Abstract
Most patients who succumb to cancer have metastases to bone that contribute to their death. Cancer cells that metastasize to bone are regularly subjected to mechanical stimuli that may affect their proliferation, growth and protein expression. Understanding why some cancer cells thrive in this environment could provide insight into new approaches to prevent or treat metastasis to bone. We used 4T1 cells as a model of breast cancer cells, and implanted them in gelatin hydrogels with moduli of 1 or 2.7 kPa to mimic the properties of bone marrow. The constructs were subjected to either perfusion of media through the hydrogel or combined perfusion and cyclic mechanical compression for 1 h d−1 for 4 d. Controls were cultured in free-swelling conditions. The cells formed spheroids during the 4 d of culture, with larger spheroids in the statically cultured constructs than in perfusion or compressed constructs. In stiffer gelatin, smaller spheroids formed in compressed constructs than perfusion alone, while compression had no effect compared to perfusion in the softer gelatin. Immunostaining indicated that the spheroids expressed osteopontin, parathyroid hormone-related protein and fibronectin, which are all hallmarks of bone metastasis. The proliferative marker Ki67 was present in all spheroids on day 4. In the 1 kPa gelatin, Ki67 staining intensity was greater in the statically cultured, free-swelling constructs than in bioreactor culture, regardless of dynamic compression. By contrast, proliferation was higher in the compressed gelatins compared to perfusion alone in the 2.7 kPa constructs, although the spheroids were smaller, on average. This suggests the stiffer gelatin may restrict spheroid growth at the same time that it enhances mechanobiological signalling during compression. Taken together, 4T1 breast cancer cells are mechanically sensitive, and mechanical stimuli can alter their proliferation and protein expression within soft materials with mechanical properties similar to bone marrow. As such, both in vivo and in vitro models of cancer metastasis should consider the role of the mechanical environment in the bone.
Keywords: metastasis, bioreactor, model system, gelatin, immunohistochemistry
1. Introduction
Over 90% of cancer-related deaths are owing to distant metastases rather than the primary tumour [1]. Bone is the most common site for breast cancer metastasis, and more than 70% of patients who die of breast cancer have bone metastases [2]. Breast cancer cells that metastasize to bone often target the axial skeleton, which comprises trabecular bone filled with an abundance of red marrow [3]. When metastatic breast cancer cells engraft in trabecular bone, they face a unique mechanical environment that may affect their progression characterized by a soft marrow that is interspersed between relatively rigid struts of bone matrix [4]. In addition to experiencing changes in substrate stiffness within the bone environment, breast cancer cells will also experience mechanical stimulation from daily activities such as walking and muscle contractions [5–11]. It has been proposed that bone tissue provides a favourable mechanical microenvironment for tumour attachment and invasion [12–16].
Several studies have examined how biophysical stimuli govern the activity of cancer cells. In particular, the role of substrate rigidity for regulating cancer cell proliferation, migration and invasion has been widely investigated in two-dimensional culture. For example, it has been reported that polyacrylamide gel stiffness was correlated with proliferation of breast cancer [17] and glioma cells [18]. Furthermore, substrate degradation and traction stresses of aggressive carcinoma cells were dependent on polyacrylamide matrix rigidity [19]. Mammary epithelial cells had more integrin adhesions and demonstrated a malignant phenotype when cultured on stiffer collagen gels compared to softer gels (170–1200 Pa) [20].
Cancer cells migrate and invade into tissues in vivo, and as such are governed by mechanical stimuli in three-dimensional environments that are not captured by such two-dimensional culture studies. There have been few studies of cancer cell activity and the three-dimensional mechanical environment. It has been shown that metastatic breast cancer cells invade and migrate through methacrylated gelatin with moduli ranging from 300 to 750 Pa [21], or form spheroids in polyethylene glycol diacrylate (PEGDA) gels with moduli between 5 and 70 kPa [22]. Moreover, interstitial flow applied in a microfluidic device increased the number and rate of migratory metastatic breast cancer cells embedded in collagen hydrogels mixed with Matrigel® [23]. Similarly, transient strain applied to a collagen-fibronectin matrix enhanced invasion of fibrosarcoma cells into the matrix when seeded on top of the hydrogel [24]. Prostate cancer cells exposed to shear stress via fluid flow increased secretion of prometastatic cytokines as well as expression of osteogenic genes, indicating a potential role for mechanobiology in metastasis [25]. The importance of the mechanical niche on cancer cells in bone has been investigated in vivo. Cyclic compression of mouse tibiae injected with metastatic breast cancer cells inhibited osteolysis and secondary tumour formation [26]. Additionally, low-intensity vibration reduced tumour burden, osteolysis and marrow necrosis in femora of mice injected with multiple myeloma [27]. These studies suggest that anabolic loading can be used to prevent osteolysis in response to bone metastases, though the effects of loading on tumour growth remain unknown. However, the combined effects of substrate stiffness and dynamic mechanical loading (matrix deformation and fluid shear stress) on cancer cell proliferation, spheroid formation and protein expression in three dimensions have not been investigated, even though dynamic three-dimensional loading is an essential component of bone physiology.
The objective of this study was to investigate the role of biophysical stimuli—including matrix stiffness, cyclic compression and perfusion—on spheroid formation, proliferation and differentiation of metastatic breast cancer cells cultured in three-dimensional hydrogels. Specifically, 4T1 cells were seeded in low- and high-modulus gelatin hydrogels to mimic stiffness ranges found in bone marrow [28] and cultured in a bioreactor that applied perfusion, with or without simultaneous compression, to the constructs. After culture, spheroid size was measured and Ki67 staining was conducted to quantify cancer cell proliferation. Parathyroid hormone-related protein (PTHrP) and osteopontin (OPN) staining were conducted to investigate the propensity for bone metastasis (osteotropism), while fibronectin immunohistology was used to identify potential differences in cancer cell adhesion.
2. Methods
2.1. Gelatin-mTGase hydrogel fabrication
We cultured cells in gelatin-mTGase hydrogels to mimic the range of mechanical properties of normal bone marrow [28]. Gelatin is a natural matrix that incorporates integrin-binding sites appropriate to the 4T1 cells [29], and is digestible under the action of matrix metalloproteinases. By contrast, Matrigel is much more compliant than bone marrow, although it contains many of the same proteins as bone marrow extra cellular matrix. Collagen and alginate matrices are much stiffer than bone marrow, and not as readily digested.
