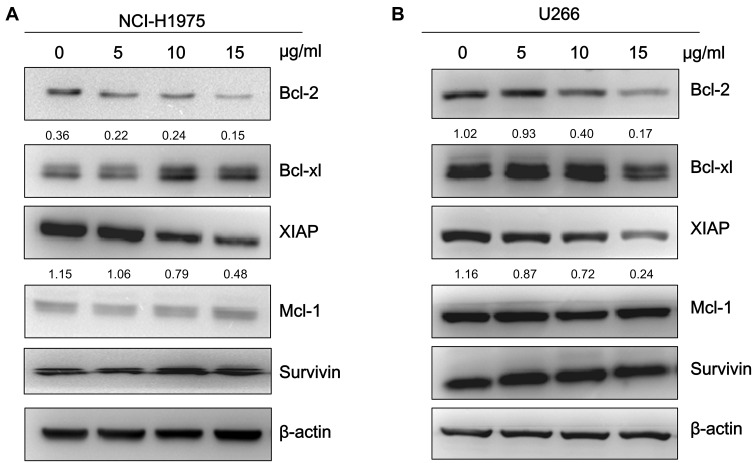
Physalis alkekengi var. franchetii Extracts Suppress the Expression of STAT3-Regulated Genes in NCI-H1975 and U266 Cells
To further investigate whether the physalins suppress STAT3-mediated downstream signaling, we determined the expression levels of the target genes of STAT3, including antiapoptotic proteins in the Bcl-2 family (Mcl-1, Bcl-2, and Bcl-xL), XIAP, and survivin by Western blot analysis. The expression levels of Bcl-2 and XIAP in NCI-H1975 and U266 cells were significantly reduced after physalin exposure for 24 h. Moreover, the decrease occurred in a concentration-dependent manner (Figure 4A, B). The band signals of both Bcl-2 and XIAP were greatly diminished after an incubation with the physalins at 15 μg/mL. However, the expression levels of the other detected genes (Mcl-1, Bcl-xL and survivin) showed no significant difference. Therefore, these results indicated that the P. alkekengi var. franchetii extracts further inhibited the STAT3-regulated downstream genes Bcl-2 and XIAP. Then, NCI-H1975 and U266 cells were transfected with siSTAT3 or scrambled siRNA and incubated for 24 h. STAT3, p-STAT3-Tyr and the downstream anti-apoptotic protein STAT3 were detected (Supplementary Figure S2). The results showed that STAT3 knockdown in NCI-H1975 and U266 cells significantly reduced Bcl2 and XIAP levels. Therefore, blocking the STAT3 signaling pathway plays an important role in promoting cell apoptosis.
Figure 4.
Physalis alkekengi var. franchetii extracts suppressed STAT3-mediated downstream genes in NCI-H1975 and U266 cells. (A) NCI-H1975 cells were incubated with various concentrations of the physalins for 24 h. The cell lysates were isolated for Western blot analysis to detect the Bcl-2 family, XIAP and survivin protein levels. β-actin was used as a loading control. (B) U266 cells were treated with physalins in a dose-dependent manner for 24 h. Western blot was performed with the anti-Bcl-2 family, anti-XIAP and anti-survivin primary antibodies. The semiquantification of protein levels was performed with ImageJ software. The relative gray values of Bcl-2 and XIAP are shown below.
