Abstract
Background and Aim:
Mastitis has been identified as the most prevalent and economically imperative disease among dairy animals. Thus, understanding its common bacterial pathogens and risk factors is necessary to improve udder health at herd, region, or country level. However, scientific research on caprine mastitis, especially on Beetal breed, has remained to be insufficient in Pakistan. Therefore, this study aimed to evaluate the epidemiology and antibiogram assay of common mastitis-causing bacterial agents, that is, Staphylococcus, Streptococcus, and Escherichia coli, in dairy goats.
Materials and Methods:
In total, 500 Beetal goats, irrespective of age and those that were not treated with any kind of antimicrobial agents during the past 120 h, were screened using California Mastitis Test in Pattoki, Kasur District, whereas epidemiological factors were recorded. The milk samples of mastitic goats were then collected and processed using standard methods. Each sample was primarily cultured on nutrient agar. Using a specific medium, each bacterial colony was separated using several streak methods. Six antibiotic disks belonging to different antibiotic groups were used for antibiogram profiling of bacterial isolates. Chi-square test was used to assess the association of baseline characteristics and mastitis occurrence. Meanwhile, multivariable logistic regression (p<0.001) was utilized to determine the risk factors associated with positive and negative dichotomous outcome of mastitis.
Results:
The results revealed that the overall prevalence of goat mastitis was 309 (61.8%), in which 260 (52%) and 49 (9.8%) cases were positive for subclinical mastitis (SCM) and clinical mastitis (CM), respectively. Streptococcus and E. coli were found to be the predominant isolates causing SCM and CM, respectively (p<0.001). It was observed that amoxicillin+clavulanic acid was highly sensitive to isolates of Staphylococcus and Streptococcus and ceftiofur sodium to isolates of Streptococcus and E. coli., while enrofloxacin was found to be sensitive to isolates of Streptococcus and E. coli. Risk factors such as herd structure, deworming, vaccination, presence of ticks, use of teat dip and mineral supplements, feeding type, age, parity, housing, blood in the milk, milk leakage, milk taste, and milk yield were found to have the strongest association with mastitis occurrence, while ease of milking has moderate association.
Conclusion:
In the area examined, cases of SCM were found to be higher compared with that of CM, and ceftiofur sodium has been identified as the preferred treatment in both clinical and subclinical forms of caprine mastitis in Beetal goats. Risk factors for mastitis that was identified in this study can form the basis for the creation of an udder health control program specific for dairy goats. We hope our findings could raise awareness of the risk factors and treatment approaches for common mastitis-causing bacterial agents.
Keywords: antibiotic disks, Beetal goats, common bacteria, epidemiology, isolates, mastitis, Pattoki
Introduction
Worldwide, goats are primarily raised for milk, meat, and fiber production. Across the globe, Pakistan ranks third among countries with the largest goat production, following after India and China. Goats are known to be the poor man’s cow in Pakistan [1-5]. This could be attributed to goat’s milk being cheap, nutritious, easily digestible, and wholesome, which is recommended not only for its nutritional benefits for growing babies but also for its therapeutic effects in many diseases [5-7]. Mastitis has been identified as one of the expensive and multifactorial diseases affecting the udder tissue of dairy animals as this will often result in the decrease in milk quality, quantity, and yield, which in turn leads to drop in the overall milk production and also cause physical, chemical, pathological, and microbiological changes in the milk composition and impaired quality, an increase in somatic cells, especially leukocytes detrimental changes, transitory to permanent blocking of milk ducts, and also having the danger for the spread of milk-related zoonotic diseases [8]. In general, mastitis can either be clinical or subclinical. Clinical mastitis (CM) is often characterized by visible symptoms such as the udder becoming swollen or hot to the touch and changes in the organoleptic properties of milk. Meanwhile, subclinical mastitis (SCM) requires somatic cell count and bacteriological culturing of milk for accurate diagnosis; thus, various tests such as Surf Field Mastitis Test and California Mastitis Test (CMT) should be performed under field conditions [9]. Bacterial pathogens, such as Staphylococcus spp., Streptococcus spp., Escherichia coli, and Pseudomonas aeruginosa, have been reported as the main causative agents of mastitis in goats [10-12]. To improve udder health, it is necessary to determine the factors associated with the increased risk of SCM [13]. Reoccurrence of infection or the risk of infection can be attributed to factors such as poor treatment protocol during late lactation [14,15], low body score, long teats, milk fever, season, and prophylactic hygiene management; furthermore, infections in health-care centers have been identified as the major contributors to mastitis prevalence throughout the world [16-18].
Today, mastitis has been determined as the most common and expensive disease that plagued the entire dairy industry, with about two-thirds of its total economic losses attributed to SCM infection. Cases of mastitis in countries like Pakistan have seen an increasing trend due to poor disease prevention and reporting system [19]. Thus, understanding its common bacterial pathogens and risk factors is necessary to improve udder health at herd, region, or country level. In Pakistan, caprine mastitis has not been given enough attention as considerable efforts and resources have been focused on the control of mastitis infection among cattle and buffaloes; furthermore, overall scientific research data on caprine mastitis, especially on the recently documented strains of Beetal goat, are meager or non-existent at all [20-23].
Thus, there is a dire need to curtail this disease to produce milk according to the standards set by the World Trade Organization. This study has been conducted to raise awareness of the prevalence of caprine mastitis, its associated risk factors, and bacterial pathogens such as Staphylococcus, Streptococcus, and E. coli in lactating dairy goats in Pattoki Tehsil, Kasur District, Punjab, Pakistan. The findings of this study will be helpful in the creation of udder health control program specific for dairy goats.
Materials and Methods
The flowchart for the Materials and Methods is provided in Figure-1.
Figure-1.
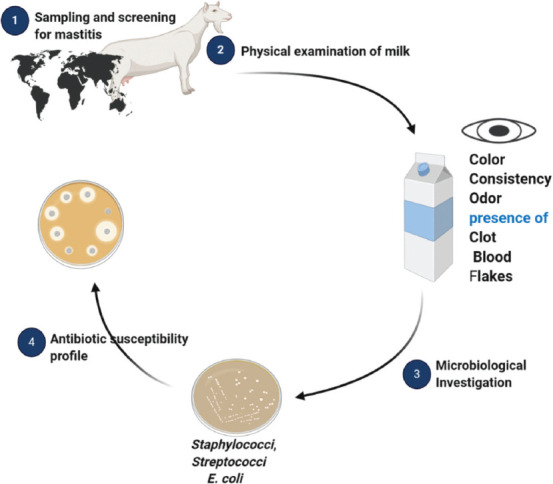
The flowchart of materials and methods.
Ethical approval and Informed consent
Ethical approval for this study was obtained from University of Veterinary and Animal Sciences (UVAS), Lahore, Pakistan.
Study period and location
The study was conducted from July 2019 to December 2019 on both public and private farms and small households of every Union Council of Pattoki Tehsil, Kasur District, Punjab, Pakistan.
Sample size
The sample size was calculated based on the expected prevalence of about 20% (known disease status), with confidence intervals (CI) of 95% and absolute desire precision 5%. The expected prevalence of 20% was deduced from the average prevalence of caprine mastitis in the previous studies reported from 2013 to 2018 (6 years) [5,22,24,25].
The sample size was then estimated using the following equation [26]:
n = 1.962 Pexp (1−Pexp)/d2
d = 1- C.I where:
n = required sample size
Pexp = expected prevalence
d = desired absolute precision.
The number of samples thus calculated was further subjected to the following equation for adjustments to reach the maximum number of samples [26]:
nadj = (N × n)/(N+n) where:
N = total population
n = calculated sample size through formula
Following the proportionate sampling strategy and assuming the highest population (70%) of Beetal goats in Punjab (5), 500 Beetal goats were screened for this study, examining the prevalence of mastitis in Pattoki Tehsil, Kasur District.
