Abstract
Background and Aim:
Food poisoning caused by Salmonella is among the most common gastrointestinal discomfort resulted from egg consumption which can produce various syndromes. The present study is a systematic review and meta-analysis investigation on the published studies about the prevalence of Salmonella contamination in the consumed eggs in Iran.
Materials and Methods:
The data were collected and analyzed from four international search databases, including PubMed, Scopus, Science Direct, and Google Scholar and four Iranian databases comprising SID, MagIran, Civilica, and IranDoc. After searching all the databases, 303 articles were found, from which 31 articles were included in the final analysis.
Results:
According to the data analysis, the highest rate of contamination was belonged to the industrial eggs (7.49%), however, the prevalence rate was reported 13.61% in the eggshell part. The overall prevalence of Salmonella contamination in consumed eggs of Iran using culture of microbial, molecular, molecular-serological, culture-molecular, culture-serological, and culture -molecular-serological methods was obtained 11.33%, 5.52%, 0.37%, 1.91%, 5.52%, and 0.73%, respectively. Prevalence in the 21 geographical areas, where studies have been conducted, ranged from 0% (Zahedan) to 29.06% (Tabriz). The studies have also showed that eight different serotypes were among the major cause of Salmonella contamination in eggs. The most common Salmonella serotype was Salmonella Enteritidis and the highest diversity in Salmonella contaminant serotypes was recorded in Talesh (including S. Enteritidis, Salmonella Gallinarum, Salmonella Virchow, and Salmonella Newport).
Conclusion:
Results of this study revealed the high prevalence of Salmonella contamination in eggs, in Iran. Therefore, disinfection and cleaning bed, cleaning of equipment and supplies, and proper maintenance temperature and humidity of the eggs are recommended. In addition, proper personal hygiene and prohibition of consuming raw egg products are essential.
Keywords: eggs, Iran, Salmonella, systematic review and meta-analysis
Introduction
Salmonella is the most common and important bacteria involved in food poisoning caused by egg consumption and can produce various syndromes, with the most common clinical manifestations of gastroenteritis and food poisoning [1]. Salmonellosis in humans and animals is generated by various serotypes through oral intake [1,2]. Salmonella Enteritidis and Salmonella Typhimurium are the most common serotypes involved in the occurrence of salmonellosis [3] through consumption of poultry meat and egg [4]. In general, there are two different routes to transfer Salmonella to the egg. The initial transmission rout is vertical which is happening through the direct contamination of the yolk, whites, membrane, or shell of the egg before ovulation. Horizontal transmission is the second rout, in which, the bacterium contaminates the shell surface through penetrating the infected intestine or stool [5]. Of two common serotypes, contamination with S. Enteritidis and S. Typhimurium was occurred during ovulation [3]. Unfortunately, massive use of antibiotics in human and in the livestock and poultry breeding industries has led to a dramatic increase in Salmonella resistance strains, which is a global dilemma [2]. This issue has doubled the importance of attention to the health of red meat, poultry meat, and eggs. The prevalence of egg contamination with Salmonella has been investigated in different studies in Iran which was varied from 0% to 99% [2,6]. Amin-Zare et al. [7] investigated the contamination to Salmonella in 100 samples of industrial eggs in Urmia by microbial culture method. The results showed that the contents of six samples were contaminated with S. Enteritidis. In addition, no evidence of Salmonella contamination in 525 industrial egg specimens was reported in Isfahan [8].
Understanding the importance of prevalence, the involved serotypes of Salmonella and also contamination of different parts of the egg to promote preventive measures of salmonellosis is crucial.
Therefore, the purpose of this systematic review and meta-analysis study was to access the above-mentioned information through a survey of accomplished articles and studies in Iran.
Materials and Methods
Ethical approval
This is a systematic review and meta-analysis of the published studies and ethical approval is not needed for this study.
Study method
The present study was a systematic review and meta-analysis on the prevalence of Salmonella contamination of the eggs consumed in Iran from 1996 to 2018.
Search strategy
In 2018, a comprehensive scientific search was carried out in four valid international databases (PubMed, Scopus, Science Direct, and Google Scholar) and four Iranian valid electronic databases (Sid, MagIran, Civilica, and IranDoc). The selected keywords for the international and national databases were “Salmonella,” “egg,” and “Iran.” The collected data were then entered into the EndNote, X8 software, to automatically delete the duplicate articles.
Screening
Initial search of studies was conducted by two authors (first and last ones). Screening of studies, extraction of results, and evaluation of the quality control of articles were performed separately by two authors (first and last ones). If there was no agreement between the two authors, the team leader (the responsible author) would declare a final comment on that article.
Inclusion and exclusion criteria
Among the extracted studies, some of them were excluded from the list of received studies: Review articles, summary of presented articles at the congress, the studies that abstract and full texts were not available, surveys that were not part of the original research, unrelated surveys to the prevalence of Salmonella contamination in consumed eggs, and studies that did not explicitly express data.
Quality control
A checklist developed by the Joanna Briggs Institute was used to check and control the quality of articles [9]. This tool consists of eight questions that are classified as Yes, No, Uncertain, and Unused. The purpose of this tool is to evaluate the methodological quality of studies, ways to access and understand the errors available in studies, design, implementation, and analysis of data.