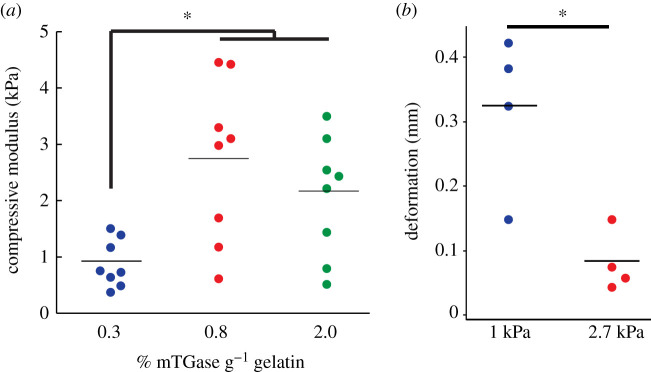
Hydrogels prepared with 0.3% mTGase g−1 of gelatin had a compressive modulus of 0.88 ± 0.42 kPa, compared to 2.7 ± 1.4 kPa and 2.1 ± 1.1 kPa for hydrogels crosslinked with 0.8 and 2% mTGase g−1 of gelatin, respectively (p < 0.02; figure 1a; electronic supplementary material, Supplementary methods). These compositions were chosen to mimic the mechanical behaviour of bone marrow, which ranges from 0.3 up to 2.4 kPa at physiologic temperature [28].
Figure 1.
(a) The elastic moduli of hydrogels crosslinked with 0.3% mTGase were lower than those crosslinked with 0.8 or 2% mTGase (*p < 0.017; n = 8 per group; ANOVA). (b) Deformation of the soft and stiff layers of the gelatin-mTGase hydrogel constructs were measured during compressive loading via image analysis. The soft hydrogels crosslinked with 0.3% mTGase experienced a significantly higher deformation than the stiff hydrogels crosslinked with 0.8% mTGase (*p = 0.04; Student's t-test).
2.2. Three-dimensional cell culture in gelatin-mTGase hydrogels
4T1 murine metastatic breast cancer cells were cultured under standard conditions (5% CO2, 37°C) in RPMI 1640 culture media containing 10% foetal bovine serum and 1% penicillin/streptomycin. The cells were expanded on tissue culture plastic, detached using trypsin-EDTA (Sigma) and suspended in media at a concentration of 106 cells ml−1 before encapsulating in the hydrogel.
Two-layer hydrogel samples were prepared in rectangular polydimethylsiloxane (PDMS) moulds (16 mm × 4 mm × 4 mm) with one stiff gel layer and one soft gel layer, to ensure the same force was applied for a fixed displacement in the bioreactor. Gelatin solution, mTGase solution and cell suspension were mixed at a 5 : 4 : 1 volume ratio to yield final concentrations of 3% gelatin, and either 0.3 or 0.8% mTGase solution and 100 000 cells ml−1. In hydrogel layers prepared without cells, the cell suspension was replaced with cell culture media. Half of the PDMS mould was filled with 300 µl of soft or stiff hydrogel solution without cells, placed in a sterile Petri dish, and allowed to polymerize at 4°C for 15 min. The remaining half of the moulds were then filled with 300 µl soft or stiff hydrogel solution containing 4T1 cells and allowed to polymerize for 15 min at 4°C. The constructs were then removed from the moulds with a sterile spatula, placed in cell culture media and allowed to equilibrate overnight at 37°C.
2.3. Bioreactor culture and mechanical stimulation of hydrogels
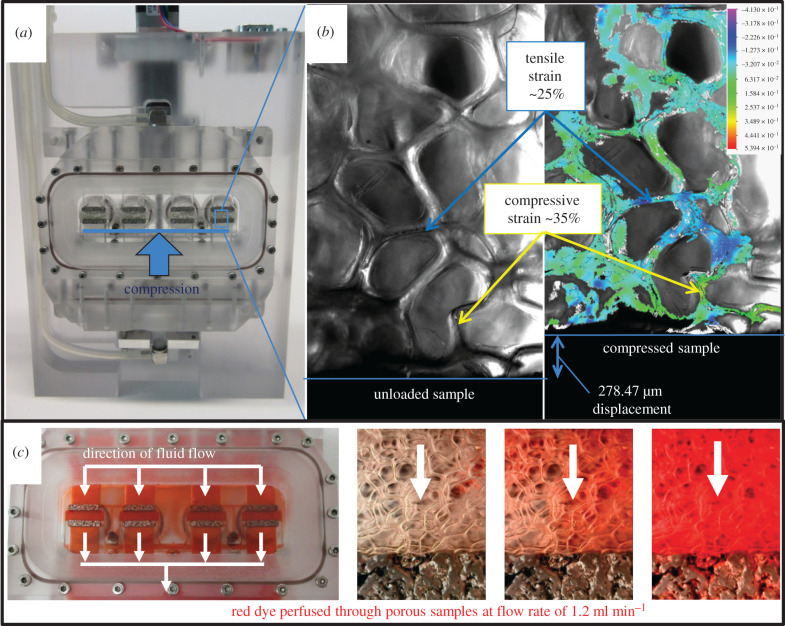
To determine the effect of mechanical stimulation on cancer cells embedded in hydrogels of different stiffness, hydrogel constructs were cultured for 4 days in a custom made, compression-perfusion bioreactor and exposed to mechanical stimulation in the form of compression and perfusion, or perfusion only, which were validated within the bioreactor (figure 2). Controlled displacements were applied to a porous scaffold sample and recorded under high magnification. The strain distribution was determined at the local level using a digital image correlation technique [30] (figure 2b). Media perfusion was validated using red dye, and was determined to have a homogeneous flow profile across all four channels (figure 2c). The porous platens did not affect overall flow symmetry within the system.
Figure 2.
(a) Compression and perfusion bioreactor holds four hydrogel constructs between porous platens. (b) Compression was verified with digital image correlation. Cyclic compression was applied to 4T1-embedded hydrogels to a maximum displacement of 0.5 mm for 1 h d−1 for 4 days (electronic supplementary material, video S1). (c) Validation of media perfusion through the bioreactor. A peristaltic pump continuously circulated media from the reservoirs to the samples at a rate of 1.2 ml min−1.