Sampling and screening for mastitis
In total, 500 goats, irrespective of age, which were not treated with any kind of antimicrobial agents in the past 120 h, were screened using CMT as per the procedure described by Saleem [4], from July 2019 to December 2019. The study was conducted on both public and private farms and small households of every Union Council of Pattoki Tehsil, Kasur District, Punjab, Pakistan. Coordinates of sample unit were taken using GPS Essential Android application, while the study spots were generated using ArcGIS version 10.3 (Figure-2). Ten milliliters of CMT-positive milk samples were collected from each animal using sterile plastic tubes with safety lids, in compliance with the National Mastitis Council (USA) procedures [27]; these were later stored in the isothermal container at 2-4°C and were immediately transported to the University Diagnostics Laboratory, Central Laboratory Complex (CLC) of UVAS Ravi Campus, Pattoki, for further analysis.
Figure-2.
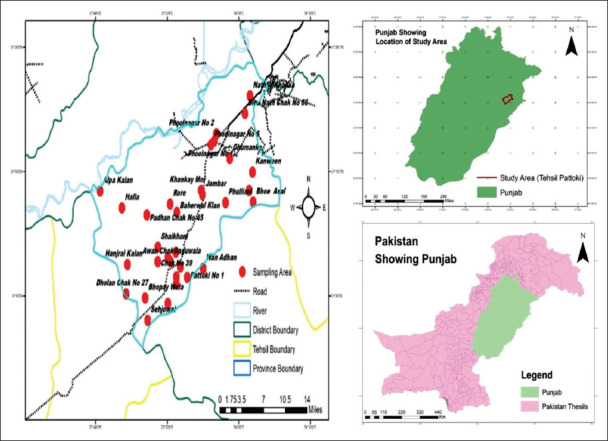
Sampling and screening for mastitis, study area, were generated with the help of ARC GIS version 10.3.
Physical examination of milk
Immediately after collection, the milk samples were subjected to naked eye examination to determine any changes in color, consistency, or odor, any presence of clot, blood or flakes, and other visible abnormalities.
Risk factor analysis
Data on the potential risk factors such as herd structure, deworming, vaccination against common diseases, presence of ticks, use of teat dip and mineral supplements, feeding type, age, parity, housing, blood in the milk, milk leakage, milk taste, milk yield, and ease of milk were obtained using the questionnaire for each sampling unit, assuming that these risk factors are determinants of the disease. Udder tick infestation was considered when more than 2 ticks were present as well as the presence of tick on teat was including ticks portion as per described by Abera et al. [28].
Microbiological investigation
Milk samples were cultured using a conventional technique as described by Khan et al. [29]. Plates were then incubated for 24 h at 37°C, where the growth of bacteria was observed after every colony was separated using the specific media as follows: Staph-110 for Staphylococcus, blood agar for Streptococcus, and eosin methylene blue agar for E. coli. Separate colonies were purified using several streaking methods. Pure bacterial isolates were determined using their baseline cultural characteristics; guidance for the microscopic study and biochemical profile was taken from the identification flowcharts of Bergey’s Manual of Systematic Bacteriology (2010). Isolates of Staphylococcus were confirmed using the procedure described by Nazia et al. [30] while the method of Gillespie and Oliver [31] was used for the confirmation of streptococci and E. coli isolates. Different isolates found on specific media are shown in Figure-3a-c.
Figure-3.
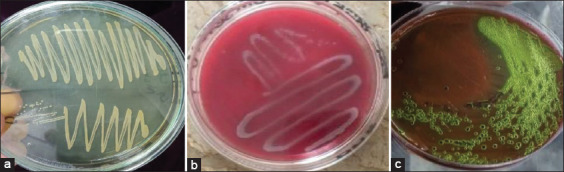
Isolated of (a) staphylococci on staph-110 agar, (b) streptococci on blood agar, and (c) Escherichia coli on eosin methylene blue agar.
Prevalence of pathogen
The prevalence of mastitis-causing bacterial pathogens was established by reviewing epidemiological surveys and treatment trials published from 1995 to 2018. The positive milk samples were cultured for analysis, and the prevalence was calculated as per the formula described by Waltner-Toews [26].
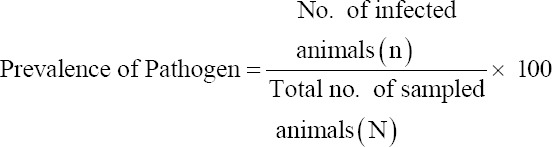
Antibiogram profile
For the antibiogram profiling of all bacterial isolates, antibiotic disks of enrofloxacin (ENO) (5 µg), amoxicillin+clavulanic acid (20/10 μg), oxytetracycline (30 μg), ceftiofur sodium (30 μg), gentamycin (10 μg), and sulfamethoxazole+trimethoprim (1.25/23.7 μg) were exposed using the disk diffusion method to test for antibiotic sensitivity [32]. The inhibition zones (mm) that have formed around disks were measured after incubation at 37°C for 24 h. The standards set by the Clinical and Laboratory Standards Institute (2020) was used to compare the inhibition zones of each disk, to determine sensitive, intermediate, or resistant susceptibility.
Statistical analysis
The collected data set was then encoded into SPSS (IBM Corp., NY, USA) version 26.0. Frequencies of baseline characteristics were reported. To determine the risk factors associated with mastitis occurrence in goats, all variables were initially tested through univariable analysis [33,34]. The Chi-square (χ2) test was used to assess the association of baseline characteristics and the occurrence of mastitis. Multivariable logistic regression (MLR) was also used to recognize risk factors associated with positive and negative dichotomous outcome of mastitis. MLR-based statistical test was based on two-sided Wald test, and 95% CI was used to highlight the odds ratio. The statistical significance was set for all statistical tests at p<0.05 (two-sided).
Results and Discussion
Baseline characteristics of the study
The baseline characteristics used in this study were selected randomly in a blinded manner. After sampling, it was observed, animals having age of 2-3 and 3-4 years were 19% in each group, 46.8% parity ≥5 times, and 78% history of fodder feeding in the studied animal, as shown in Table-1.
Table-1.
Baseline characteristics of the study.
| Characteristics | *n | % |
|---|---|---|
| Age (years) | ||
| 2-3 | 95 | 19 |
| 3-4 | 95 | 19 |
| 4-5 | 72 | 14.4 |
| 5-6 | 67 | 13.4 |
| 6-7 | 64 | 12.8 |
| 7-8 | 56 | 11.2 |
| ≥9 | 51 | 10.2 |
| Parity | ||
| 1-2 | 129 | 25.8 |
| 3-4 | 137 | 27.8 |
| ≥5 | 234 | 46.8 |
| Feeding | ||
| Fodder | 390 | 78 |
| Fodder+Concentrate | 51 | 10.2 |
| Concentrate | 59 | 11.8 |
n=500 respondents
Epidemiology of Beetal goat mastitis in Pattoki Tehsil
Goat mastitis has not been given enough attention in Pakistan [20,21]. Thus, our analysis has revealed that the overall prevalence of mastitis was 309 (61.8%), in which 260 (52%) and 49 (9.8%) were positive for SCM and CM, respectively, whereas 191 (38.2%) were found to be negative in the study area (Table-2). These findings are consistent with that of Sori et al. [35] and Matios et al., [36], who reported that the prevalence range of mastitis is about 52.8-64.4%. In Peshawar, the mastitis incidence rate in lactating goats has been reported to be 80.0%, 26.7%, 33.3%, 57.1%, and 42.0%, respectively, for five successive years (1997-2001) [37]. It is reported that the overall prevalence of goat mastitis is 40.4% [38], having an average of 18.3% occurrence rate among different goat breeds in Pakistan [22]. It is reported that the prevalence range of goat mastitis is 7-40%, 5-30%, and 20-50%, respectively [39-41], while 33%, 30.6%, 44.6%, 36%, 33.9%, and >50% prevalence are, respectively, reported by Aqib et al. [15], Ali et al. [21], Hall and Rycroft [42], Islam et al. [43], and Bourabah et al. [44]. This variation could be due to environmental factors and varying animal breed, fluctuation of immune response, housing and management system, all other farmers’ and host’s-related determinants, usage of different diagnostic methods and different levels of expertness for diagnosis, and the varying interpretation of the results [45].