Data extraction
From each article, some information including authors’ name, type of egg (local or industrial), method of detection, relevant Salmonella serotypes, egg contamination site, frequency rate of positive cases, as well as areas contaminated with Salmonella were entered into the pre-designed tables (Table-1) [1-8,10-32]. Then, the data were classified and statistically analyzed.
Table-1.
Information of included studies in the meta-analysis of the prevalence of Salmonella spp. in eggs in Iran.
| Egg type | N a | p (%) | 95% confidence interval | Egg Cs | Method | Location | Serotype | References |
|---|---|---|---|---|---|---|---|---|
| Industrial | 120 | 3.3 | 0.2-6.4 | Contents | Culture | Mashhad | Spp. | [1] |
| Local | 120 | 7.5 | 2.9-12.1 | Contents | Culture | Mashhad | Spp. | |
| Industrial | 120 | 22.5 | 15.2-29.8 | Shell | Culture | Mashhad | Spp. | |
| Local | 120 | 39.1 | 30.6-47.6 | Shell | Culture | Mashhad | Spp. | |
| Industrial | 100 | 5 | 1.1-8.9 | Shell | Culture | Qom | Spp. | [15] |
| Industrial | 100 | 1 | 0-2.8 | Contents | Culture | Qom | Spp. | |
| Industrial | 100 | 0 | 0-4.5 | Contents | Culture | Talesh | Spp. | [2] |
| Local | 100 | 0 | 0-4.5 | Contents | Culture | Talesh | Spp. | |
| Industrial | 100 | 19 | 11.5-26.5 | Shell | Culture | Talesh | Enteritidis | |
| Local | 100 | 4 | 0.7-7.3 | Shell | Culture | Talesh | Spp. | |
| Local | 210 | 3.3 | 1.4-5.2 | Shell | PCR | Kohgiluyeh and Boyer-Ahmad | Spp. | [13] |
| Local | 210 | 3.3 | 1.4-5.2 | Contents | PCR | Kohgiluyeh and Boyer-Ahmad | Spp. | |
| Industrial | 150 | 1.3 | 0-2.8 | Contents/shell | Culture/PCR | Shahrud | Spp. | [16] |
| Local | 150 | 2.5 | 0.7-4.5 | Contents/shell | Culture/PCR | Shahrud | Spp. | |
| Industrial | 186 | 1.6 | 0.1-3.1 | Contents | Culture/PCR | Esfahan | Enteritidis | [17] |
| Local | 500 | 0.4 | 0.1-0.7 | Shell | Culture/PCR/Serology | Birjand | Spp. | [4] |
| Local | 500 | 0.2 | 0-0.5 | Contents/shell | Culture/PCR/serology | Birjand | Spp. | |
| Industrial | 100 | 6 | 1.5-10.5 | Contents | Culture | Urmia | Enteritidis | [7] |
| Industrial | 120 | 56.6 | 47.9-65.1 | Shell | Culture/serology | Zanjan | Spp. | [18] |
| Industrial | 120 | 0 | 0-2.9 | Contents | Culture/serology | Zanjan | Spp. | |
| Local | 54 | 1.8 | 0-5.3 | Contents | PCR | Mazandaran | Enteritidis | [19] |
| Local | 54 | 1.8 | 0-5.3 | Shell | PCR | Mazandaran | Enteritidis | |
| Industrial | 625 | 4 | Contents | Culture/PCR/serology | Tehran | Enteritidis | [11] | |
| Industrial | 1680 | 14.2 | 12.6-15.8 | Contents | Culture | Gilan Zanjan… | Spp. | [20] |
| Industrial | 100 | 2 | 0.1-3.9 | Contents | Culture/serology | Tehran | Enteritidis | [21] |
| Industrial | 100 | 8 | Mar-13 | Shell | Culture/serology | Tehran | Enteritidis | |
| Industrial | 120 | 15.8 | 9.4-22.2 | Contents | Culture | Tabriz | Spp. | [6] |
| Industrial | 120 | 99.1 | 97.5-100 | Shell | Culture | Tabriz | Spp. | |
| Industrial | 40 | 22.5 | 16.2-28.8 | Contents | PCR | Karaj | Enteritidis | [10] |
| Local | 100 | 5 | 1.1-8.9 | Contents | Culture | Ahvaz | Spp. | [22] |
| Local | 100 | 4 | 0.7-7.3 | Shell | Culture | Ahvaz | Spp. | |
| Industrial | 180 | 0 | 0-2.4 | Shell | Culture/PCR/serology | Khorramabad | Spp. | [5] |
| Local | 180 | 0 | 0-2.4 | Contents | Culture/PCR/serology | Khorramabad | Spp. | |
| Local | 180 | 1.1 | 0-2.6 | Contents | Culture/PCR/serology | Khorramabad | Spp. | |
| Industrial | 180 | 0 | 0-2.4 | Shell | Culture/PCR/serology | Khorramabad | Spp. | |