Physalis alkekengi var. franchetii Extracts Induce the Apoptosis of NCI-H1975 and U266 Cells
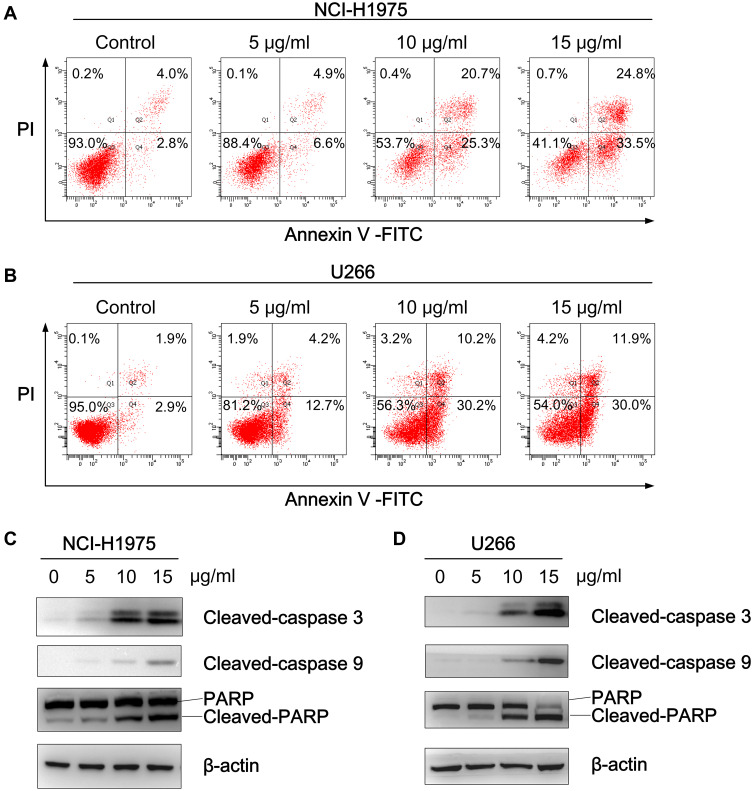
Since Bcl-2 and XIAP are known to be potent inhibitors of apoptosis, an Annexin V-FITC/PI assay was subsequently performed to investigate whether physalins induce the apoptosis of NCI-H1975 and U266 cells. As expected, the flow cytometry results showed that physalins significantly elevated the apoptotic rate of the NCI-H1975 and U266 cells in a concentration-dependent manner (Figure 5A and B). Compared with the control groups, the proportion of apoptotic NCI-H1975 cells (the upper right panel for the late stage and the lower right panel for the early stage of apoptosis in Figure 5A) increased from 6.8±1.2% to 11.5±3.1%~58.3±6.1% after physalin treatment at doses of 5~15 μg/mL for 24 h. Similarly, the percentage of apoptotic U266 cells in the physalin-treated groups (18.6±3.6%~43.4±10.4%) were much higher than that in the control group (4.5±1.0%). Correspondingly, the apoptosis related proteins cleaved caspase 3, cleaved caspase 9 and cleaved poly (ADP-ribose) polymerase (PARP) were significantly increased in the physalin-administered groups (24 h treatment) (Figure 5C and D). Collectively, these results demonstrated that P. alkekengi var. franchetii extracts promoted the apoptosis of NCI-H1975 and U266 cells by regulating the STAT3 signaling pathway.
Figure 5.
Physalis alkekengi var. franchetii extracts induced the poptosis of NCI-H1975 and U266 cells. (A) NCI-H1975 cells and (B) U266 cells were treated with different concentrations of physalins for 24 h, and then, the cells were collected and labeled with Annexin V-FITC/PI followed by flow cytometric analysis. (C) NCI-H1975 cells and (D) U266 cells were treated with physalins in a dose-dependent manner for 24 h. Western blotting was performed with anti-cleaved caspase-9, anti-cleaved caspase-3 and anti-PARP antibodies.
Furthermore, physalin A, physalin O and physalin L (26.03%, 52.06% and 12.92%, in the physalin extract, respectively) were used to treat NCI-H1975 cells alone (3.9 µg/mL, 7.8 µg/mL and 1.9 µg/mL) or in combination for 24 h. Cells were collected and analyzed by Annexin V-FITC/PI assay. FACS analysis showed that physalin A lead to approximately 19.7% apoptotic cells; however, physalin O and physalin L weakly induced apoptosis. Interestingly, the combination of physalin A, physalin O and physalin L drove 41.8% of the cells to apoptosis (Supplementary Figure S3A). Western blot analysis showed that combined treatment with the three physalins strongly blocked STAT3 activation. Physalin A partially inhibited STAT3 activation; however, physalin O and physalin L had no obvious effect (Supplementary Figure S3B).