Table-2.
Prevalence of bacterial species in SCM and CM in Beetal goats.
| Bacterial isolate† | CM (9.8%) | SCM (61.8%) |
|---|---|---|
| Staphylococci | 46.9 | 15 |
| Streptococci | 14.3 | 86.5* |
| Escherichia coli | 91.8* | 48.5 |
There were 309 positive samples out of 500 (total samples). 260/500 SCM and 49/500 CM. SCM=Subclinical mastitis, CM=Clinical mastitis
Our findings of SCM are close to that of Najeeb et al. [24], Oliveira et al., [46], who have reported that the prevalence rate of SCM in goats using CMT in Thika East Sub-county, Kenya, was 53% and 50.9%, respectively. Similarly, our findings of CM are close to that of Ferdous et al. [47], who reported 11.67% and 8% prevalence rate for CM in Bangladesh [38]. Oppositely to our findings, the prevalence of SCM is 76.7% and 15-79%, respectively, in goats, when screened with CMT [48,49]. Similarly, prevalence SCM is 32.4%, 39.2%, 38%, 40.2%, 47%, 14.3%, 38.8%, 19.9%, and 35.5%, respectively, by Saleem [4], Ali et al. [21], Altaf et al. [33], Danmallam and Pimenov [38], Moroni et al. [47], Hussain et al. [50], Ferdous et al. [51], Mishra et al. [52], and McDougall et al. [53], while opposite to the study prevalence finding of CM, which is 3.6% [54]. This prevalence can vary depending on the place and its conditions.
Prevalence of pathogens
Among goats, bacterial pathogens such as Staphylococcus spp., Streptococcus spp., and E. coli were reported as the main causative organisms of mastitis [12]. As it was studied that SCM in goats is mainly of bacterial origin, these three species of bacteria from milk samples positive for mastitis were isolated [41]. The percentages of Staphylococcus, Streptococcus, and E. coli in CMT-positive samples were 46.9%, 14.3%, and 91.8%, respectively, for CM and 15.0%, 86.5%, and 48.5%, respectively, for SCM, as shown in Table-2. It was further revealed that Streptococcus was predominant in SCM and E. coli in CM as p<0.005. These findings are close to that of Dieser et al. [55], who reported that in SCM, Streptococcus prevalence was 57.3%; meanwhile, Bradley and Green [56] reported E. coli as the predominant pathogen isolated on all farms in all months of the year, which is opposite to our findings. On the other hand, Hussain et al. [51] reported Staphylococcus as the most common pathogen (51.4%) isolated. The results regarding isolated organisms were consistent with the results reported as Staphylococcus aureus (45.3%), Streptococcus spp. (22.7%), E. coli (11.6%), and Klebsiella spp. (3.7%) [21]. Similar findings were also reported by Islam et al. [44], with 20.8-46.6% prevalence of S. aureus as a pathogen of SCM. In another study, it was concluded that most SC intramammary infections were caused by coagulase-negative Staphylococcus spp. [21]. It was reported that Staphylococcus is more dominant in Pakistan in terms of SCM infections [4]. Similarly, it was reported that S. aureus was predominant in milk samples [21], while Manser (2000) recorded the incidence of catalase-negative Staphylococcus and catalase-positive staphylococci at 80% and 16%, respectively. It was also reported that 38.98%, 27.1%, and 10.2% of Staphylococcus spp., E. coli, and Bacillus spp., respectively, were found to be in goats positive for SCM, which was opposite to our results [49]. It was further reported that staphylococci were the major etiological agents of SCM in Bangladesh and Pakistan [21,57], thus identifying staphylococci as the predominant isolate for SCM cases [58]. In total, 38.98%, 27.1%, and 10.2% of Staphylococcus spp., E. coli, and Bacillus spp., respectively, were isolated from goats positive for SCM [49].
As E. coli is mostly found on farm animals, it has been recognized as the most common environmental contaminant [59,60]. Our findings are very close to that of Moroni et al. [49], who reported E. coli as the second most common pathogen found in milk samples positive for SCM. Meanwhile, Staphylococcus was also found to be the highest in Kenya (60.3%), Spain (70%), and the USA (38.2%) [61-63]. These differences could be attributed to management conditions or geographical locations of the farm [64].
Antibiotic susceptibility profile
It was observed that amoxicillin+clavulanic acid was highly sensitive to 58/62 isolates of Staphylococcus (p<0.001), with mean±standard deviation (SD) (18.3±0.2), and 221/232 isolates of Streptococcus with mean±SD (20.2±1.3). Similarly, ceftiofur sodium was determined to be highly sensitive to 221/232 isolates of Streptococcus, with mean±SD (22.3±0.7), and 162/171 isolates of E. coli with mean±SD (21.3±0.02). Meanwhile, ENO was found to be sensitive to 217/232 isolates of Streptococcus, with mean±SD (25±2.5), and 138/171 isolates of E. coli with mean±SD (22.3±0.8; p<0.01). The sensitivity zones of the antibiotic disks to bacterial isolates are shown in Table-3, whereas the prevalence and antibiotic susceptibility of the isolated Staphylococcus, streptococci, and E. coli are shown in Table-4.
Table-3.
Sensitivity zone (mean±SD) in mm of antibiotic disk against bacterial isolates.
| Antibiotic disk | Staphylococci | Streptococci | Escherichia coli |
|---|---|---|---|
| Mean±SD | Mean±SD | Mean±SD | |
| ENO-5 | 21.4±0.2 | 25±2.5 | 22.3±0.8 |
| AMC-5 | 18.3±0.2 | 20.2±1.3 | 20.4±1.3 |
| T-30 | 8.9±7.3 | 28±1.3 | 26.7±0.5 |
| XNL-30 | 22±0.7 | 22.3±0.7 | 21.3±0.2 |
| GM-10 | 16.1±0.8 | 16.4±0.9 | 17±1.3 |
| SXT | 17.8±0.6 | 17.05±0.7 | 17.3±0.2 |
Six different antibiotics are used to determine the sensitivity zone of bacteria. ENO-5=Enrofloxacin (5 μg), AMC-5=Amoxicillin+Clavulanic acid (20/10 μg), T-30=Oxytetracycline (30 μg), XNL-30=Ceftiofur sodium (30 μg), GM-10=Gentamycin (10 μg), and SXT=Sulfamethoxazole trimethoprim (1.25/23.7 μg). Data are presented as mean±SD.
Table-4.
Prevalence and antibiotic susceptibility of the isolated staphylococci, streptococci, and Escherichia coli.