| Industrial | 775 | 0.6 | 0.1-1.1 | Shell | Culture | Ahvaz | Spp. | [23] |
| Industrial | 775 | 0.1 | 0-0.3 | Contents | Culture | Ahvaz | Spp. | |
| Industrial | 775 | 0.1 | 0-0.3 | Contents/shell | Culture | Ahvaz | Spp. | |
| Industrial | 230 | 0 | 0-1.9 | Contents | Culture | Zahedan | Spp. | [24] |
| Industrial | 100 | 20 | 12.2-27.8 | Shell | Culture | Urmia | Spp. | [25] |
| Local | 100 | 50 | 40.2-59.8 | Shell | Culture | Urmia | Spp. | |
| Industrial | 100 | 2 | 0.1-3.9 | Contents | Culture | Urmia | Spp. | |
| Local | 100 | 29 | 20.3-37.7 | Contents | Culture | Urmia | Spp. | |
| Local | 300 | 0.3 | 0-0.9 | Shell | Culture/serology | Gilan | Spp. | [14] |
| Local | 300 | 1 | 0-2.1 | Contents | Culture/serology | Gilan | Spp. | |
| Local | 60 | 1.6 | 0-4.3 | Contents | Culture/serology | Qom | Spp. | [12] |
| Industrial | 60 | 0 | 0-7.02 | Contents | Culture/serology | Qom | Spp. | |
| Industrial | 34 | 0 | 0-12.2 | Contents | Culture/PCR | Tehran | Spp. | [26] |
| Local | 200 | 5 | 2.3-7.7 | Contents | Culture/PCR | Fasa | Enteritidis | [27] |
| Industrial | 500 | 0.4 | 0-0.7 | Shell | Culture | Shiraz | Spp. | [28] |
| Industrial | 500 | 0.2 | 0-0.5 | Contents | Culture | Shiraz | Spp. | |
| Local | 120 | 1.6 | 0-3.8 | Contents | Culture/serology | Zanjan | Spp. | [29] |
| Industrial | 120 | 0 | 0-3.7 | Shell | Culture/serology | Zanjan | Spp. | |
| Local | 120 | 0 | 0-3.7 | Shell | Culture/serology | Zanjan | Spp. | |
| Industrial | 120 | 0 | 0-3.7 | Contents | Culture/serology | Zanjan | Spp. | |
| Local | 50 | 10 | 5.8-14.2 | Contents | Culture/PCR | Shiraz | Enteritidis | [30] |
| Industrial | 150 | 1.3 | 0-3.1 | Shell | Culture/PCR | Tabriz | Spp. | [31] |
| Industrial | 150 | 0 | 0-2.9 | Contents | Culture/PCR | Tabriz | Spp. | |
| Industrial | 100 | 0 | 0-4.4 | Contents | Culture | Shahrekord | Spp. | [32] |
| Industrial | 250 | 1.6 | 0.1-3.1 | Shell | Culture/PCR | Mashhad | Spp. | [3] |
| Industrial | 250 | 0 | 0-1.7 | Contents | Culture/PCR | Mashhad | Spp. | |
| Industrial | 525 | 0 | 0-0.7 | Contents | Culture | Isfahan | Spp. | [8] |
| Industrial | 525 | 0 | 0-0.7 | Shell | Culture | Isfahan | Spp. |
=Number of samples,
b= Number of positive samples, PCR=Polymerase chain reaction
Risk of bias between studies
Egger’s test was used to investigate the risk of propagation bias [33].
Statistical analysis
Chi-square test with a significance level of 0.05, I2 >50% was used to assess the degree of heterogeneity among the included studies. If there was heterogeneity, the random effect model was used with the inverse variance method. If not, the fixed effect model was applied. All analyses were performed using the statistical software STATA, version 13 (StataCorp LLC, College Station, Texas, USA).
Results
Systematic review results
Search results and selection of studies
After investigating the international and internal databases, 303 relevant articles were chosen, 272 articles were undergone an assessment of titles and abstracts, after excluding the duplicate articles. After assessment titles and abstracts, 35 articles entered the next stage, in which, the full text of the articles was investigated and 31 articles were approved and entered into the final analysis. During screening stages, some studies were excluded from investigation due to the unrelated subject, the study population, and duplicate results. The flowchart of the included studies is shown in Figure-1.
Figure-1.
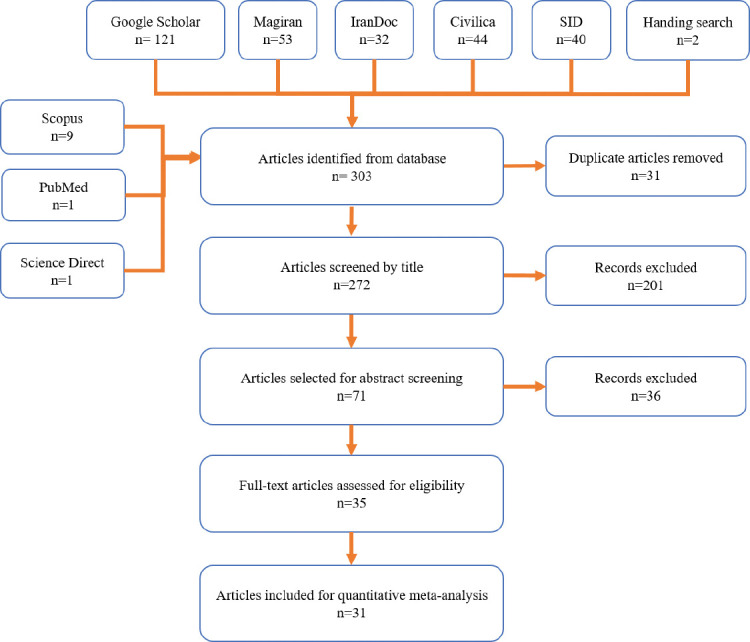
Flowchart of the included eligible studies in systematic review and meta-analysis.