Hydrogel deformation in the bioreactor was validated by measuring the deformation of each layer under the experimentally applied compression regime (cyclic compression at a frequency of 0.5 Hz to a maximum deformation of 0.5 mm). To measure the deformation of each layer, eight hydrogel constructs without cells were prepared as described previously. The hydrogels were compressed to a total deformation of 0.5 mm and filmed with an endoscope (electronic supplementary material, video S1). The height of the top layer of each construct, which contained cells in the experimental studies, was measured from the images at zero and peak compression via ImageJ (NIH) to calculate the resulting deformation in each layer during loading. The average deformation in the soft layer of the hydrogel constructs was 0.32 ± 0.12 mm, which was significantly higher than 0.08 ± 0.05 mm in the stiff layer (p = 0.04; figure 1b). Therefore, cells embedded in the soft layer experienced significantly more deformation during compressive loading in the bioreactor compared to cells in the stiff layer, while the applied stress on both constructs was the same.
Non-loaded samples were cultured in a six-well plate, and day 0 samples were fixed immediately (table 1). Hydrogel constructs consisted of two layers of enzymatically linked gelatin with moduli of approximately 1 and 2.7 kPa with 4T1 cells seeded at 100 000 cells ml−1 in one of the layers. Media perfusion was applied at 1 ml min−1 to each sample in the bioreactor via a peristaltic pump. Samples were compressed at a frequency of 0.05 Hz to a maximum deformation of 0.5 mm—equivalent to a stretch ratio of 0.88—for 1 h d−1 for 4 d. Four constructs were tested for each group, and this 4 day culture experiment was repeated four times for a total sample size (n) of 16 per group.
Table 1.
Gelatin-mTGase hydrogel constructs were cultured under various loading conditions as described below. (Constructs consisted of a stiff and soft layer, with cells embedded in one of the layers.)
| sample nomenclature | culture condition | layer containing cells | number of constructs |
|---|---|---|---|
| stiff compression | bioreactor: compression and perfusion | stiff | 16 |
| soft compression | bioreactor: compression and perfusion | soft | 16 |
| stiff perfusion | bioreactor: perfusion | stiff | 16 |
| soft perfusion | bioreactor: perfusion | soft | 16 |
| stiff static | six-well plate | stiff | 16 |
| soft static | six-well plate | soft | 16 |
| day 0 stiff | fixed immediately | stiff | 16 |
| day 0 soft | fixed immediately | soft | 16 |
2.4. Viability assays
Cell viability was verified in four hydrogel constructs from one culture experiment using a live/dead assay (ThermoFisher Scientific) following the manufacturer's instructions. Briefly, hydrogels were removed from culture and rinsed twice with sterile phosphate buffered saline (PBS). Samples were then incubated with live/dead solution containing calcein AM and ethidium homodimer-1 (EthD-1) at concentrations of 8 µM each for 2 h at 37°C. Following incubation, samples were rinsed twice with PBS and immediately imaged with a confocal microscope (Olympus Fluoview 1000) at 60× magnification.
Cell metabolism was measured using an Alamar Blue assay (ThermoFisher) performed on four hydrogel constructs from one culture experiment following the manufacturer's instructions. Briefly, constructs were removed from culture and placed in a 24-well plate containing media with 10% Alamar Blue reagent. Hydrogels without cells were used as controls. Samples were incubated for 18 h at 37°C protected from light. Media supernatant was pipetted in triplicate into a 96-well plate and the absorbance was read at 570 nm and 600 nm on a spectrophotometer (Synergy HT, Biotek) to calculate the per cent reduction of Alamar Blue reagent.
2.5. Cell spheroid analysis
Cells formed spheroids in the crosslinked gelatin after 4 d of culture and these were measured under light microscopy. Following culture, hydrogels were rinsed with PBS, fixed in 4% paraformaldehyde in PBS for 24 h at 4°C and stored in PBS at 4°C until imaging. Five micrographs per hydrogel were captured on a bright-field microscope (Olympus BX53) at 100×. The diameters of spheroids that were focused in plane were measured manually using ImageJ (NIH).
2.6. Immunostaining
Immunohistochemical staining was conducted to investigate the presence of proteins implicated in cell proliferation (Ki67), bone metastasis (PTHrP and OPN) and mechanotransduction (fibronectin). After fixation, samples were embedded in an optimal cutting temperature compound (VWR) and cooled at 4°C for 20 min, then kept at −20°C overnight and then stored at −80°C until sectioning. All samples were sectioned at a thickness of 20 µm using a cryomicrotome (Leica) and mounted on SuperFrost Plus™ glass slides (Fisher Scientific). After 10 min of permeabilization, samples were blocked for 1 h using 0.5% bovine serum albumin in PBS, and then incubated overnight with the relevant primary antibody diluted at 1 : 200 for rabbit anti-OPN (AB1870, Sigma), rabbit anti-Ki67 (AB9260, Sigma) and rabbit anti-fibronectin (ab2413, Abcam), or at 1 : 100 for mouse anti-PTH/PTHrP (05–517, Sigma) in the blocking solution. After washing three times for 10 min with PBS, slides were incubated with the respective secondary antibody at 1 : 200 for 1 h protected from light. Donkey anti-rabbit FITC (711-095-152, Jackson ImmunoResearch Labs) was used for fibronectin, Ki67 and OPN, while goat anti-mouse Alexa 488 (A11029, Invitrogen) was used for PTH/PTHrP. The samples were washed with PBS, and nuclei were labelled with a DAPI solution for 5 min (100 ng ml−1). Finally, samples were washed three times with PBS and the slides were mounted using Fluoroshield mounting media (Sigma). Fluorescence emission was detected using a confocal laser scanning microscopy (Olympus Fluoview 1000).
2.7. Statistical analysis
Statistical analysis was performed on Minitab 17 (State College, PA). ANOVA was used to compare compressive moduli data across groups followed by multiple comparison procedure (Tukey's HSD test). Alamar blue assay, spheroid diameter and Ki67 expression data were compared with a two-factor ANOVA to examine the effects of hydrogel stiffness and mechanical stimulation. Results were expressed as mean ± s.d.