| Antibiotic disks | Staphylococci (62 a) | Streptococci (232 b)) | Escherichia coli (171 c)) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | |
| ENO-5 | 5 (8.1) | 2 (3.2) | 55 (88.5) | 217 (93.5)* | 10 (4.3) | 5 (2.2) | 138* (80.7) | 21 (12.3) | 12 (7) |
| AMC-5 | 58 (93.5)** | 3 (4.8) | 1 (1.6) | 221 (95.3)** | 8 (3.4) | 3 (1.3) | 2 (1.2) | 0 (0.0) | 169 (98.8) |
| T-30 | 3 (4.8) | 7 (11.3) | 52 (83.9) | 28 (12.1) | 27 (11.6) | 177 (76.3) | 3 (1.8) | 7 (4.1) | 161 (94.2) |
| XNL-30 | 11 (17.7) | 10 (16.1) | 41 (66.1) | 221 (95.3)** | 10 (4.3) | 1 (0.4) | 162 (94.7)** | 6 (3.5) | 3 (1.8) |
| GM-10 | 4 (6.5) | 13 (21) | 45 (72.6) | 25 (10.8) | 54 (23.3) | 153 (65.9) | 4 (2.3) | 8 (4.7) | 159 (93) |
| SXT | 2 (3.2) | 9 (14.5) | 51 (82.3) | 2 (0.9) | 56 (24.1) | 174 (75) | 24 (14) | 24 (14) | 123 (71.9) |
There were 39 and 23, 225 and 7, 126 and 45;
in SCM and CM, respectively;
highly sensitive,
sensitive,
ENO-5=Enrofloxacin (5 μg), AMC-5=Amoxicillin+Clavulanic acid (20/10 μg), T-30=Oxytetracycline (30 μg), XNL-30=Ceftiofur sodium (30 μg), GM-10=Gentamycin (10 μg), and SXT=Sulfamethoxazole trimethoprim (1.25/23.7 μg). “S” stands for sensitive, “I” for intermediate, and “R” for resistant
Amoxicillin+clavulanic acid was determined to be the drug of choice against Staphylococcus; amoxicillin+clavulanic acid, ceftiofur sodium, and ENO against Streptococcus; and ceftiofur sodium and ENO against E. coli. The percentage efficacy of the different antibiotics is shown in Figure-4a-c. Our findings are very close to that of Aqib et al. [15] and Joshi et al. [65], who reported that amoxicillin was the most effective and isolates of Streptococcus and E. coli have shown higher susceptibility to ceftiofur sodium and ENO, respectively, as these drugs are not regularly used for treatment in this region which might explain for their higher efficacy. However, this was a counterstatement to Ali et al. [21], who claimed that the efficacy of gentamicin was higher than other antibiotics against in vitro mastitis bacteria. It was studied that in vitro efficacy of tetracycline was highest (90.4%) against bacterial isolates of milk [66]. Similarly, the sensitivity of tetracycline was reported to be at 80.7% by Ali et al. [21] and 93.7% by Rola et al. [67]. Furthermore, tetracycline was identified to have the highest efficacy against mastitis bacteria found in goats [66,68,69]. Similarly, findings of Ahmed et al. [37], Sumathi et al. [70], Mir et al. [71], and Ceniti et al., [72] are opposite to our study. There has been difficulty in comparing different studies as the criteria for interpretation and methods of resistance or susceptibility used vary [72].
Figure-4.
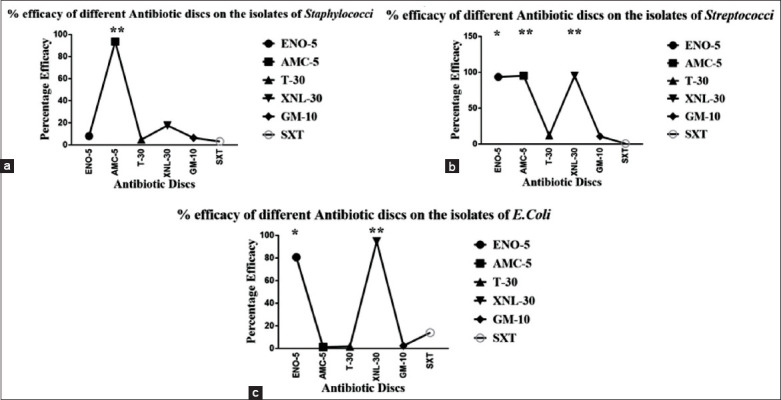
Antibiotic susceptibility profile of (a) staphylococci, (b) streptococci, and (c) Escherichia coli.
Risk factors
To improve udder health, it is necessary to determine the risk factors associated with SCM [13]. Reoccurrence of infection or the risk of infection can be attributed to factors such as poor treatment protocol during late lactation [14,15], low body score, long teats, milk fever, season, and prophylactic hygiene management; furthermore, infections in health-care centers have been identified as the major contributors to mastitis prevalence throughout the world [16-18].
This study has determined the association of several risk factors such as herd structure, deworming, vaccination, presence of ticks, use of teat dip and supplements (mineral), feeding type, age, housing, blood in the milk, milk leakage, milk taste, parity, milk yield, and ease of milking in the occurrence of mastitis in goats. These factors were analyzed statistically to determine their association (Table-1). Then, the association of risk factors and the occurrence of mastitis were analyzed using the MLR model. Initially, 14 variables that produced p<0.001 in the univariate analysis (Table-5) were included in the MLR model. However, the final model only contained 11 statistically significant variables (Table-6).
Table-5.
Association of baseline variables with mastitis.
| Variable | Group | Mastitis positive (%) | Mastitis negative (%) | χ2 | df | p-value |
|---|---|---|---|---|---|---|
| Herd structure | Goats | 44 (8.8) | 140 (28) | 177.6 | 3 | <0.001 |
| Goats+Sheep | 202 (41) | 37 (7.4) | ||||
| Goats+Bovine | 63 (12) | 14 (2.8) | ||||
| Deworming | Yes | 55 (11) | 254 (50.8) | 10.7 | 1 | <0.001 |
| No | 55 (11.6) | 133 (26.6) | ||||
| Vaccination | Yes | 39 (7.8) | 67 (13.4) | 35.6 | 1 | <0.001 |
| No | 270 (54) | 124 (24.8) | ||||
| Ticks | Yes | 58 (11.6) | 16 (3.2) | 10.1 | 1 | <0.001 |
| No | 251 (50.2) | 175 (35) | ||||
| Use of teat dip | Yes | 6 (1.2) | 42 (8.4) | 54.7 | 1 | <0.001 |
| No | 303 (60.6) | 149 (29.8) | ||||
| Supplement (mineral) | Yes | 4 (0.8) | 44 (8.8) | 64.3 | 1 | <0.001 |
| No | 305 (61) | 147 (29.4) | ||||
| Feeding type | Fodder | 284 (56.8) | 106 (21.2) | 99.7 | 2 | <0.001 |
| Fodder+concentrate | 19 (3.8) | 32 (6.4) | ||||
| Concentrate | 6 (1.2) | 53 (10.6) | ||||
| Age (years) | 2-3 | 33 (6.6) | 62 (12.4) | 70.6 | 6 | <0.001 |
| 3-4 | 42 (8.4) | 51 (10.2) | ||||
| 4-5 | 45 (9) | 27 (5.4) | ||||
| 5-6 | 48 (9.6) | 19 (3.8) | ||||
| 6-7 | 51 (10.2) | 13 (2.6) | ||||
| 7-8 | 46 (9.2) | 10 (2) | ||||
| >9 | 42 (8.4) | 9 (1.8) | ||||
| Parity | 2-3 | 87 (17.4) | 75 (15) | 55.4 | 2 | <0.001 |
| 3-4 | 131 (26.2) | 65 (13) | ||||
| >5 | 91 (18.2) | 51 (10.2) | ||||
| Housing | Shed | 4 (0.8) | 96 (19.2) | 302.0 | 3 | <0.001 |
| Backyard shed | 1 (0.2) | 46 (9.2) | ||||
| Tethering | 85 (17) | 19 (3.8) | ||||
| Loose | 219 (43.3) | 30 (6) | ||||
| Blood in milk | Yes | 51 (10.2) | 1 (0.2) | 32.3 | 1 | <0.001 |
| No | 258 (51.6) | 190 (38) | ||||
| Milk leakage | Yes | 33 (6.6) | 1 (0.2) | 18.5 | 1 | <0.001 |
| No | 277 (55.4) | 190 (38) | ||||
| Milk taste | Sweet | 121 (24.2) | 46 (9.2) | 115.4 | 2 | <0.001 |
| Bitter | 42 (8.4) | 112 (22.4) | ||||
| Salt | 146 (29.2) | 33 (6.6) | ||||
| Milk yield | Normal | 196 (39.2) | 180 (36) | 60.1 | 1 | <0.001 |
| Decreased | 113 (22.6) | 11 (2.2) | ||||
| Ease of milking | Positive | 11 (2.2) | 21 (19.2) | 10.9 | 1 | 0.010 |
| Negative | 298 (59.6) | 170 (34) |
Table-6.