Characteristics of studies and data extraction
In this study, the contamination rates were investigated in 21 geographical regions of Iran. However, most studies were accomplished in Zanjan and Mashhad (six studies) (Table-2). Of the studies, 29, 9, and 3 cases were, respectively, examined the contamination of the contents, shell, and the contents and eggshell with Salmonella (Table-1). Six different methods, including the culture of microbial, molecular, molecular culture, serological-molecular, culture-serological-molecular, and culture-serological have been used to detect the microorganism (Table-3). The highest prevalence was ranged from 99.1% and 0% in seven studies conducted on egg contamination with Salmonella (Table-1).
Table-2.
Prevalence of Salmonella subgrouped by location.
| Location | Total inputs | Total sample size | Overall prevalence (%) | 95% confidence interval | I2 (%) | p for χ2 |
|---|---|---|---|---|---|---|
| Tehran | 4 | 859 | 3.50 | 1.39-5.60 | 57.3 | 0.071 |
| Tabriz | 4 | 540 | 29.06 | 30.94-89.06 | 100 | 0.000 |
| Shiraz | 3 | 1050 | 0.73 | 0.20-1.66 | 90.6 | 0. 000 |
| Esfahan | 3 | 1236 | 0.14 | 0.33-0.61 | 52.6 | 0.121 |
| Urmia | 5 | 500 | 20.78 | 7.22-34.35 | 97.0 | 0.000 |
| Shahrekord | 1 | 100 | 0.00 | −2.50 2.5 | 0 | 0 |
| Talesh | 4 | 400 | 4.45 | −0.51 9.42 | 88.3 | 0.000 |
| Mashhad | 6 | 980 | 10.24 | 5.14-15.35 | 95.9 | 0.000 |
| Qom | 4 | 320 | 1.60 | −0.02-3.21 | 24.3 | 0.266 |
| Kohgiluyeh and Boyer-Ahmad | 2 | 420 | 3.30 | 1.96-4.64 | 0.0 | 1.000 |
| Birjand | 2 | 1000 | 0.30 | 0.09-0.51 | 0.0 | 0.356 |
| Zanjan | 6 | 720 | 7.35 | 1.84-12.85 | 97.0 | 0.000 |
| Shahrood | 2 | 300 | 1.81 | 0.57-3.06 | 9.7 | 0.293 |
| Mazandaran | 2 | 108 | 1.80 | −0.67-4.27 | 0.0 | 1.000 |
| Gilan | 2 | 600 | 0.49 | −0.12-1.10 | 16.6 | 0.274 |
| Karaj | 1 | 40 | 22.50 | 16.20-28.80 | 0 | 0 |
| Khorramabad | 4 | 720 | 0.25 | −0.46-0.96 | 0.0 | 0.659 |
| Ahvaz | 4 | 1750 | 0.32 | −0.06-0.69 | 72.9 | 0.005 |
| Zahedan | 1 | 230 | 0.00 | −1.10-1.10 | 0 | 0 |
| Fasa | 1 | 200 | 5.00 | 2.30-7.70 | 0 | 0 |
| Gilan, Zanjan, Kermanshah, Azerbaijan-Shargi, Mazandaran | 1 | 1680 | 14.20 | 12.60-15.80 | 0 | 0 |
Table-3.
Prevalence of Salmonella in eggs subgrouped by method of the detection of Salmonella.
| Method of detection | Total inputs | Total sample size | Overall prevalence (%) | 95% confidence interval | I2 (%) | p for χ2 |
|---|---|---|---|---|---|---|
| Culture | 29 | 8405 | 11.33 | 7.88-14.78 | 93.9 | 0.000 |
| PCR | 4 | 568 | 5.52 | 1.63-9.42 | 89.1 | 0.000 |
| Culture/PCR | 10 | 1570 | 1.91 | 0.75-3.07 | 74.0 | 0.000 |
| Culture/PCR/serology | 7 | 2345 | 0.73 | 0.08-1.38 | 81.8 | 0.000 |
| Culture/serology | 8 | 960 | 5.52 | 2.36-8.69 | 96.0 | 0.000 |
| PCR/serology | 4 | 480 | 0.37 | −0.69-1.43 | 0.0 | 0.669 |
PCR=Polymerase chain reaction
In the accomplished study, local and industrial eggs have been considered, which the highest prevalence has been reported for industrial eggs. Local eggs are produced by fed on organic diet without any supplementation of chemicals and antibiotic with free access to the outdoors, and industrial eggs produced by hens confined indoors and living in group cages fed on a formulated diet with some supplementation. Salmonella distinct serotypes from eggs including Salmonella Enteritidis, Salmonella Gallinarum, Salmonella Virchow, Salmonella Newport, Salmonella Typhi A, Salmonella Agona, Salmonella Paratyphi, and Salmonella Typhimurium (Table-1).
Meta-analysis results
Overall prevalence
The results of meta-analysis showed that the overall prevalence of Salmonella in eggs was 6.89% (CI: 4.96%-8.82%) (Figure-2).
Figure-2.