3. Results
3.1. Cancer cell viability within gelatin-mTGase hydrogels
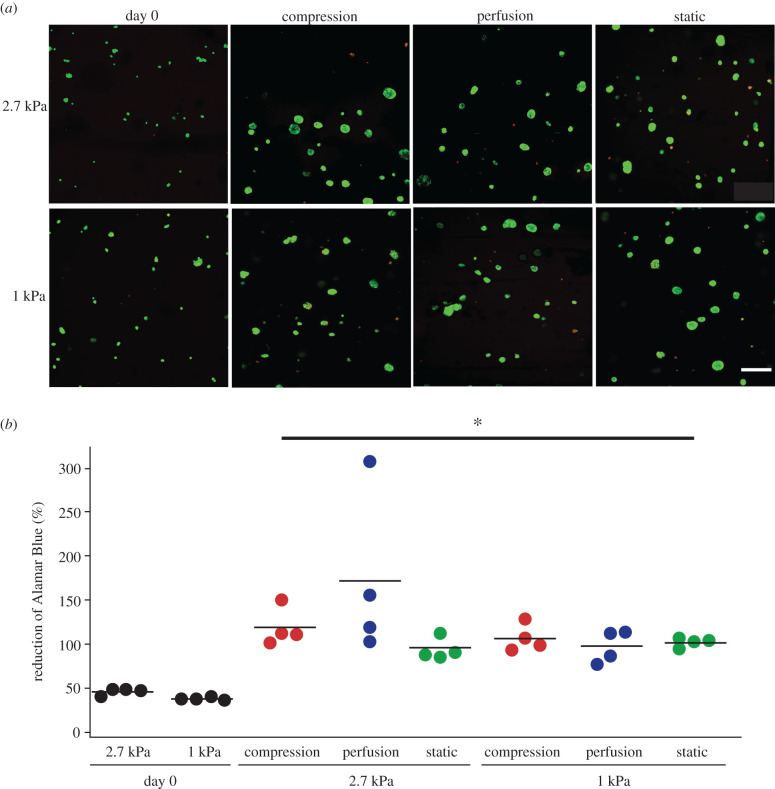
Nearly all of the 4T1 cells within the hydrogels were viable. Ubiquitous calcein staining was present throughout the constructs in the live/dead stain (figure 3a). Cell metabolism within gelatin constructs was higher after 4 days of culture compared to day 0 samples, as quantified by Alamar Blue (p < 0.03; figure 3b). Hydrogel stiffness and mechanical stimulation did not have an effect on cell metabolism (two-factor ANOVA; p > 0.28; figure 3b), indicating that cell viability remained relatively constant among all groups during the 4 days of culture.
Figure 3.
(a) Representative images of live/dead stain throughout the depth of the hydrogel showed that 4T1 cells remained viable in all culture conditions during the experiment (scale bar = 200 µm). Almost no dead cells (EthD-1 staining) were visible. (b) After 4 days of culture, cell metabolic activity was approximately two times higher than the day 0 cultures (*p < 0.03; Student's t-tests). There was no difference in metabolic activity between groups after 4 days of culture, indicating similar numbers of cells in all conditions (p > 0.28; two-factor ANOVA).
3.2. Cancer cell spheroid diameter in response to stiffness and mechanical stimulation
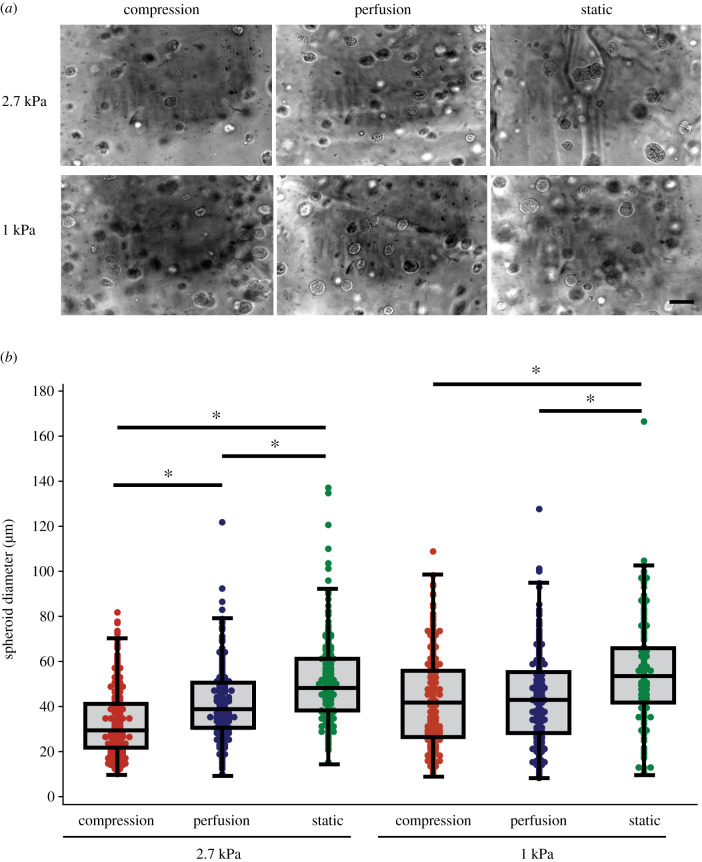
The cells formed spheroids during 4 days of culture under all culture conditions (figure 4a; electronic supplementary material, video S2). day 0 hydrogels had a mean diameter of 19.6 ± 6.2 µm, which was less than all of the 4 d culture groups (p < 0.001). After 4 days of culture, cells cultured in well plates under static conditions exhibited the largest spheroids with a mean diameter of 52.8 ± 18.9 µm, independent of the stiffness of the seeding layer (figure 4b). However, constructs that were exposed to compression for 4 days had an average spheroid size of 32.8 ± 14.1 µm when cells were embedded in stiff hydrogels compared to 41.8 ± 19.1 µm in soft hydrogels (p = 0.0001; figure 4b). Moreover, there was a high proportion of spheroids under 30 µm (50%) in stiff hydrogels that underwent compression, whereas those in static culture had a higher proportion of spheroids larger than 70 µm (16%) regardless of the gelatin stiffness (electronic supplementary material, figure S1). Cells cultured in soft hydrogels under compression compared to perfusion alone had similar spheroid sizes (p = 0.09; figure 4b). Both hydrogel stiffness and mechanical stimulation significantly affected spheroid diameter (two-factor ANOVA; p = 0.001).
Figure 4.
(a) Representative phase-contrast images of 4T1 spheroids in gelatin hydrogels from each culture condition (scale bar = 100 µm). (b) Spheroids in stiff hydrogels cultured with daily compression were smaller than those cultured in the bioreactor without loading or in cell culture plates (*p < 0.001). In soft hydrogels, the spheroid size was larger in hydrogels cultured in culture plates, but did not differ between loaded and unloaded constructs in the bioreactor. Both hydrogel stiffness and loading condition significantly affect spheroid diameter (p < 0.001; two-factor ANOVA).