Summary of key risk factors associated with the occurrence of mastitis in Beetal goats: variables included in final logistic regression model.
| Characteristics | *OR | 95%** CI | p-value |
|---|---|---|---|
| Herd structure | |||
| Goats | 34.4 | 7.2-164.2 | 0.00 |
| Goats+Sheep | 44.6 | 4.3-464.5 | 0.01 |
| Goats+Bovines | 1 | ||
| Housing | |||
| Shed | 1 | ||
| Backyard shed | 0.002 | 0.03 | 0.00 |
| Tethering | 0.002 | 0-0.3 | 0.012 |
| Loose | 1.2 | 0.3-4.8 | 0.77 |
| Deworming | |||
| No | 3.97 | 0.25-0.45 | 0.11 |
| Yes | 1 | ||
| Vaccine | |||
| No | 4.853 | 0.9-23.57 | 0.05 |
| Yes | 1 | ||
| Ticks | |||
| No | 0.25 | 0.05-1.25 | 0.09 |
| Yes | 1 | ||
| Feeding | |||
| Fodder | 0.48 | 0.07-3.09 | 0.44 |
| Fodder+Concentrate | 0.001 | 0.00-0.06 | 0.001 |
| Concentrate | 1 | ||
| Supplement (mineral) | |||
| No | 0.5 | 0.003-0.88 | 0.04 |
| Yes | 1 | ||
| Age | |||
| 2-3 years | 0.48 | 0.08-2.9 | 0.41 |
| 3-4 years | 4.6 | 0.52-40.11 | 0.17 |
| 4-5 years | 3 | 0.44-20.23 | 0.27 |
| 5-6 years | 2.8 | 0.18-45.2 | 0.47 |
| 6-7 years | 1.4 | 0.09-21.7 | 0.8 |
| 7- 8 years | 21.95 | 0.78-615.5 | 0.07 |
| 9 and above year | |||
| Parity | |||
| 1-2 years | 1 | ||
| 3-4 years | 0.09 | 0.02-0.44 | 0.003 |
| More than 5 | 1.28 | 0.7-1.7 | 0.08 |
| Teat dip | |||
| No | 0.14 | 0.02-1.01 | 0.05 |
| Yes | 1 | ||
| Blood in milk | |||
| No | 37.6 | 5.12-274.2 | 0.000 |
| Yes | 1 | ||
| Milk leakage | |||
| No | 49.8 | 1.3-1924 | 0.036 |
| Yes | 1 | ||
| Milk taste | |||
| Sweet | 1 | ||
| Bitter | 0.61 | 0.15-2.45 | 0.48 |
| Salt | 0.18 | 0.4-0.85 | 0.3 |
| Milk yield | |||
| Normal | 49.8 | 2.7-932.3 | 0.009 |
| Decrease | 1 | ||
| Ease of milk | |||
| No | 0.04 | 0.004-0.38 | 0.005 |
| Yes | 1 |
OR=Odds ratio;
CI=Confidence interval
In Pakistan, it is common that milking of goats is done by hands as herds are smaller in number or are not mechanized, increasing the risk for mastitis due to poor management and hygiene practices of milk handlers [21,43,73]. In the herd, a diseased animal increases the chances of intramammary infection, which means treatment and culling policy can overcome SCM prevalence. It is reported that if culling of animals positive for CM is not done, then it will lead to the further spread of contagious mastitis in the herd [74]. Our findings on herd structure are consistent with that of Aqib et al. [15] while opposite to that of Megersa et al. [18], who stated that there is no significant difference between herd structure and occurrence of mastitis in goats. It was observed that dairy farmers do not follow the management and biosecurity standard operating procedures; for example, there is often lack of proper deworming in newborn animals [75]. It is reported that in cattle, deworming has a significant correlation with the occurrence of mastitis [76]; however, it was contrary to the findings observed by Kao et al. [5].
Due to vaccines against mastitis lacking widespread efficacy, new vaccine development is under way [77]. The current study revealed a strong association of vaccination of common diseases with the occurrence of mastitis due to common diseases, immune status of the animal becomes low. Therefore, occurrence of mastitis chances becomes high, while finding of Kao et al. [5] is opposite to the current study that stated that there is no significant difference among vaccinations against common diseases with the occurrence of mastitis in goats.
The current study showed a strong association of ticks with the occurrence of mastitis as ticks mostly infest udder causing teat and skin lesions, help in the entry of bacteria, and mark permanent damage of tissue, which may increase gradually to teats and udders. It results in a significant portion of the udder lesion and infestation of ticks was positive with mastitis. It is reported that when there was 72% infestation of ticks on the udder than mastitis occurrence. It was 30% more than non-infested udder [78]. Similarly, it was observed that the prevalence of mastitis and the infestation of ticks and lesions of the udder are correlated [79]. Thus, mastitis and other problems of the udder in animals can be reduced using acaricide to control ticks. It is suggested that during the non-lactation period of animals, ticks should be removed from the body of animals by gentle rotation and firm move without damaging the skin of the udder [80].
It is observed that animals having long teats, pendulous and deep udder, late lactation, low body condition scoring, and poor management and hygiene are more susceptible to udder infection such as mastitis and udder inflammation as compared to others [81]. Our finding on how teat dip and mastitis occurrence are correlated is similar to that in Casu et al. [82] and Barkema et al. [83]. The immunity that comes from supplements like minerals has a beneficial effect on the health of the udder [84]. Mineral supplementation is recommended throughout the year as it can enhance the immunity of dairy animals [85]. For example, selenium has been identified of having high beneficial effect on immune cell activity in cows and, rarely, in heifers [86]. Our findings of mineral supplementation are very close to that in Coe et al. [85], and findings related to feeding and occurrence of mastitis are similar to the findings of Aqib et al. [15], and Altaf et al., [33], while opposite to Kao et al. [5].
Determination age and parity have been identified as the major factors of SCM; this as age and parity increase, occurrence of mastitis also increases due to the increase in SCC [21,87]. This was consistent with the findings of Megersa et al. [18], Ferdous et al. [47], Da Silva et al. [68], Piepers et al., [84] who demonstrated the association of goat mastitis prevalence and gradual increase in age and parity number. Oppositely, it is reported that there is no significant association of mastitis with parity, which is opposite to our findings [5,15,33,87].
Our findings on how housing and occurrence of mastitis are associated and consistent with Kao et al. [5], Aqib et al. [15] and Kumar et al., [66]. Ease of milking, milk leakage, blood in the milk, milk taste, and milk yield have been determined to have the strongest connection with the occurrence of mastitis, showing a highly significant effect (p<0.05); this as mastitis is detected, a marked hardness of teat during milking, milk leakage, blood in milk in a severe attack of mastitis, and 20% milk yield decrease are also noted. The findings on these parameters are strongly supported by Kao et al. [5].
Conclusion
Our results show that SCM among Beetal goats caused by bacterial organisms is predominant in the study area, and the percentage prevalence of Streptococcus and E. coli is predominant in SCM and CM. Amoxicillin+Clavulanic acid (AMC-5) has the highest sensitivity against Staphylococcus isolates, while AMC-5, XNL-30, and ENO-5 have the highest sensitivity against Streptococcus. XML-30 and ENO-5 have the highest sensitivity against E. coli.
Herd structure, deworming, vaccination, presence of ticks, use of teat dip, mineral supplementation, feeding type, age, housing, blood in the milk, milk leakage, milk taste, parity, and milk yield have been determined to have a strong association with the occurrence of mastitis; meanwhile, ease of milking has a moderate association with mastitis occurrence. We hope our findings have raised awareness on the risk factors and treatment approaches against common mastitis-causing bacterial agents.
Authors’ Contributions
MHS, IA, and AH designed the project. AJ, MI and MZI did sampling, data collection, processing, and interpretation of results. MQ and MA analyzed the data. AJ, HS, MMT, and HAN drafted the manuscript and reviewed by all. All the authors read the manuscript and approved the content.
Acknowledgments
The authors highly acknowledged the Central Laboratory complex, University of Veterinary and Animal Sciences, Ravi Campus, Pattoki, Pakistan, for providing research facilities to carry out some part of this work and Prof. Md. Tanvir Rahman from The Department of Microbiology and Hygiene, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh, for the critical review. The authors would like to thank Enago (www.enago.com) for the English language review.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published map and institutional affiliation.