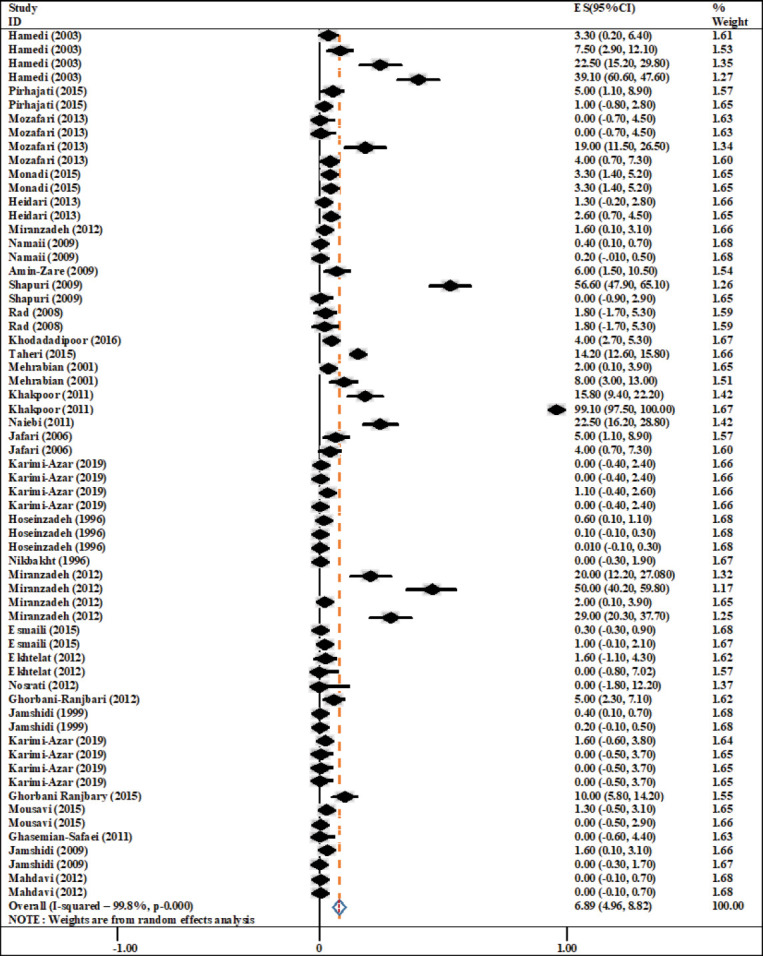
Forest plot of total prevalence outbreak of Salmonella in eggs.
Salmonella serotypes
The results showed that the contamination of eggs with Salmonella spp. (6.87%) was higher than S. Enteritidis (6.20%) (Table-4).
Table-4.
Prevalence of salmonella in eggs subgrouped by type of Salmonella.
| Serotypes | Total inputs | Total sample size | Overall prevalence (%) | 95% confidence interval | I2 (%) | p for χ2 |
|---|---|---|---|---|---|---|
| Salmonella. Spp. | 52 | 12,606 | 6.87 | 4.74-9.00 | 99.8 | 0.000 |
| Salmonella Enteritidis | 11 | 1609 | 6.20 | 3.84-8.55 | 87.0 | 0.000 |
Egg contamination site
The meta-analysis revealed that the highest and lowest contaminations were belonged to part of the eggshells (13.61%) and eggshell plus egg contents (0.35%) (Table-5).
Table-5.
Prevalence of Salmonella in eggs subgrouped by egg contamination site of Salmonella.
| Egg contamination site | Total inputs | Total sample size | Overall prevalence (%) | 95% confidence interval | I2 (%) | p for χ2 |
|---|---|---|---|---|---|---|
| Contents | 35 | 7909 | 2.53 | 1.77-3.29 | 93.5 | 0.000 |
| Shell | 24 | 5044 | 13.61 | 7.85-19.37 | 99.9 | 0.000 |
| Shell/contents | 4 | 1575 | 0.35 | −0.09-0.78 | 66.5 | 0.030 |
Method of detection
Among the six methods of detection, the highest prevalence rate of eggs has been confirmed by the conventional microbial culture (11.33%) and the lowest prevalence rate of eggs was observed in combined serological-molecular method (0.37%) (Table-3).
Geographical location
The highest prevalence rate has been reported in north and northwestern cities (29.06%), Karaj (22.50%), and Urmia (20.78%), moreover, the prevalence rate of 0% has been reported from Sistan-Baluchistan province (Table-2).
Discussion
The present study showed a relatively high rate of overall prevalence of Salmonella (6.89%) in the industrial Iranian eggs (Figure-2). The contamination rate in the eggshell was higher than the contents and the lowest contamination rate was associated with the whole eggs (Table-5). The obtained results showed that in the collected studies, six different methods have been used to detect Salmonella with different findings so that the highest and lowest prevalence were, respectively, reported in the microbial culture and the combined serological-PCR methods (Table-3). Eight different serotypes of Salmonella were involved in egg contamination with the highest prevalence of S. Enteritidis (Table-4). In addition, the highest diversity of Salmonella contaminant serotypes (including S. Enteritidis, S. Gallinarum, S. Virchow, and S. Newport) was reported from Talesh [2]. Among industrial and local eggs, the highest prevalence was reported in the industrial ones. However, no significant association was observed between prevalence rate and geographic areas (Table-2).