Physalis alkekengi var. franchetii Extracts Inhibit Tumor Growth in a Human NSCLC Xenograft Model
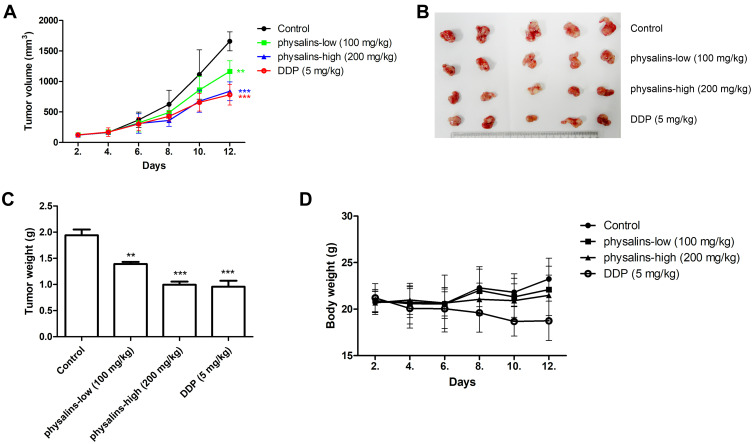
Finally, NCI-H1975 xenograft models were used to evaluate the antitumor activity of the physalins. Compared to the control group, tumor growth (tumor volume) was significantly inhibited in the high-dose physalin-treated group (200 mg/kg) and the cisplatin (DDP)-treated (5 mg/kg) group after 12 days of survival (Figure 6A and C). Then, the mice were sacrificed, showing that the tumor volume was reduced in the low-dose physalin-treated group (100 mg/kg) (P=0.0057, F=3.677) and was obviously decreased in the high-dose physalin-treated group (P<0.0001, F=10.71) and the DDP-treated group (P<0.0001, F=13.06) (Figure 6A). The physalins significantly reduced the body weight compared with the control and physalin-treated groups, as indicated by ANOVA (P<0.0001, F=38.58). The tumor weight was reduced in the low-dose physalin-treated group (100 mg/kg) (P=0.0016) and was obviously decreased in the high-dose physalin-treated group (P<0.0001) and the DDP-treated group (P=0.0003) (Figure 6B). The weights of the physalin-treated groups displayed no significant difference compared with the control group, but the DDP-treated group showed weight loss (P=0.01) (Figure 6D). Taken together, these results demonstrated that the antitumor activity of physalins was comparable to that of cisplatin, a chemotherapy agent, but physalins mitigated the side effects of body weight loss.
Figure 6.
Physalis alkekengi var. franchetii extracts inhibited tumor growth in a human NSCLC xenograft model. (A) Tumor volumes were measured at the indicated times using Vernier calipers. Tumor volume = length × width2/2. ** for P<0.01 and *** for P<0.001. The P values were analyzed by ANOVA with a post hoc test. (B) After 10 days, all the mice were sacrificed, and the tumors were arranged and photographed. (C) The average tumor weights were analyzed in each group. The statistical analysis was performed using Student’s t-test and one-way ANOVA, ** for P<0.01 and *** for P < 0.001. (D) The body weights were measured at the indicated times.
Discussion
Natural compounds derived from food, herbs and plants have been used as antitumor agents.17 Population studies suggest that potential natural cancer preventive compounds are safe, practical, economical, and effective for cancer treatment.18 For example, P. alkekengi var. franchetii is an herb that has captured interest due to its therapeutic properties.1 Previous studies have demonstrated that several extracts from the calyces of P. alkekengi var. franchetii are vital in combating cancer.4,6 For example, in different cancers, physalin A inhibits tumor cell growth, promotes apoptosis and autophagy via Nrf2 signaling, JAK-STAT3 signaling or ROS generation.4,19–21
In our study, the main components from the calyces of P. alkekengi var. franchetii extracts were five physalins, that were identified by UPLC. Physalin species are considered of great medicinal value since these compounds display various biological activities, such as antimicrobial, antitumor, anti-inflammatory, immunomodulatory, immunosuppressive, cytotoxic, trypanocidal, and molluscicidal effects.7,22–27 Previously, we reported that the antitumor growth and apoptosis-promoting effects of physalin A were dependent on suppressing STAT3 activity in NSCLC cell lines.4 Our current study focused on the antitumor effect of the combination of five physalins from the calyces of P. alkekengi var. franchetii. This antitumor effect was initially evaluated in seven types of cell lines and the results showed that the physalins inhibited the growth of all the tested cell lines, which suggested that the physalins exhibited nonselective antitumor activity. Moreover, the antitumor molecular mechanism of the physalins was explored in vitro using representative solid tumor (NCI-H1975) and hematological tumor (U266) cell lines.