3.3. Cancer cell expression of proteins associated with proliferation and bone metastasis
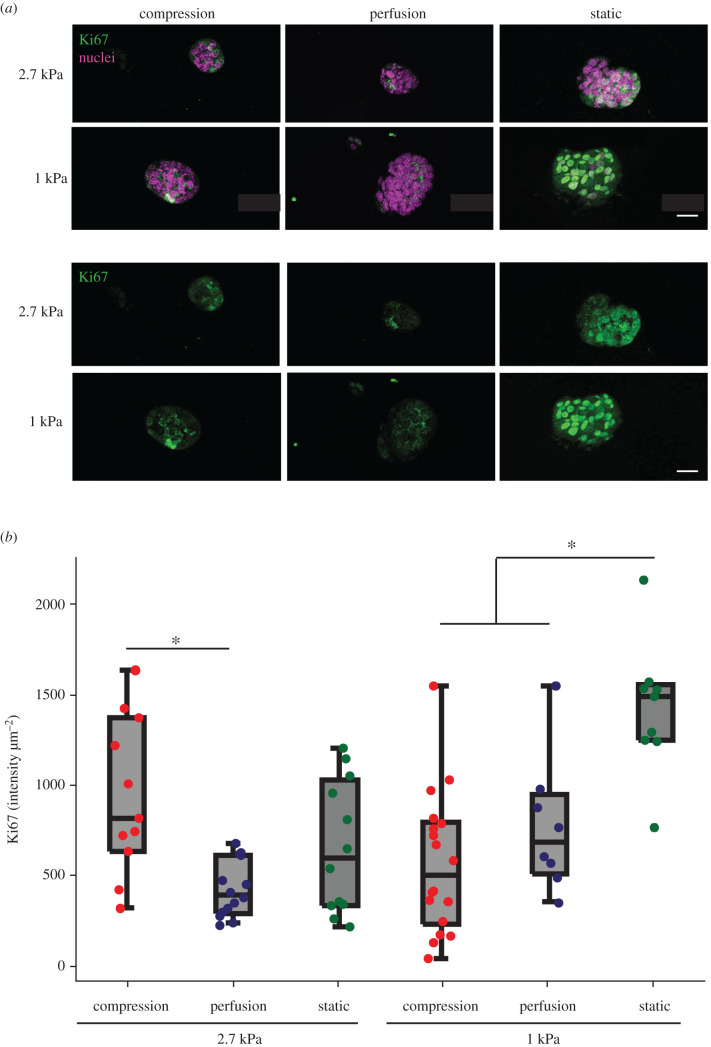
Hydrogel stiffness and mechanical stimulation significantly affected Ki67 expression (two-factor ANOVA; p = 0.01). After 4 days of culture, the fluorescence intensity of immunostaining for the proliferative marker Ki67 was present in all spheroids (figure 5a). Ki67 staining intensity was expressed more prevalently in the statically cultured soft constructs compared to those that were mechanically loaded or perfused (p = 0.002; figure 5b). However, in stiff hydrogels, Ki67 expression was not different between static and compression cases (figure 5b), although stiff compression constructs exhibited more Ki67 than stiff perfusion constructs (p = 0.003; figure 5b).
Figure 5.
(a) Representative images of proliferation marker Ki67 expression. Scale bars are 20 µm. (b) Compressed constructs expressed more Ki67 compared to constructs exposed to perfusion in stiff hydrogels (ANOVA; *p = 0.003), while statically cultured explants exhibited the highest Ki67 expression in soft hydrogels (ANOVA; *p = 0.002). Both hydrogel stiffness and loading condition had significant effects on Ki67 expression (two-factor ANOVA; *p < 0.01).
After 4 days of culture, 4T1 cells from all experimental culture conditions exhibited immunostaining for PTHrP and OPN (figure 6a,b). The fluorescence intensity of those two proteins was qualitatively higher in the 1 kPa stiffness hydrogels when compared to 2.7 kPa gels. There was no difference in fibronectin expression between culture conditions (figure 6c).
Figure 6.
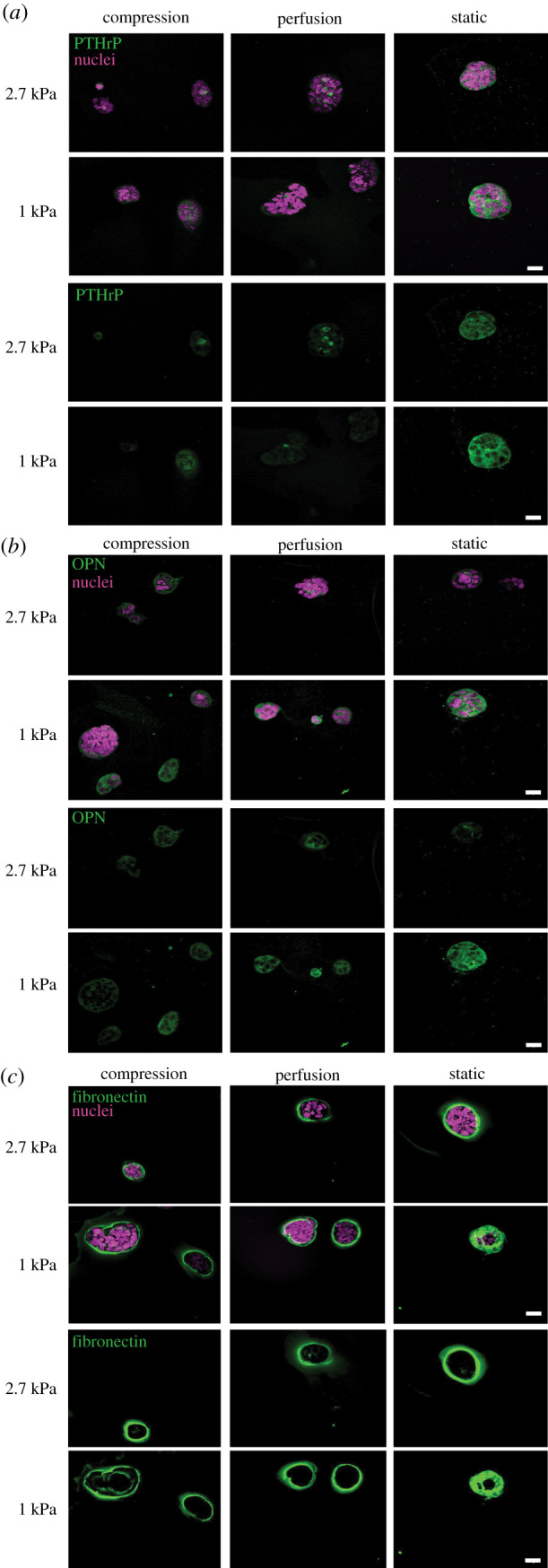
Representative images of 4T1 spheroids stained for PTHrP (a), OPN (b), and fibronectin (c) in stiff (2.7 kPa) and soft (1 kPa) hydrogels. Expression of PTHrP and OPN appears to be higher in 1 kPa gels compared to 2.7 kPa gels, and highest in statically cultured gels compared to those exposed to perfusion or compression. There was no observable difference in fibronectin expression between conditions. Scale bars are 20 µm.