References
- 1.Pirzada M. Prevalence of subclinical mastitis in dairy goats caused by bacterial species. J. Anim. Health Prod. 2016;4(2):55–59. [Google Scholar]
- 2.Khan M.S, Rehman Z.U, Khan M.A, Sohail A. Genetic resources and diversity in Pakistani cattle. Pak. Vet. J. 2011;28(2):95–102. [Google Scholar]
- 3.Haenlein G.F. Goat milk in human nutrition. Small Rumin. Res. 2004;51(2):155–163. [Google Scholar]
- 4.Saleem M.I. Epidemiological study of mastitis in three different strains of beetal goat in selected districts of Punjab, Pakistan. Pak. Vet. J. 2019;39(3):389–394. [Google Scholar]
- 5.Kao H.F, Wang Y.C, Tseng H.Y, Wu L.S, Tsai H.J, Hsieh M.H, Chen P.C, Kuo W.S, Liu L.F, Liu Z.G, Wang J.Y. Goat milk consumption enhances innate and adaptive immunities and alleviates allergen-induced airway inflammation in offspring mice. Front. Immunol. 2020;11:184. doi: 10.3389/fimmu.2020.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miranda-de la Lama G.C, Mattiello S. The importance of social behaviour for goat welfare in livestock farming. Small Rumin. Res. 2010;90(1-3):1–10. [Google Scholar]
- 7.Panicker S.S. Oestrous response and conception rate in Malabari cross-bred goats following two different oestrus synchronization protocols. J. Anim. Health Prod. 2015;3(2):39–42. [Google Scholar]
- 8.Maréchal C.L, Thiéry R, Vautor E, Loir Y.L. Mastitis impact on technological properties of milk and quality of milk products a review. Dairy Sci. Technol. 2011;91(3):247–282. [Google Scholar]
- 9.Bachaya H.A, Iqbal Z, Muhammad G, Yousaf A, Ali H.M. Mastitis in buffaloes in attock district of Punjab (Pakistan) Pak. Vet. J. 2005;25(3):134–136. [Google Scholar]
- 10.Paape M.J, Poutrel B, Contreras A, Marco J.C, Capuco A.V. Milk somatic cells and lactation in small ruminants. J. Dairy Sci. 2001;84(E. Suppl.):E237–E244. [Google Scholar]
- 11.Virdis S, Scarano C, Cossu F, Spanu V, Spanu C, De Santis E.P. Antibiotic resistance in Staphylococcus aureus and coagulase negative staphylococci isolated from goats with subclinical mastitis. Vet. Med. Int 2010. 2010:517060. doi: 10.4061/2010/517060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Liu H, Zhao X, Gao Y, Zhang M, Chen D. Prevalence and pathogens of subclinical mastitis in dairy goats in China. Trop. Anim. Health Prod. 2014;47(2):429–435. doi: 10.1007/s11250-014-0742-y. [DOI] [PubMed] [Google Scholar]
- 13.Koop G, Collar C.A, Toft N, Nielen M, Van Werven T, Bacon D, Gardner I.A. Risk factors for subclinical intramammary infection in dairy goats in two longitudinal field studies evaluated by Bayesian logistic regression. Prev. Vet. Med. 2013;108(4):304–312. doi: 10.1016/j.prevetmed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Koop G, Islam M.N, Rahman M.M, Khatun M, Ferdous J, Sayeed M.A, Islam S, Ahaduzzaman M, Akter S, Mannan A, Hassan M.M, Dissanayake R, Hoque M.A. Risk factors and therapy for goat mastitis in a hospital-based case-control study in Bangladesh. Prev. Vet. Med. 2016;124:52–57. doi: 10.1016/j.prevetmed.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Aqib A.I, Nighat S, Ahmed R, Sana S, Jamal M.A, Kulyar M.F, Khan N.U, Sarwar M.S, Hussain M.A, Ullah A, Rahman A, Rahman A. Drug susceptibility profile of Staphylococcus aureus isolated from mastitic milk of goats and risk factors associated with goat mastitis in Pakistan. Pak. J. Zool. 2019;51(1):307–315. [Google Scholar]
- 16.Nikbakht M, Nahaei M.R, Akhi M.T, Asgharzadeh M, Nikvash S. Molecular fingerprinting of meticillin-resistant Staphylococcus aureus strains isolated from patients and staff of two Iranian hospitals. J. Hosp. Infect. 2008;69(1):46–55. doi: 10.1016/j.jhin.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 17.Koop G, Nielen M, van Werven T. Bulk milk somatic cell counts are related to bulk milk total bacterial counts and several herd-level risk factors in dairy goats. J. Dairy Sci. 2009;92(9):4355–4364. doi: 10.3168/jds.2009-2106. [DOI] [PubMed] [Google Scholar]
- 18.Megersa B, Tadesse C, Abunna F, Regassa A, Mekibib B, Debela E. Occurrence of mastitis and associated risk factors in lactating goats under pastoral management in Borana, Southern Ethiopia. Trop. Anim. Health Prod. 2010;42(6):1249–1255. doi: 10.1007/s11250-010-9557-7. [DOI] [PubMed] [Google Scholar]
- 19.Javaid S.B, Gadahi J.A, Khaskeli M, Bhutto M.B, Kumbher S, Panhwar A.H. Physical and chemical quality of market milk sold at Tando Jam, Pakistan. Pak. Vet. J. 2009;29(1):27–31. [Google Scholar]
- 20.Arshad M, Muhammed G, Siddique M, Ashraf M, Khanh A. Staphylococcal mastitis in bovines and some properties of staphylococcal isolates. Pak. Vet. J. 2006;26(1):20–22. [Google Scholar]
- 21.Ali Z, Muhammad G, Ahmad T, Khan R, Naz S, Anwar H, Farooqi F.A, Manzoor M.N, Usama A.R. Prevalence of caprine sub-clinical mastitis, its etiological agents and their sensitivity to antibiotics in indigenous breeds of Kohat, Pakistan. Pak. J. Life Soc. Sci. 2010;8(1):63–67. [Google Scholar]
- 22.Rizwan M, Zameer A, Ijaz M, Kashif M, Firyal S. Veterinaria clinio-bacterialogical investigation of sub-clinical and clinical mastitis in dairy goats. Veterinaria. 2016;4(1):4–6. [Google Scholar]
- 23.Mekonnen S.A, Koop G, Melkie S.T, Getahun C.D, Hogeveen H, Lam T.J.G. Prevalence of subclinical mastitis and associated risk factors at cow and herd level in dairy farms in North-West Ethiopia. Prev. Vet. Med. 2017;145:23–31. doi: 10.1016/j.prevetmed.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Najeeb M.F, Anjum A.A, Ahmad M.U.D, Khan H.M, Ali M.A, Sattar M.M.K. Bacterial etiology of subclinical mastitis in dairy goats and multiple drug resistance of the isolates. J. Anim. Plant Sci. 2013;23(6):1541–1544. [Google Scholar]
- 25.Rashid M, Saleem M.I, Deeba F, Khan M.S, Mahfooz S.A, Butt A.A, Abbas M.W. Effect of season on occurrence of caprine mastitis in beetal in faisalabad premises. Matrix Sci. Med. 2017;1(1):19–21. [Google Scholar]
- 26.Waltner-Toews D. Veterinary epidemiology. Prev. Vet. Med. 1988;5(3):233–236. [Google Scholar]
- 27.Oliver S.P Microbiological procedures for the diagnosis of bovine udder infection and determination of milk quality. Verona: NMC; 2004. p. 47. [Google Scholar]
- 28.Abera M, Abdi O, Abunna F, Megersa B. Udder health problems and major bacterial causes of camel mastitis in Jijiga, Eastern Ethiopia:Implication for impacting food security. Trop. Anim. Health Prod. 2010;42(3):341–347. doi: 10.1007/s11250-009-9424-6. [DOI] [PubMed] [Google Scholar]
- 29.Khan A, Rind R, Shoaib M, Kamboh A.A, Mughal G.A, Lakho S.A, Malhi K.K, Nizamani A.R, Yousaf A. Isolation, Identification and antibiogram of Escherichia coli from table eggs. J. Anim. Health Prod. 2015;4(1):1–5. [Google Scholar]