STAT3 is vital in the initiation and progression of various cancers, such as proliferation, antiapoptosis, invasion and immune surveillance evasion.28–30 It is activated by the phosphorylation of its tyrosine or serine residues by modulating upstream regulators.31,32 The phosphorylation of STAT3 induces dimerization through the interactions of reciprocal phosphotyrosine-Src homology domain 2 (SH2). Activated STAT3 dimers initiate the transcription of target genes by translocating to the nucleus and interacting with the promoter sequence.31,33 Parent STAT3 activation is related to a wide spectrum of cancers, including NSCLC and MM, and is positively associated with a poor prognosis.34–37 A wide range of preclinical studies have shown that phosphorylated-STAT3 (pSTAT3) is a well-known characteristic of various cancers.38–40 Accumulating evidence shows that constitutive STAT3 activation occurs frequently in a wide range of tumor cells, including MM and in more than 22%~65% of NSCLC cases.41–44 This indicates that aberrant STAT3 signaling is an important process in malignant progression. Therefore, the inhibition of STAT3 signaling is considered a novel and potential target to treat cancers.
STAT3 is activated by Tyr705 phosphorylation in response to membrane factors such as interleukin-6 (IL-6).45 It has also been shown that Ser727 of STAT3 is phosphorylated by various kinds of kinases in various cancers.46–49 However, some reports have shown that the Ser727 site phosphorylation suppresses STAT3 activity and cell proliferation.50,51 In our study, we reported that P. alkekengi var. franchetii extracts repressed Tyr705 phosphorylation of STAT3 in H1975 and U266 cells but have increased Ser727 phosphorylation of STAT3 in H1975 cells.
In our previous study, we illustrated that physalins acted as Michael reaction factors and directly induced a rapid drop in the concentration of intracellular glutathione (GSH), thereby triggering the S-glutathionylation of STAT3 and inhibiting the tyrosine phosphorylation of STAT3.13,52 In this study, P. alkekengi var. franchetii extracts inhibited the level of STAT3 tyrosine phosphorylation and further suppressed the expression of STAT3 downstream target genes. Bcl-2 and XIAP are both anti-apoptotic proteins. Following Bcl-2 downregulation, the proapoptotic proteins are subsequently activated, resulting in mitochondrial outer membrane permeabilization (MOMP). Then, cytochrome c and second mitochondria-derived activator of caspase (SMAC) are released from the mitochondria, which results in the activation of caspase 9. Caspase 9 then activates procaspase 3 and procaspase 7, leading to cell death.53 XIAP, X-linked inhibitor of apoptosis, inhibits caspase 9.54 Another apoptotic marker, cleaved PARP, is catalyzed by caspase-3.55 In brief, P. alkekengi var. franchetii extracts inhibit STAT3-targeted anti-apoptotic proteins, which leads to the cleavage of both caspase-3 and PARP, resulting in increased apoptosis rates of both NCI-H1975 and U266 cells. Therefore, suppressing the continuous activation of STAT3 may be an effective target for cancer therapy. It is meaningful and valuable to discover and develop effective STAT3 inhibitors as antitumor components in the treatment of cancer. However, the inhibitory specificity and selectivity of the physalins are still unknown and will be further clarified by a molecular docking assay and competitive inhibition experiment.
Notably, based on the results from the in vitro experiments, including cell proliferation and apoptosis, we are aware that the effects of P. alkekengi var. franchetii extracts on NCI-H1975 and U266 cells are almost identical, which suggests that the physalins from P. alkekengi var. franchetii may have similar or identical antitumor activity on solid tumors and hematological tumors. Moreover, the antitumor effects of the P. alkekengi var. franchetii extracts were tested using a human xenograft model of lung cancer. Consistent with the in vitro results, the data showed that the physalins from P. alkekengi var. franchetii inhibited tumor growth, as indicated by decreases in tumor volume and weight in the xenograft model.
In the human NSCLC xenograft model, the body weights of the physalin-treated groups were not significantly different compared with the control group, but the DDP-treated group showed weight loss. Moreover, the physalin-treated groups did not have an adverse reaction during the observation period; therefore, P. alkekengi var. franchetii extracts are safe, induce low-toxicity and no obvious side effects. P. alkekengi L. extracts are more convenient for large-scale preparation and extraction than purified physalin monomers. Moreover, herbal cocktail therapy can partially attenuate single drug resistance. After isolating the main physalins in the extracts, we found that the combination of physalin A, physalin O and physalin L can increase cell death and inhibit STAT3 activation. The detailed mechanism of this combination will be further explored in the context of therapy our next paper.
In summary, the P. alkekengi var. franchetii extracts inhibited tumor proliferation and promoted apoptosis of NSCLC and MM cells by repressing STAT3 signaling. The findings of this study will aid in the development of the physalins from P. alkekengi var. franchetii as a promising antitumor drugs. Clinical evaluation and application were the main limitations of our study, and these can be future research directions.