Eggs are produced both locally and industrially, and bacterial contamination occurs in both local and industrially produced eggs. In the present study, the highest prevalence rate was reported for industrial eggs (7.49%) (Table-6). Some of the important reasons are including the misuse of antibiotics (which make Salmonella resistant to unfavorable environmental conditions) in poultry farms, high density at industrial poultry breeding location, feeding methods, and different slaughter methods [2].
Table-6.
Prevalence of Salmonella subgrouped by egg type.
| Egg type | Total inputs | Total sample size | Total sample size | 95% confidence interval | I2 (%) | p for χ2 |
|---|---|---|---|---|---|---|
| Industrial | 39 | 10,500 | 10,500 | 4.65-10.33 | 99.8 | 0 |
| Local | 24 | 4028 | 4028 | 2.22-4.11 | 92.4 | 0 |
Each egg contains two parts: The shell and contents, which both parts can be contaminated to an important pathogen such as Salmonella [1]. In the present study, the highest prevalence rate of Salmonella was reported in eggshells (13.61%) (Table-5). Naturally, neglecting the health of poultry farm staff (direct contact of hand with the eggshell), inappropriate substrates, contact of chicken feces with the eggshell, and improper maintenance conditions of temperature can lead to grow Salmonella on the egg surface [1,5]. In the study of Suresh et al. [34] of the 492 eggs studied in South India, 38 cases were positive, with the highest prevalence of the eggshells (29 cases). This finding was consistent with the present study. In another study, more than 5700 eggs from 15 flock contaminated with S. Enteritidis were tested, in which the contents of 32 eggs (0.6%) were contaminated. It was also shown the lower prevalence rate in the contents of the eggs than our studies. Different prevalence reported before was probably related to the maintenance conditions of chicken and eggs and overall sanitation [7].
The most important bacterial species involved in eggs are different Salmonella serotypes. In this study, Salmonella serotypes were divided into two main groups, including S. Enteritidis and other Salmonella serotypes. S. Enteritidis was the dominant serotype involved in the contamination of eggs (Table-1). In various accomplished studies in the world, the most common Salmonella serotype separated from contaminated eggs is S. Enteritidis [4], which may be due to the important role of flagella in this bacterium to transmit and survive in the host cells [10].
To identify Salmonella serotypes, different methods have been used. In this study, six different methods were used to identify different Salmonella serotypes. Microbial culture was the most common method and combined culture-serological-PCR assay was the least methods to identify Salmonella. The prevalence rate of Salmonella in each method was reported differently; for instance the highest prevalence rate was related to microbial culture (11.33%) and the lowest prevalence was associated with the combined serological-PCR method (0.37%) (Table-3). Using microbial culture is common in laboratories due to its easy access and low cost method [35], even though the disadvantage of such techniques is the impairment to detect small numbers of bacteria, time-consuming, and lack of sufficient diagnostic specificity [36]. Since the molecular method has multiple applications [11], it is highly accurate and sensitive and is considered fast and less expensive method, it is also one of the new approaches to confirm the conventional techniques [2,37].
To identify the pathogenic aerobic bacteria from egg shell and its contents, contamination to Salmonella serotypes among 6.67% of eggshell was detected using microbial culture method, while none of the eggs contents were found positive. However, seven samples of egg contents and 14 samples of eggshells were reported positive, using PCR technique, the difference was corresponds to the employment of different methods of identification [5]. The difference was due to high sensitivity and accuracy of the molecular methods [37]. The overall prevalence of Salmonella in the Iranian eggs was 6.89%. The low prevalence was probably due to the conventional culture used in our study. The results obtained from this study also approve this hypothesis that the prevalence of Salmonella was higher if more accurate methods such as combined conventional culture and PCR procedures were employed. Moreover, no significant difference was observed between various geographical areas in Iran. The highest and lowest prevalence were, respectively, reported from Tabriz (29.06%) and Zahedan (0%) (Table-2). The difference was likely due to the inappropriate temperature and humidity [4].
This is the first study that investigates the overall prevalence of Salmonella in Iranian eggs. The contamination rate of different parts of egg, common sites of contamination, and the most common serotypes involved are among the most important factors involving in egg contamination. In this study, both local and industrial eggs were examined. In addition, the contamination was reported from many geographical areas of the country. All feasible methods for the diagnosis of Salmonella were analyzed and the prevalence of Salmonella was also determined in each method. The contamination with all serotypes of the bacteria was identified. The present study was composed of four English and four Persian databases.
In this study, the contamination of shell and contents in the industrial and local eggs was not investigated separately. Few recently published data and Ph.D. thesis are also not included in our analysis.
Conclusion and Recommendation
The present study was performed based on a systematic review and meta-analysis to evaluate the overall prevalence of Salmonella in Iranian eggs. The obtained results showed a relatively high prevalence rate. S. Enteritidis was also the most common contaminant serotype. The prevalence of Salmonella in the industrial eggs was higher than the local eggs and it was higher in the eggshells. The contamination was observed in most provinces. The results also revealed that six different methods were commonly used to diagnose the bacteria. In most studies, the conventional culture was used which showed higher contamination rate compared, whereas the lowest prevalence was obtained in the molecular-serological method.
To prevent the occurrence of Salmonella in the eggs, the following schemes need to be carefully investigated; personal hygiene, disinfection and cleaning bed (in traditional breeding) and cages (in industrial breeding), disinfection and cleaning of equipment and supplies which is in contact with eggs, and observance of proper maintenance conditions (temperature and humidity) [12]. The misuse of antibiotics in the poultry farms should be avoided [13] and also consuming raw and medium (half-cooked) eggs should be prohibited, and if in case, the raw eggs are used in sauces or desserts, lowering pH of the product is strongly recommended [14].