- 30.Nazia M.K, Durrani N.U, Kamboh A.A, Lakho S.A, Rind R, Abro S.H, Soomro N.M. Prevalence of septic arthritis caused by Staphylococcus aureus in poultry birds at Tandojam, Pakistan. J. Anim. Health Prod. 2015;3(3):73–77. [Google Scholar]
- 31.Gillespie B.E, Oliver S.P. Simultaneous detection of mastitis pathogens, Staphylococcus aureus, Streptococcus uberis, and Streptococcus agalactiae by multiplex real-time polymerase chain reaction. J. Dairy Sci. 2005;88(10):3510–3518. doi: 10.3168/jds.S0022-0302(05)73036-8. [DOI] [PubMed] [Google Scholar]
- 32.Bhatt V.D, Patel M.S, Joshi C.G, Kunjadia A. Identification and antibiogram of microbes associated with bovine mastitis. Anim. Biotechnol. 2011;22(3):163–169. doi: 10.1080/10495398.2011.570132. [DOI] [PubMed] [Google Scholar]
- 33.Altaf M, Ijaz M, Iqbal M.K, Rehman A, Avais M, Ghaffar A, Ayyub R.M. Molecular characterization of methicillin resistant Staphylococcus aureus (MRSA) and associated risk factors with the occurrence of goat mastitis. Pak. Vet. J. 2020;40(1):1–6. [Google Scholar]
- 34.Bursac Z, Gauss C.H, Williams D.K, Hosmer D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008;3(1):17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sori H, Zerihum A, Abdicho S. Dairy cattle mastitis in and around Sebeta, Ethiopia. J. Appl. Res. Vet. Med. 2005;3(4):332–338. [Google Scholar]
- 36.Matios L, Tadele T, Worku T. Prevalence and major bacterial causes of bovine mastitis in Asella, South Eastern Ethiopia. Trop. Anim. Health Prod. 2009;41(7):1525–1530. doi: 10.1007/s11250-009-9343-6. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed A, Muhammad A, Muhammad Q, Muhammad A, Usman, Muhammad T, Khan A. Bacteriology of mastitic milk and in vitro antibiogram of the isolates. Pak. Vet. J. 2004;24(4):161–164. [Google Scholar]
- 38.Danmallam F.A, Pimenov N.V. Study on prevalence, clinical presentation, and associated bacterial pathogens of goat mastitis in Bauchi, plateau, and Edo states, Nigeria. Vet. World. 2019;12(5):638–645. doi: 10.14202/vetworld.2019.638-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox L.K, Gay J.M. Contagious mastitis. Vet. Clin. North Am. Food Anim. Pract. 1993;9(3):475–487. doi: 10.1016/s0749-0720(15)30615-0. [DOI] [PubMed] [Google Scholar]
- 40.Gelasakis A.I, Angelidis A, Giannakou R, Arsenos G. Bacterial subclinical mastitis and its effect on milk quality traits in low-input dairy goat herds. Vet Rec. 2018;183(14):449–449. doi: 10.1136/vr.104804. [DOI] [PubMed] [Google Scholar]
- 41.Bergonier D, de Crémoux R, Rupp R, Lagriffoul G, Berthelot X. Mastitis of dairy small ruminants. Vet. Res. 2003;34(5):689–716. doi: 10.1051/vetres:2003030. [DOI] [PubMed] [Google Scholar]
- 42.Hall S.M, Rycroft A.N. Causative organisms and somatic cell counts in subclinical intramammary infections in milking goats in the UK. Vet. Rec. 2007;160(1):19–22. doi: 10.1136/vr.160.1.19. [DOI] [PubMed] [Google Scholar]
- 43.Islam M.R, Ahamed M.S, Alam M.S, Rahman M.M, Sultana T, Roh Y.S, Kim B. Identification and antibiotic sensitivity of the causative organisms of subclinical mastitis in sheep and goats. Pak. Vet. J. 2012;32(2):179–182. [Google Scholar]
- 44.Bourabah A, Ayad A, Boukraa L, Hammoudi S.M, Benbarek H. Prevalence and etiology of subclinical mastitis in goats of the Tiaret Region, Algeria. Glob. Vet. 2013;11(5):604–608. [Google Scholar]
- 45.Mangi M.H. Seroprevalence of brucellosis in Holstein-Friesian and indigenous cattle breeds of Sindh Province, Pakistan. J. Anim. Health Prod. 2015;3(4):82–87. [Google Scholar]
- 46.Oliveira A, Melo B, Seixas L, Azevedo C, Teixeira M, Melo O, Emidio S, Oliveira C. Mastitis and milk composition in first partum Santa Ines ewes. J. Vet. Adv. 2013;3(8):220. [Google Scholar]
- 47.Ferdous J, Rahman M, Khan M, Khan M, Rima U. Prevalence of clinical and subclinical caprine mastitis of northern region in Bangladesh. Prog. Agric. 2018;29(2):127–138. [Google Scholar]
- 48.Mbilu T.J.N. Status of mastitis in lactating goats at Sokoine University of agriculture and neighbouring smallholder farms in Morogoro Municipality, Tanzania. Livest. Res. Rural Dev. 2007;19(3):54–60. [Google Scholar]
- 49.Sarker H, Samad M. Udder-halve-wise comparative prevalence of clinical and sub-clinical mastitis in lactating goats with their bacterial pathogens and antibiotic sensitivity patterns in Bangladesh. Bangladesh J. Vet. Med. 2013;9(2):137–143. [Google Scholar]
- 50.Moroni P, Pisoni G, Antonini M, Ruffo G, Carli S, Varisco G, Boettcher P. Subclinical mastitis and antimicrobial susceptibility of Staphylococcus caprae and Staphylococcus epidermidis isolated from two Italian goat herds. J. Dairy Sci. 2005;88(5):1694–1704. doi: 10.3168/jds.S0022-0302(05)72841-1. [DOI] [PubMed] [Google Scholar]
- 51.Hussain M, Yaqoob M, Riaz A, Umar S, Kashif J, Memon J, Shaheen S. Prevalence, bacteriology and antibiotic sensitivity profile of sub-clinical mastitis in goats in District Jhelum. Pak. J. Sci. 2017;69(3):240–245. [Google Scholar]
- 52.Mishra A.K, Sharma N, Singh D.D, Gururaj K, Abhishek Kumar V, Sharma D.K. Prevalence and bacterial etiology of subclinical mastitis in goats reared in organized farms. Vet. World. 2018;11(1):20–24. doi: 10.14202/vetworld.2018.20-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDougall S, Pankey W, Delaney C, Barlow J, Murdough P.A, Scruton D. Prevalence and incidence of subclinical mastitis in goats and dairy ewes in Vermont USA. Small Rumin. Res. 2002;46(2-3):115–121. [Google Scholar]
- 54.Gabli Z, Djerrou Z, Gabli A.E, Bensalem M. Prevalence of mastitis in dairy goat farms in Eastern Algeria. Vet. World. 2019;12(10):1563–1572. doi: 10.14202/vetworld.2019.1563-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dieser S.A, Vissio C, Lasagno M.C, Bogni C.I, Larriestra A.J, Odierno L.M. Prevalence of pathogens causing subclinical mastitis in Argentinean dairy herds. Pak. Vet. J. 2014;34(1):124–126. [Google Scholar]
- 56.Bradley A.J, Green M.J. Aetiology of clinical mastitis in six Somerset dairy herds. Vet. Rec. 2001;148(22):683–686. doi: 10.1136/vr.148.22.683. [DOI] [PubMed] [Google Scholar]
- 57.Akter S, Rahman M.M, Sayeed M.A, Islam M.N, Hossain D, Hoque M.A, Koop G. Prevalence, aetiology and risk factors of subclinical mastitis in goats in Bangladesh. Small Rumin. Res. 2020;184:106046. [Google Scholar]
- 58.Batavani R.A, Mortaz E, Falahian K, Dawoodi M.A. Study on frequency, etiology and some enzymatic activities of subclinical ovine mastitis in Urmia, Iran. Small Rumin. Res. 2003;50(1-2):45–50. [Google Scholar]