4. Discussion
Bone is primarily a load-bearing tissue, and metastatic cancer cells that home to the bone marrow experience a unique mechanical environment. Mechanical cues play a crucial role in metastatic progression [12,31]. As such, the goal of this study was to quantify the interacting effects of matrix mechanical properties and three-dimensional dynamic mechanical loading on tumour spheroid formation. We found that the mechanobiological effects attenuated spheroid growth in a three-dimensional crosslinked gelatin culture. Media perfusion, which may cause gel consolidation and induce fluid shear stress on the cell membranes, and constraint within the bioreactor resulted in smaller spheroids in comparison to those that formed under static, free-swelling culture. Dynamic mechanical compression of the hydrogels further decreased the spheroid size, but only in stiffer hydrogels. This may be because stiffer hydrogels induce more deformation in the spheroids. Taken together, our results demonstrate that dynamic mechanical stimuli can inhibit tumour spheroid growth, which may provide an insight into the biophysical conditions that enable the development of metastases and inform intervention targets to minimize metastatic tumour growth.
Our study has several notable strengths. We used a novel bioreactor system that allows cell seeded hydrogels to be cultured while subjected to controlled mechanical compression and perfusion simultaneously. We further demonstrated that the 4T1 cells remained viable throughout the culture. Our gelatin cross-linking protocol produced hydrogels with two different stiffness comparable to bone marrow [28], as validated through mechanical testing. Most importantly, this is, to our knowledge, the first study that has investigated the combined effects of substrate stiffness and dynamic mechanical stimulation on tumour spheroid formation in three-dimensional culture. Because mechanical deformation is an inherent feature of the bone and marrow microenvironment, its role in tumour spheroid growth is essential to understanding bone metastasis.
There are several limitations to this study. Firstly, the spheroid size could have been affected by non-homogeneous dispersion of cells in suspension from the outset. However, cell seeding densities were consistent between groups, cell suspensions were mixed thoroughly before and after mixing with gelatin, and similar techniques were used for all conditions and thus should not influence the comparisons between groups. Bright-field micrographs of the constructs showed that cells were evenly dispersed into single cells at day 0, and spheroids were uniformly dispersed at day 4. Secondly, the compressive moduli used in this study were significantly lower than that of bone, which is in the order of 18 GPa [32]. However, metastatic breast cancer cells often home to the bone marrow [4], which has a modulus between 0.3 and 2.4 kPa at physiological temperatures, similar to that of the gelatin constructs used in this study [28]. Similarly, the compressive loading regime employed may not have been representative of cancer cell stimulation when it resides in the bone microenvironment in vivo. Computational models show that trabecular bone experiences between 2000 and 3000 µstrain compression during walking, which induces shear strains in the marrow on the order of 1000 µstrain [33,34]. This is much lower than the 40 000–140 000 µstrain applied to the stiff and soft hydrogels, respectively. Similarly, the applied stress in the constructs was in the order of 100 Pa, while marrow experiences a shear stress of approximately 5 Pa during walking [33,34]. Although the shear stress owing to media perfusion was approximately 80 µPa in this study, an increase in shear stress is expected in compressed samples as fluid leaves the constructs in response to loading. We were able to demonstrate changes in spheroid size with the compression regime applied, providing important information regarding how cancer cells respond to superphysiologic mechanical stimulation. Finally, the dynamic compression loading could have altered our expected behaviour because of viscoelastic effects in the hydrogel. While the slow loading rate we used should have limited these effects, experiments that control viscoelastic response could provide more insight into dynamic loading.
Previous studies have shown that cells cultured in hydrogels of different stiffness migrated toward lower modulus regions. For example, metastatic MDA-MB-231 cells migrated from stiff to soft regions in methacrylated gelatin hydrogels, while non-metastatic MCF7 cells formed clusters [21]. Substrate stiffness has also been shown to affect proliferation, invasion and migration of metastatic cancer cells in two dimensions [17–20,35]. By contrast, the metastatic 4T1 cells used in this three-dimensional study did not migrate, but instead formed spheroids with average diameters between 25 and 50 µm in all culture conditions. This may be owing to cell seeding density. Peela et al. seeded breast cancer cells at a density 60 times higher than in this study, which may have influenced paracrine signalling and cell migration [21]. Additionally, a lack of migration may have been associated with the lack of cancer cell adhesion to the gelatin substrate. Fibronectin, a cellular adhesion marker, was expressed around the exterior of 4T1 spheroids, which is a hallmark of epithelial to mesenchymal transition, which is often associated with cell migration. However, the formation of 4T1 spheroids has also been observed in previous studies using different hydrogel systems [22,36], suggesting that 4T1 cells preferentially form spheroids in three-dimensional culture. Here, we provide an advanced understanding of how the biophysical environment governs the tumour spheroid formation.
In PEGDA gels, 4T1 spheroids had an average diameter of 37.6 µm in 2.5 kPa gels, which agrees with our data [22,36]. Yang et al. demonstrated that 4T1 spheroid formation was biphasic within PEGDA hydrogels with matrix moduli between 2.5 and 69 kPa, with the largest spheroids and highest cancer stem cell CD44 marker expression observed in matrix with a stiffness of 5 kPa [36]. Breast cancer cell spheroid size increased with increasing elastic modulus from 0.17 to 1.20 kPa in collagen hydrogels [20], while spheroid growth was inhibited by higher stiffness agarose gels [37]. In our soft hydrogels, spheroid size was higher in the conditions with greater expression of the proliferation marker Ki67, suggesting that substrate stiffness in addition to mechanical stimulation affected cell proliferation to control spheroid size. However, in the stiff hydrogels, the compressed hydrogels exhibited smaller spheroids relative to the perfused case, but expressed more Ki67. The higher gel stiffness would more efficiently transfer force and deformation to the cells and spheroids. This mechanical signal may have postponed cell proliferation, with higher Ki67 expression at day 4 reflecting a delay in the cell cycle progression in those spheroids.