Authors’ Contributions
EB: Study design; review relevant articles, analysis, and interpretation of data; drafting and finalizing the manuscript; and study supervision. BH, MN, and SH: Review relevant articles, analysis, and interpretation of data; drafting the manuscript. SMM, SH, LE, and MZ: Analysis and interpretation of data; drafting the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This study was entirely financed by Shiraz University of Medical Sciences (SUMS) (project no. 98-01-84-21312). The authors would like to thank the Vice-chancellor for Health of SUMS for their kind assistance during sampling for this project.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Hamedi A, Ganaat J. Investigation of Salmonella infection in two types of local and machine eggs in Ghaem hospital laboratory in Mashhad. Mashhad J. Med. Sci. 2003;76(45):67–71. [Google Scholar]
- 2.Mozafari M, Rahmani Z, Isazadeh K.H. Investigating the level of contamination of red meat, chicken and industrial and local eggs in Salmonella species in Talesh city and evaluating the pattern of antibiotic resistance in them. J. Qom. Med. Sci. 2013;7(5):60–65. [Google Scholar]
- 3.Jamshidi A, Kalidari G.A, Hedayati M. Isolation and identification of Salmonella Enteritidis and Salmonella Typhimurium from the eggs of retail stores in Mashhad, Iran usingconventional culture method and multiplex PCR assay. J. Food Saf. 2009;30(3):558–568. [Google Scholar]
- 4.Namaii M, Ziyaii M. The prevalence of Salmonella contamination in local (non-industrial) eggs produced in Birjand (2006) J. Birjand Univ. Med. Sci. 2009;16(2):37–41. [Google Scholar]
- 5.Dolat A, Mahzunie M, Shams N, Etemadfar L. Investigating the prevalence and comparison of Salmonella serotypes in native and industrial poultry eggs of Khorramabad city using culture and PCR. Iran. J. Med. Microbiol. 2018;12(2):88–95. [Google Scholar]
- 6.Khakpoor M, Bozorgnia M. Determination of bacterial agents in eggshells and yolks of eggs with infected shells and eggs with clean shells supplied in Tabriz. Food Hyg. J. 2011;1(2):17–27. [Google Scholar]
- 7.Amin-Zare M, Neyriz-Naqdehi M, Rasooli S, Delshad R. Separation of Salmonella from the yolks of local eggs in Urmia. J. Vet. Med. 2009;3(7):51–55. [Google Scholar]
- 8.Mahdavi M, Jalali M, Ghasemian-Safaei H, Shamloo E. Microbial quality and prevalence of Salmonella and Listeria in eggs. Int. J. Environ. Health Eng. 2012;6(1):16–20. [Google Scholar]
- 9.The Joanna Briggs Institute. Critical Appraisal Tools for Use in JBI Systematic Reviews Checklist for Analytical Cross-Sectional Studies. Australia: The Joanna Briggs Institute; 2017. [Google Scholar]
- 10.Naiebi N, Goreishi S.A, Herzandi N, Shamsara M, Tabraee B, Bakhtiari A. Evaluation of PCR method for diagnosis of Salmonella Enteritidis bacterial infection in poultry products in Karaj city. J. Med. Sci. 2011;21(1):32–37. [Google Scholar]
- 11.Khodadipoor T, Mahmoudi R. Evaluation of virulence and enterotoxin gene in Salmonella Enteritidis Strains isolated from meat and egg samples by multiplex PCR. J. Food Microbiol. 2016;3(2):25–33. [Google Scholar]
- 12.Ekhtelat M, Rezvani S.J, Safarloo A. Investigation of contamination to Salmonella and Staphylococcus aureus in native and industrial hen's eggs in Qhom areas. J. Vet. Lab. Res. 2012;4(2):309–314. [Google Scholar]
- 13.Monadi M, Kargar M, Najafi A, Mohamadi R. Molecular identification of Salmonella serotype isolated from local eggs of Kohgiluyeh and Boyer-Ahmad province using PCR technique and evaluation of drug resistance. Lab. Sci. J. 2015;9(1):18–23. [Google Scholar]
- 14.Esmaili H, Hamedi M. Salmonella serotypes in native eggs of Gilan province. Quart. J. Infect. Trop. Dis. 2015;19(65):39–45. [Google Scholar]
- 15.Pirhajati-Mahabadi R, Taybi M, Yagubi S, Bakhtiarizadeh M, Saadat-Mousavi N. Investigation of antibiotic resistance of bacteria isolated from the contents and shell of industrial eggs in Qom. Qom Med. Sci. J. 2015;9(11):69–75. [Google Scholar]
- 16.Heidari A, Javid A.B, Ghanbarian M, Roohafzaiy M. Prevalence of Salmonella Infection in Local and Industrial Eggs in Shahrood. Iran: 16th National Conference on Environmental Health of Iran; 2013. [Google Scholar]
- 17.Miranzadeh H, Zahraie-Salehi T, Karimi V. Enumeration of mesophilic aerobic microorganisms and isolation of Salmonella bacteria from eggs consumed in Isfahan in 2010. Vet. Med. 2012;25(1):32–35. [Google Scholar]