Conclusions
Collectively, our study demonstrates that the extracts from Physalis alkekengi var. franchetii have antitumor effects against NSCLC and MM. The results reported that the P. alkekengi var. franchetii extracts inhibited tumor growth and induced the apoptosis of human NSCLC and MM cell lines by repressing the STAT3 signaling pathway. In addition, the physalins significantly reduced tumor growth in xenograft models of lung cancer without weight loss. Importantly, these findings imply that the extracts of Physalis alkekengi var. franchetii might be a safe and promising agents for use in cancer treatment.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China [grant numbers: 81603340, 81773945, 81802887, 81873276, 81503297, 81803776], Medical Health Science and Technology Project of Zhejiang Provincial Health Commission [grant numbers: 2019RC228, 2019RC229], the Zhejiang Provincial Natural Science Foundation of China [grant numbers: LY21H030002, LY17H280006], the Opening Project of Zhejiang Provincial First-rate Subject (Integrated Traditional and Western Medicine) of the Zhejiang Chinese Medical University [grant numbers: ZXYJH2018004]; Traditional Chinese Medicine Scientific Research Foundation of Zhejiang Province [grant numbers: 2018ZB044].
Abbreviations
P. alkekengi var. franchetii, Physalis alkekengi var. franchetii; NSCLC, non-small cell lung cancer; MM, multiple myeloma; ROS, reactive oxygen species; PARP, poly (ADP-ribose) polymerase; DDP, cisplatin; GSH, glutathione.
Data Sharing Statement
The materials and data of this study are available to other researchers upon request.
Ethics Approval and Consent to Participate
The animal study was approved by the ethics committee for research on laboratory animal use of the Animal Experimental Center, Zhejiang Academy of Medical Sciences. For an animal carrying one tumor, the diameter should not exceed 2.0 cm in mice for therapeutic studies according to the guidelines from the University of Pennsylvania Institutional Animal Care and Use Committee. This study was carried out in accordance with the local guidelines for the welfare of laboratory animals of the Animal Experimental Center, Zhejiang Academy of Medical Sciences.
Consent for Publication
All authors have provided consent for publication.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interests.
References
- 1.Guo Y, Liu H, Ding L, Oppong M, Pan G, Qiu F. LC-MS/MS method for simultaneous determination of flavonoids and physalins in rat plasma: application to pharmacokinetic study after oral administration of Physalis alkekengi var. franchetii (Chinese lantern) extract. Biomed Chromatography. 2017;31(10):e3970. doi: 10.1002/bmc.3970 [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Ding AW. On the subsequent revision of pharmacopoeia of the People’s Republic of China: volume Ⅰ (2010 Edition)——Based on the Regulation of Crude Drugs Standard in the Japanese Pharmacopoeia(16th Edition). J Nanjing Univ Trad Chinese Med. 2012;28(4):301–365. [Google Scholar]
- 3.Wang ZY, Su -T-T, Li N. Study on extraction and antimicrobial activity of extracts from physalis alkekengil l. var. francheti(mast.) makino. Food Sci Technol. 2009. [Google Scholar]
- 4.Zhu F, Dai C, Fu Y, et al. Physalin A exerts anti-tumor activity in non-small cell lung cancer cell lines by suppressing JAK/STAT3 signaling. Oncotarget. 2016;7(8):9462–9476. doi: 10.18632/oncotarget.7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang H, Kwon SR, Choi HY. Inhibitory effect of Physalis alkekengi L Var Franchetii Extract and Its Chloroform Fraction on LPS or LPS/IFN-?-Stimulated inflammatory response in peritoneal macrophages. J Ethnopharmacol. 2011;135(1):95–101. [DOI] [PubMed] [Google Scholar]
- 6.Magalhães HIF, Torres MR, Costa-Lotufo LV, et al. In-vitro and in-vivo antitumour activity of physalins B and D from Physalis angulata. J Pharm Pharmacol. 2006;58(2):235–241. doi: 10.1211/jpp.58.2.0011 [DOI] [PubMed] [Google Scholar]