Qualitatively, PTHrP immunostaining appeared stronger in 1 kPa gels compared to the 2.7 kPa gels. This is contrary to a study which reported an increase in PTHrP expression by metastatic breast cancer cells cultured on increasing substrate stiffness ranging from 1 kPa to 1 GPa [38]. However, their stiff substrates were made with polyurethane films which have moduli on the order of GPa, which is three orders of magnitude higher than the hydrogels in our study. As mechanically transduced signals have been shown to affect PTHrP expression [39], it is likely that the difference in stiffness between our hydrogels was not large enough to substantially alter PTHrP expression by the 4T1 cells. The expression of PTHrP in the spheroids is notable, as it is an essential feature of bone metastases. PTHrP expression is associated with higher metastatic progression owing to increased bone resorption, and may differentially affect the behaviour of cells in the primary tumour. For example, increased PTHrP expression by primary breast tumours correlated to improved survival and reduced development of bone metastasis in patients, suggesting a role for PTHrP in breast cancer cell behaviour independent of its ability to induce bone resorption [40,41]. This is further supported by a mouse mammary carcinoma model where conditional deletion of PTHrP led to higher tumour incidence [42]. These studies indicate that PTHrP-negative breast cancer cells may be more metastatic in nature, and upon reaching the bone marrow, environmental factors may enhance PTHrP production to increase bone resorption and promote metastatic progression [43].
Expression of OPN also qualitatively appeared to be higher in 1 kPa hydrogels compared to 2.7 kPa hydrogels. This is consistent with a previous study that demonstrated increased OPN expression in metastatic breast cancer MDA-MB-231 cells cultured on 1 kPa polyacrylamide hydrogels compared to stiffer substrates [38]. Other studies have associated OPN expression in plasma [44] and MDA-MB-231 cells [45] with increased incidence of bone metastasis, which may indicate a difference in metastatic behaviour between cells cultured in 1 kPa versus 2.7 kPa gels in our study. However, there may be other factors present in the metastatic microenvironment in vivo that need to be considered to draw such a conclusion.
Fibronectin formed a shell around the spheroids in all conditions. Fibronectin has been shown to be a marker of poor prognosis for breast cancer metastasis [46], and extracellular matrix changes including fibronectin are markers of malignancy in cancer [47]. Fibronectin can undergo post-translational modification citrullination during pathological extracellular matrix modification, which affects its binding to the integrin α5β1 and αvβ3 to influence cell migration [48]. It would be interesting to examine citrullination in our system and to assess how mechanical stimulation alters post-translational modification of fibronectin during cancer cell spheroid formation.
This is, to our knowledge, the first study that has investigated the formation and growth of tumour spheroids in three-dimensional culture with simultaneous mechanical loading. The bioreactor culture system provided a unique means of mechanically compressing three-dimensional gelatin hydrogel constructs while supplying media perfusion. This enabled the effects of mechanical stimulation to be investigated simultaneously with hydrogel stiffness on metastatic cancer cell proliferation. Spheroid growth was inhibited in mechanically compressed hydrogels when compared to unloaded hydrogels with perfusion, which were in turn of the same stiffness. At the same time, spheroid sizes were comparable within unstimulated hydrogels of different stiffness. A possible explanation for this is that compression of the stiff hydrogel decreases cell division simply owing to confinement of the spheroid. Additionally, compression may have altered gap junction expression between cancer cells, which has been shown to affect proliferation in pancreatic cancer cells [49]. Mechanical stimulation of metastatic bone tumours has been shown to reduce tumour progression in vivo. For example, compressive loading reduced metastatic breast cancer tumour formation in mouse tibiae in vivo [26], and low-intensity vibration also reduced myeloma tumour burden in mouse femora in vivo [27]. As cancer cells are subjected to mechanical stimuli in vivo [50], it is important to mechanically stimulate cancer cells in a three-dimensional culture system. Although compression has been employed to examine the inflammatory response of human monocytes embedded in three-dimensional agarose [51], we demonstrated, for the first time to our knowledge, the ability to mechanically compress cancer cells in three-dimensional hydrogels with stiffnesses similar to that of bone marrow. The results indicate that mechanical stimuli and the mechanical properties of the microenvironment play key roles in cancer cell spheroid formation and growth. Mechanobiological effects should be considered in studies investigating cancer metastasis to bone.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Funding
This work was supported by the Irish Research Council (IRC) Consolidator grant (MEMETic), and Science Foundation Ireland (SFI) co-funded under the European Regional Development fund under grant no. 14/IA/2884. K.J.C. was supported by the Orthopaedic Research Society Collaborative Exchange Grant, the Harper Cancer Research Institute and Walther Cancer Foundation Interdisciplinary Interface Training Program, and the Advanced Diagnostics & Therapeutics Leiva Graduate Fellowship in Precision Medicine.
Data accessibility
The data are publicly available at doi:10.7274/r0-ng8g-4b75.
Authors' contributions
K.J.C. performed the bioreactor and mechanical characterization experiments, performed statistical analysis and drafted the manuscript; J.S. performed immunohistochemistry and confocal imaging; M.J.M. designed the bioreactor and assisted with culture; V.K. developed gelatin protocols and assisted with gelatin culture; L.M.M. and G.L.N. directed the research; all authors contributed to results interpretation and reviewed the final manuscript.
Competing interests
We declare we have no competing interests.