- 59.Ansari A.R.M. Prevalence and antimicrobial resistance profile of Escherichia coli and Salmonella isolated from diarrheic calves. J. Anim. Health Prod. 2014;2(1):12–15. [Google Scholar]
- 60.Begum S. Prevalence of Escherichia coli from pigs and cattle. J. Anim. Health Prod. 2014;2(3):38–39. [Google Scholar]
- 61.Sánchez A, Contreras A, Corrales J.C. Parity as a risk factor for caprine subclinical intramammary infection. Small Rumin. Res. 1999;31(3):197–201. [Google Scholar]
- 62.White E.C, Hinckley L.S. Prevalence of mastitis pathogens in goat milk. Small Rumin. Res. 1999;33(2):117–121. [Google Scholar]
- 63.Ndegwa E.N, Mulei C.M, Munyua S.J.M. Prevalence of microorganisms associated with udder infections in dairy goats on small-scale farms in Kenya. J. S. Afr. Vet. Assoc. 2001;72(2):97–98. doi: 10.4102/jsava.v72i2.627. [DOI] [PubMed] [Google Scholar]
- 64.Silas A.F. Effect of stocking density and quantitative feed restriction on growth performance, digestibility, haematological characteristics and cost of starting broiler chicks. J. Anim. Health Prod. 2014;2(4):60–64. [Google Scholar]
- 65.Joshi V, Gupta V.K, Dimri U, Alam S, Bhanuprakash A.G. Antibiogram studies of bacterial isolates from nasal swabs of respiratory diseases affected calves. Intas Polivet. 2017;18(1):4–6. [Google Scholar]
- 66.Kumar R, Gupta D.K, Bansal K, Singh S, Sharma S, Kumar A, Uppal S.K. Prevalence, current antibiogram and risk factors associated with mastitis in dairy goats in Punjab. Int. J. Sci. Environ. Technol. 2016;5(6):4580–4593. [Google Scholar]
- 67.Rola J.G, Sosnowski M, Ostrowska M, Osek J. Prevalence and antimicrobial resistance of coagulase-positive staphylococci isolated from raw goat milk. Small Rumin. Res. 2015;123(1):124–128. [Google Scholar]
- 68.Da Silva E.R, Siqueira A.P, Martins J.C.D, Ferreira W.P.B, Da Silva N. Identification and in vitro antimicrobial susceptibility of Staphylococcus species isolated from goat mastitis in the Northeast of Brazil. Small Rumin. Res. 2004;55(1-3):45–49. [Google Scholar]
- 69.Begum M, Hossain M, Ershaduzzaman M, Alam M. Epidemiological studies on subclinical mastitis in dairy goats in Northern Regions of Bangladesh. Bangladesh J. Livest. Res. 2016;19(1-2):112–122. [Google Scholar]
- 70.Sumathi B.R, Veeregowda B.M, Gomes A.R. Prevalence and antibiogram profile of bacterial Isolates from clinical bovine mastitis. Vet. World. 2008;1(8):237–238. [Google Scholar]
- 71.Mir A.Q, Bansal B.K, Gupta D.K. Subclinical mastitis in machine milked dairy farms in Punjab:Prevalence, distribution of bacteria and current antibiogram. Vet. World. 2014;7(5):291–294. [Google Scholar]
- 72.Ceniti C, Britti D, Santoro A.M.L, Musarella R, Ciambrone L, Casalinuovo F, Costanzo N. Phenotypic antimicrobial resistance profile of isolates causing clinical mastitis in dairy animals. Ital. J. Food Saf. 2017;6(2):84–87. doi: 10.4081/ijfs.2017.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Popov L, Kovalski J, Grandi G, Bagnoli F, Amieva M.R. Three-dimensional human skin models to understand Staphylococcus aureus skin colonization and infection. Front. Immunol. 2014;5:41. doi: 10.3389/fimmu.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schukken Y.H, Wilson D.J, Welcome F, Garrison-Tikofsky L, Gonzalez R.N. Monitoring udder health and milk quality using somatic cell counts. Vet. Res. 2003;34(5):579–596. doi: 10.1051/vetres:2003028. [DOI] [PubMed] [Google Scholar]
- 75.Zadoks R.N, Middleton J.R, McDougall S, Katholm J, Schukken Y.H. Molecular epidemiology of mastitis pathogens of dairy cattle and comparative relevance to humans. J. Mammary Gland Biol. Neoplasia. 2011;16(4):357–372. doi: 10.1007/s10911-011-9236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan A, Durrani A.Z, Yousaf A, Khan J.A, Chaudhry M, Fatima Z, Khan A. Epidemiology and antimicrobial susceptibility of methicillin-resistant Staphylococcus aureus in cattle of Pothohar Region, Pakistan. Pak. Vet. J. 2019;39(3):438–442. [Google Scholar]
- 77.Castagliuolo I, Piccinini R, Beggiao E, Palù G, Mengoli C, Ditadi F, Vicenzoni G, Zecconi A. Mucosal genetic immunization against four adhesins protects against Staphylococcus aureus induced mastitis in mice. Vaccine. 2006;24(20):4393–4402. doi: 10.1016/j.vaccine.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 78.Almaw G, Molla B. Prevalence and etiology of mastitis in camels (Camelus dromedarius) in Eastern Ethiopia. J. Camel Pract. Res. 2000;7(1):97–100. [Google Scholar]
- 79.Bekele T, Molla B. Mastitis in lactating camels (Camelus dromedarius) in Afar Region, North-Eastern Ethiopia. Berl. Munch. Tierarztl. Wochenschr. 2001;114(5-6):169–172. [PubMed] [Google Scholar]
- 80.Abdurahman O.A.S. Udder health and milk quality among camels in the Errer valley of Eastern Ethiopia. Livest. Res. Rural Dev. 2006;18(8):32–38. [Google Scholar]
- 81.Contreras A, Sánchez A, Corrales J.C, Sierra D, Gonzalo C, Paape M.J Mastitis in small ruminants. In:Mastitis in Dairy Production:Current Knowledge and Future Solutions. Wageningen, Netherlands: Wageningen Academic Publishers; 2005. pp. 67–74. [Google Scholar]
- 82.Casu S, Sechi S, Salaris S.L, Carta A. Phenotypic and genetic relationships between udder morphology and udder health in dairy ewes. Small Rumin. Res. 2010;88(2-3):77–83. [Google Scholar]
- 83.Barkema H.W, Schukken Y.H, Lam T.J.G, Beiboer M.L, Benedictus G, Brand A. Management practices associated with low, medium, and high somatic cell counts in bulk milk. J. Dairy Sci. 1998;81(7):1917–1927. doi: 10.3168/jds.S0022-0302(98)75764-9. [DOI] [PubMed] [Google Scholar]
- 84.Piepers S, Opsomer G, Meyer E, Demeyere K, Barkema H.W, de kruif A, de Vliegher S. Heifer and quarter characteristics associated with periparturient blood and milk neutrophil apoptosis in healthy heifers and in heifers with subclinical mastitis. J. Dairy Sci. 2009;92(9):4330–4339. doi: 10.3168/jds.2009-2029. [DOI] [PubMed] [Google Scholar]
- 85.Coe P.H, Maas J, Reynolds J, Gardner I. Randomized field trial to determine the effects of oral selenium supplementation on milk production and reproductive performance of Holstein heifers. J. Am. Vet. Med. Assoc. 1993;202(6):875–881. [PubMed] [Google Scholar]
- 86.Clark S, García M.B.M. A 100-year review:Advances in goat milk research. J. Dairy Sci. 2017;100(12):10026–10044. doi: 10.3168/jds.2017-13287. [DOI] [PubMed] [Google Scholar]
- 87.Boscos C, Stefanakis A, Alexopoulos C, Samartzi F. Prevalence of subclinical mastitis and influence of breed, parity, stage of lactation and mammary bacteriological status on coulter counter counts and California mastitis test in the milk of Saanen and autochthonous Greek goats. Small Rumin. Res. 1996;21(2):139–147. [Google Scholar]