- 7.Soares MB, Bellintani MC, Ribeiro IM, Tomassini TC, DSR Ribeiro. inhibition of macrophage activation and lipopolysaccaride-induced death by seco-steroids purified from Physalis Angulata L. Eur J Pharmacol. 2003;459(1):107. [DOI] [PubMed] [Google Scholar]
- 8.Castro DP, Figueiredo MB, Ribeiro IM, Tomassini TC, Azambuja P, Garcia ES. Immune depression in Rhodnius prolixus by seco-steroids, physalins. J Insect Physiol. 2008;54(3):555–562. doi: 10.1016/j.jinsphys.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Chen Y, Ren Y, Luan L, Wu Y. Quantitative and transformation product analysis of major active physalins from Physalis alkekengi var. franchetii (Chinese lantern) using ultraperformance liquid chromatography with electrospray ionisation tandem mass spectrometry and time-of-flight mass spectrometry. Phytochem Anal. 2012;23(4):337–344. doi: 10.1002/pca.1363 [DOI] [PubMed] [Google Scholar]
- 10.Hsu CC, Wu YC, Farh L, et al. Physalin B from Physalis angulata triggers the NOXA-related apoptosis pathway of human melanoma A375 cells. Food Chem Toxicol. 2012;50(3–4):619–624. doi: 10.1016/j.fct.2011.12.017 [DOI] [PubMed] [Google Scholar]
- 11.Wu SY, Leu YL, Chang YL, et al. Physalin F induces cell apoptosis in human renal carcinoma cells by targeting NF-kappaB and generating reactive oxygen species. PLoS One. 2012;7(7):e40727. doi: 10.1371/journal.pone.0040727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helvaci S, Kokdil G, Kawai M, Duran N, Duran G, Guvenc A. Antimicrobial activity of the extracts and physalin D from Physalis alkekengi and evaluation of antioxidant potential of physalin D. Pharm Biol. 2010;48(2):142–150. doi: 10.3109/13880200903062606 [DOI] [PubMed] [Google Scholar]
- 13.Ji L, Yuan Y, Luo L, et al. Physalins with anti-inflammatory activity are present in Physalis alkekengi var. franchetii and can function as Michael reaction acceptors. Steroids. 2012;77(5):441–447. doi: 10.1016/j.steroids.2011.11.016 [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, Ma X, Sun W, Cui P, Lu Z. Down-regulated FSTL5 promotes cell proliferation and survival by affecting Wnt/β-catenin signaling in hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8(3):3386–3394. [PMC free article] [PubMed] [Google Scholar]
- 15.Naito S, von Eschenbach AC, Giavazzi R, Fidler IJ. Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Res. 1986;46(8):4109–4115. [PubMed] [Google Scholar]
- 16.Koo MY, Park J, Lim JM, et al. Selective inhibition of the function of tyrosine-phosphorylated STAT3 with a phosphorylation site-specific intrabody. Proc Natl Acad Sci U S A. 2014;111(17):6269–6274. doi: 10.1073/pnas.1316815111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubió L, Motilva MJ, Romero MP. Recent advances in biologically active compounds in herbs and spices: a review of the most effective antioxidant and anti-inflammatory active principles. Crit Rev Food Sci Nutr. 2013;53(9):943–953. doi: 10.1080/10408398.2011.574802 [DOI] [PubMed] [Google Scholar]
- 18.Gullett NP, Ruhul Amin AR, Bayraktar S, et al. Cancer Prevention with Natural Compounds. 2010:258–281. [DOI] [PubMed] [Google Scholar]
- 19.He H, Zang LH, Feng YS, et al. Physalin A induces apoptotic cell death and protective autophagy in HT1080 human fibrosarcoma cells. J Nat Prod. 2013;76(5):880–888. doi: 10.1021/np400017k [DOI] [PubMed] [Google Scholar]
- 20.Shin JM, Lee KM, Lee HJ, Yun JH, Nho CW. Physalin A regulates the Nrf2 pathway through ERK and p38 for induction of detoxifying enzymes. BMC Complement Altern Med. 2019;19(1):101. doi: 10.1186/s12906-019-2511-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He H, Zang LH, Feng YS, et al. Physalin A induces apoptosis via p53-Noxa-mediated ROS generation, and autophagy plays a protective role against apoptosis through p38-NF-kappaB survival pathway in A375-S2 cells. J Ethnopharmacol. 2013;148(2):544–555. doi: 10.1016/j.jep.2013.04.051 [DOI] [PubMed] [Google Scholar]