- 18.Shapuri R, Rahnama M, Iqbalzadeh S.H. Prevalence of Salmonella serotypes in chicken and egg and determination of their antibiotic susceptibility in Zanjan. J. Anim. Physiol. Dev. 2009;2(3):63–71. [Google Scholar]
- 19.Rad M, Kalidry G.H.A, Kordjazi S.H. Identification of different Salmonella species in the breeding center of native poultry. Res. Contract. J. 2008;81(4):88–93. [Google Scholar]
- 20.Taheri H, Peighambari S.M, Morshed R, Barin A. Isolation amount of Salmonella and Escherichia coli from breeder herds in different provinces of Iran. Iran. J. Vet. Clin. Sci. 2015;9(2):3–10. [Google Scholar]
- 21.Mehrabian S, Tabatabaiy-Rafiy R, Hajian A. Evaluation of type and amount of drug resistance of Salmonella isolated from food. J. Sci. Teach. Train. Univ. 2001;1(3-4):193–199. [Google Scholar]
- 22.Jafari R, Fazlara A, Delirannia A. Investigation of Salmonella contamination in indigenous eggs consumed in Ahvaz. Iran. J. Vet. Med. 2006;2(2):58–63. [Google Scholar]
- 23.Hoseinzadeh SH Investigation of Salmonella contamination of eggs consumed in Ahvaz and its importance in terms of public health. Msc Thesis. Ahvaz, Iran: Chamran University; 1996. [Google Scholar]
- 24.Nikbakht B, Heidarzadeh M, Hosseini M, Sargazi G.H, Irani M Investigation of Salmonella Infection in Eggs of Laying Hen Farms in Zahedan City. Iran: 23rd National Congress of Food Science and Technology of Iran; 1996. [Google Scholar]
- 25.Mehdizadeh T, Farhadi M, Tajik H Comparative Study of Salmonella Contamination in Local and Industrial Eggs in Urmia. United States: 21st National Congress of Iranian Food Science and Technology; 2013. [Google Scholar]
- 26.Nosrati S.H, Sabokbar A, Dezfulian M, Tabraee B, Fallah F. Investigating the prevalence of Salmonella Typhi, mumps, typhoid and enteritis serotypes in food at Mofid hospital. J. Med. School Res. 2012;36(1):43–48. [Google Scholar]
- 27.Ghorbani-Ranjbari A, Ghorbani-Ranjbari N, Ghorbani-Ranjbari Z, Asmarian S.H. Study of Salmonella drug resistance separated from indigenous eggs of Fasa county. J. Modern Vet. Res. 2012;4(11):1–7. [Google Scholar]
- 28.Jamshidi A.A. Investigation of Shiraz eggs in terms of Salmonella contamination. J. Babol. Med. Sci. 1999;2(1):21–25. [Google Scholar]
- 29.Karimi-Azar F, Soltanpour M.S, Aminzare M, Hassanzada-Zar H. Prevalence genotyping, serotyping, and antibiotic resistance of isolated Salmonella strains from industrial and local eggs in Iran. J. Food Saf. 2019;39(1):e12585. [Google Scholar]
- 30.Ghorbani-Ranjbary A. Study of drug resistance in Salmonella spp. Isolated from native eggs of Iran's Southern region. J. Pure Appl. Microbiol. 2015;9(5):175–179. [Google Scholar]
- 31.Mousavi M.H, Esmaeili S, Bagheri-Amiri F, Mostafavi E, Zahraei-Salehi T. Detection of Salmonella spp. in commercial eggs in Iran. Iran. J. Microbiol. 2015;7(1):50–54. [PMC free article] [PubMed] [Google Scholar]
- 32.Ghasemian-Safaei H, Jalali M, Hosseini A, Narimani T, Sharifzadeh A, Raheimi E. The prevalence of bacterial contamination of table eggs from retail markets by Salmonella spp Listeria monocytogenes, Campylobacter jejuni and Escherichia coli in Shahrekord, Iran. Jundishapur J. Microbiol. 2011;4(4):249–253. [Google Scholar]
- 33.Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suresh T, Hatha A.A.M, Sreenivasan D, Sangeetha N, Lashmanaperumalsamy P. Prevalence and antimicrobial resistance of Salmonella Enteritidis and other Salmonellas in the eggs and egg-storing trays from retail markets of Coimbatore, South India. J. Food Microbiol. 2006;23(3):294–299. doi: 10.1016/j.fm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Talebzadeh S, Zaker-Bostanabad S, Nazari R. Study and comparison of Brucella diagnostic methods (culture-molecular) in samples isolated from patients suspected of having brucellosis in different areas of Tehran. New Cell. Mol. Biotechnol. J. 2016;6(23):105–109. [Google Scholar]
- 36.Shakeri M.A, Shahidi F, Mortazavi S.A, Bahrami A.R, Nasiri M.R. A Review of Molecular Methods for Identifying and Counting Probiotic Bacteria in Dairy Products. The First Snack Conf. 2014;1:1. [Google Scholar]
- 37.Dareh-Kordi A, Rezazadeh-Zarandi E, Reza-Hoseini O, Asar S.H.A, Asar S. Diagnosis of bacterial infections;traditional and molecular methods:A narrative study. J. Rafsanjan Univ. Med. Sci. 2018;17(9):865–880. [Google Scholar]