- 22.Chiang HC, Jaw SM, Chen CF, Kan WS. Antitumor agent, physalin F from Physalis angulata L. Anticancer Res. 1992;12(3):837–843. [PubMed] [Google Scholar]
- 23.Nagafuji S, Okabe H, Akahane H, Abe F. Trypanocidal constituents in plants 4. Withanolides from the Aerial Parts of Physalis Angulata. Biol Pharm Bull. 2004;27(2):193–197. [DOI] [PubMed] [Google Scholar]
- 24.Wu SJ, Ng LT, Lin DL, Huang SN, Wang SS, Lin CC. Physalis peruviana extract induces apoptosis in human Hep G2 cells through CD95/CD95L system and the mitochondrial signaling transduction pathway. Cancer Lett. 2004;215(2):199–208. doi: 10.1016/j.canlet.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 25.Wu SJ, Ng LT, Chen CH, Lin DL, Wang SS, Lin CC. Antihepatoma activity of Physalis angulata and P. peruviana extracts and their effects on apoptosis in human Hep G2 cells. Life Sci. 2004;74(16):2061–2073. doi: 10.1016/j.lfs.2003.09.058 [DOI] [PubMed] [Google Scholar]
- 26.Lee CC, Houghton P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J Ethnopharmacol. 2005;100(3):237–243. doi: 10.1016/j.jep.2005.01.064 [DOI] [PubMed] [Google Scholar]
- 27.Choi JY, Lee SJ, Lee SJ, et al. Analysis and tentative structure elucidation of new anthocyanins in fruit peel of Vitis coignetiae Pulliat (meoru) using LC-MS/MS: contribution to the overall antioxidant activity. J Sep Sci. 2010;33(9):1192–1197. doi: 10.1002/jssc.200900748 [DOI] [PubMed] [Google Scholar]
- 28.Darnell JE Jr. STATs and gene regulation. Science. 1997;277(5332):1630–1635. doi: 10.1126/science.277.5332.1630 [DOI] [PubMed] [Google Scholar]
- 29.Bromberg J, Darnell JE. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19(21):2468–2473. doi: 10.1038/sj.onc.1203476 [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Xu X, Feng X, Zhang B, Wang J. Adenovirus-mediated delivery of bFGF small interfering RNA reduces STAT3 phosphorylation and induces the depolarization of mitochondria and apoptosis in glioma cells U251. J Exp Clin Cancer Res. 2011;30(1):80. doi: 10.1186/1756-9966-30-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klemm JD, Schreiber SL, Crabtree GR. Dimerization as a regulatory mechanism in signal transduction. Annu Rev Immunol. 1998;16:569–592. doi: 10.1146/annurev.immunol.16.1.569 [DOI] [PubMed] [Google Scholar]
- 32.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19(49):5662–5679. doi: 10.1038/sj.onc.1203925 [DOI] [PubMed] [Google Scholar]
- 33.Banerjee K, Resat H. Constitutive activation of STAT3 in breast cancer cells: A review. Int J Cancer. 2016;138(11):2570–2578. doi: 10.1002/ijc.29923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez JV, Greulich H, Sellers WR, Meyerson M, Frank DA. Signal transducer and activator of transcription 3 is required for the oncogenic effects of non–small-cell lung cancer–associated mutations of the epidermal growth factor receptor. Cancer Res. 2006;66(6):3162–3168. doi: 10.1158/0008-5472.CAN-05-3757 [DOI] [PubMed] [Google Scholar]
- 35.Yin W, Cheepala S, Roberts JN, Sysonchan K, Digiovanni J, Clifford JL. Active Stat3 is required for survival of human squamous cell carcinoma cells in serum-free conditions. Mol Cancer. 2006;5(1):15. doi: 10.1186/1476-4598-5-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kusaba T, Nakayama T, Yamazumi K, et al. Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol Rep. 2006;15(6):1445–1451. [PubMed] [Google Scholar]
- 37.Morikawa T, Baba Y, Mai Y, et al. STAT3 expression, molecular features, inflammation patterns and prognosis in a database of 724 colorectal cancers. Clin Cancer Res off J Am Assoc Cancer Res. 2011;17(6):1452–1462. doi: 10.1158/1078-0432.CCR-10-2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jove R. STAT proteins: novel molecular targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8(5):409–422. doi: 10.1517/14728222.8.5.409 [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Crowe PJ, Goldstein D, Yang JL. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers (review). Int J Oncol. 2012;41(4):1181–1191. doi: 10.3892/ijo.2012.1568 [DOI] [PubMed] [Google Scholar]
- 40.CatlettFalcone R, Dalton WS, Jove R. STAT proteins as novel targets for cancer therapy. Curr Opin Oncol. 1999;11(6):490–496. doi: 10.1097/00001622-199911000-00010 [DOI] [PubMed] [Google Scholar]
- 41.Gouilleux-Gruart V, Gouilleux F, Desaint C, et al. STAT-related transcription factors are constitutively activated in peripheral blood cells from acute leukemia patients. Blood. 1996;87(5):1692–1697. doi: 10.1182/blood.V87.5.1692.1692 [DOI] [PubMed] [Google Scholar]
- 42.WeberNordt RM, Egen C, Wehinger J, et al. Constitutive activation of STAT proteins in primary lymphoid and myeloid leukemia cells and in Epstein-Barr virus (EBV)-related lymphoma cell lines. Blood. 1996;88(3):809–816. doi: 10.1182/blood.V88.3.809.809 [DOI] [PubMed] [Google Scholar]
- 43.Harada D, Takigawa N, Kiura K. The role of STAT3 in non-small cell lung cancer. Cancers. 2014;6(2):708–722. doi: 10.3390/cancers6020708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10(1):105–115. doi: 10.1016/S1074-7613(00)80011-4 [DOI] [PubMed] [Google Scholar]
- 45.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8(4):241–252. doi: 10.1016/S1359-6101(98)80005-1 [DOI] [PubMed] [Google Scholar]
- 46.Tkach M, Rosemblit C, Rivas MA, et al. p42/p44 MAPK-mediated Stat3Ser727 phosphorylation is required for progestin-induced full activation of Stat3 and breast cancer growth. Endocr Relat Cancer. 2013;20(2):197–212. doi: 10.1530/ERC-12-0194 [DOI] [PubMed] [Google Scholar]
- 47.Aziz MH, Hafeez BB, Sand JM, et al. Protein kinase Cvarepsilon mediates Stat3Ser727 phosphorylation, Stat3-regulated gene expression, and cell invasion in various human cancer cell lines through integration with MAPK cascade (RAF-1, MEK1/2, and ERK1/2). Oncogene. 2010;29(21):3100–3109. doi: 10.1038/onc.2010.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin H, Chen MC, Chiu CY, Song YM, Lin SY. Cdk5 regulates STAT3 activation and cell proliferation in medullary thyroid carcinoma cells. J Biol Chem. 2007;282(5):2776–2784. doi: 10.1074/jbc.M607234200 [DOI] [PubMed] [Google Scholar]
- 49.Kim JH, Kim JE, Liu HY, Cao W, Chen J. Regulation of interleukin-6-induced hepatic insulin resistance by mammalian target of rapamycin through the STAT3-SOCS3 pathway. J Biol Chem. 2008;283(2):708–715. doi: 10.1074/jbc.M708568200 [DOI] [PubMed] [Google Scholar]
- 50.Wakahara R, Kunimoto H, Tanino K, et al. Phospho-Ser727 of STAT3 regulates STAT3 activity by enhancing dephosphorylation of phospho-Tyr705 largely through TC45. Genes Cells. 2012;17(2):132–145. doi: 10.1111/j.1365-2443.2011.01575.x [DOI] [PubMed] [Google Scholar]
- 51.Shi X, Zhang H, Paddon H, Lee G, Cao X, Pelech S. Phosphorylation of STAT3 serine-727 by cyclin-dependent kinase 1 is critical for nocodazole-induced mitotic arrest. Biochemistry. 2006;45(18):5857–5867. doi: 10.1021/bi052490j [DOI] [PubMed] [Google Scholar]
- 52.Butturini E, Cavalieri E, de Prati AC, et al. Two naturally occurring terpenes, dehydrocostuslactone and costunolide, decrease intracellular GSH content and inhibit STAT3 activation. PLoS One. 2011;6(5):e20174. doi: 10.1371/journal.pone.0020174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov. 2017;16(4):273–284. doi: 10.1038/nrd.2016.253 [DOI] [PubMed] [Google Scholar]
- 54.Schimmer AD, Welsh K, Pinilla C, et al. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell. 2004;5(1):25–35. doi: 10.1016/S1535-6108(03)00332-5 [DOI] [PubMed] [Google Scholar]
- 55.Boulares AH, Yakovlev AG, Ivanova V, et al. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-Resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274(33):22932–22940. [DOI] [PubMed] [Google Scholar]