References
- 1.Barney LE, Jansen LE, Polio SR, Galarza S, Lynch ME, Peyton SR. 2016. The predictive link between matrix and metastasis. Curr. Opin. Chem. Eng. 11, 85–93. ( 10.1016/j.coche.2016.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biswas S, et al. 2011. Anti-transforming growth factor ß antibody treatment rescues bone loss and prevents breast cancer metastasis to bone. PLoS ONE 6, e27090 ( 10.1371/journal.pone.0027090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman RE. 2006. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 12, 6243s–6249s. ( 10.1158/1078-0432.CCR-06-0931) [DOI] [PubMed] [Google Scholar]
- 4.Templeton ZS, et al. 2015. Breast cancer cell colonization of the human bone marrow adipose tissue niche. Neoplasia N. Y. N 17, 849–861. ( 10.1016/j.neo.2015.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eimori K, Endo N, Uchiyama S, Takahashi Y, Kawashima H, Watanabe K. 2016. Disrupted bone metabolism in long-term bedridden patients. PLoS ONE 11, e0156991 ( 10.1371/journal.pone.0156991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritton JC, Myers ER, Wright TM, van der Meulen MCH. 2005. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone 36, 1030–1038. ( 10.1016/j.bone.2005.02.013) [DOI] [PubMed] [Google Scholar]
- 7.Frost HM. 2003. Bone's mechanostat: a 2003 update. Anat. Rec. A. Discov. Mol. Cell. Evol. Biol. 275, 1081–1101. ( 10.1002/ar.a.10119) [DOI] [PubMed] [Google Scholar]
- 8.Robling AG, Turner CH. 2009. Mechanical signaling for bone modeling and remodeling. Crit. Rev. Eukaryot. Gene Expr. 19, 319–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin C, Xu G, Judex S. 2001. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 15, 2225–2229. ( 10.1096/fj.01-0166com) [DOI] [PubMed] [Google Scholar]
- 10.Silva MJ, Brodt MD, Lynch MA, Stephens AL, Wood DJ, Civitelli R. 2012. Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PLoS ONE 7, e34980 ( 10.1371/journal.pone.0034980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff J. 1892. Das Gesetz der transformation der Knochen. Berlin, Germany: Verlag August Hirschwald. [Google Scholar]
- 12.Coughlin T, Moreno RR, Mason D, Nystrom L, Boerckel J, Niebur G, Littlepage LE. 2016. Bone: a fertile soil for cancer metastasis. Curr. Drug Targets 18, 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenner J, Stacer AC, Winterroth F, Johnson TD, Luker KE, Luker GD. 2014. Macroscopic stiffness of breast tumors predicts metastasis. Sci. Rep. 4, 5512 ( 10.1038/srep05512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fidler IJ. 2003. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 3, 453–458. ( 10.1038/nrc1098) [DOI] [PubMed] [Google Scholar]
- 15.Paget S. 1889. The distribution of secondary growths in cancer of the breast. The Lancet 133, 571–573. ( 10.1016/S0140-6736(00)49915-0) [DOI] [PubMed] [Google Scholar]
- 16.Steeg PS. 2006. Tumor metastasis: mechanistic insights and clinical challenges. Nat. Med. 12, 895–904. ( 10.1038/nm1469) [DOI] [PubMed] [Google Scholar]
- 17.Tilghman RW, Cowan CR, Mih JD, Koryakina Y, Gioeli D, Slack-Davis JK, Blackman BR, Tschumperlin DJ, Parsons JT. 2010. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS ONE 5, e12905 ( 10.1371/journal.pone.0012905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umesh V, Rape AD, Ulrich TA, Kumar S. 2014. Microenvironmental stiffness enhances glioma cell proliferation by stimulating epidermal growth factor receptor signaling. PLoS ONE 9, e101771 ( 10.1371/journal.pone.0101771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jerrell RJ, Parekh A. 2014. Cellular traction stresses mediate extracellular matrix degradation by invadopodia. Acta Biomater. 10, 1886–1896. ( 10.1016/j.actbio.2013.12.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paszek MJ, et al. 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254. ( 10.1016/j.ccr.2005.08.010) [DOI] [PubMed] [Google Scholar]
- 21.Peela N, Sam FS, Christenson W, Truong D, Watson AW, Mouneimne G, Ros R, Nikkhah M. 2016. A three dimensional micropatterned tumor model for breast cancer cell migration studies. Biomaterials 81, 72–83. ( 10.1016/j.biomaterials.2015.11.039) [DOI] [PubMed] [Google Scholar]
- 22.Yang X, Sarvestani SK, Moeinzadeh S, He X, Jabbari E. 2013. Effect of CD44 binding peptide conjugated to an engineered inert matrix on maintenance of breast cancer stem cells and tumorsphere formation. PLoS ONE 8, e59147 ( 10.1371/journal.pone.0059147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haessler U, Teo JCM, Foretay D, Renaud P, Swartz MA. 2012. Migration dynamics of breast cancer cells in a tunable 3D interstitial flow chamber. Integr. Biol. Quant. Biosci. Nano Macro 4, 401–409. ( 10.1039/c1ib00128k) [DOI] [PubMed] [Google Scholar]
- 24.Gasparski AN, Ozarkar S, Beningo KA. 2017. Transient mechanical strain promotes the maturation of invadopodia and enhances cancer cell invasion in vitro. J. Cell Sci. 130, 1965–1978. ( 10.1242/jcs.199760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González Á, García de Durango C, Alonso V, Bravo B, Rodríguez de Gortázar A, Wells A, Forteza J, Vidal-Vanaclocha F. 2017. Distinct osteomimetic response of androgen-dependent and independent human prostate cancer cells to mechanical action of fluid flow: prometastatic implications. Prostate 77, 321–333. ( 10.1002/pros.23270) [DOI] [PubMed] [Google Scholar]
- 26.Lynch ME, Brooks D, Mohanan S, Lee MJ, Polamraju P, Dent K, Bonassar LJ, van der Meulen MCH, Fischbach C. 2013. In vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J. Bone Miner. Res. 28, 2357–2367. ( 10.1002/jbmr.1966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagnotti GM, Chan ME, Adler BJ, Shroyer KR, Rubin J, Bain SD, Rubin CT. 2016. Low intensity vibration mitigates tumor progression and protects bone quantity and quality in a murine model of myeloma. Bone 90, 69–79. ( 10.1016/j.bone.2016.05.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansen LE, Birch NP, Schiffman JD, Crosby AJ, Peyton SR. 2015. Mechanics of intact bone marrow. J. Mech. Behav. Biomed. Mater. 50, 299–307. ( 10.1016/j.jmbbm.2015.06.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui X, Hartanto Y, Zhang H. 2017. Advances in multicellular spheroids formation. J. R. Soc. Interface 14, 20160877 ( 10.1098/rsif.2016.0877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verbruggen SW, Mc Garrigle MJ, Haugh MG, Voisin MC, McNamara LM. 2015. Altered mechanical environment of bone cells in an animal model of short- and long-term osteoporosis. Biophys. J. 108, 1587–1598. ( 10.1016/j.bpj.2015.02.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar S, Weaver VM. 2009. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 28, 113–127. ( 10.1007/s10555-008-9173-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayraktar HH, Morgan EF, Niebur GL, Morris GE, Wong EK, Keaveny TM. 2004. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J. Biomech. 37, 27–35. [DOI] [PubMed] [Google Scholar]
- 33.Metzger TA, Kreipke TC, Vaughan TJ, McNamara LM, Niebur GL. 2015. The in situ mechanics of trabecular bone marrow: the potential for mechanobiological response. J. Biomech. Eng. 137, 011006 ( 10.1115/1.4028985) [DOI] [PubMed] [Google Scholar]
- 34.Metzger TA, Schwaner SA, Laneve AJ, Kreipke TC, Niebur GL. 2015. Pressure and shear stress in trabecular bone marrow during whole bone loading. J. Biomech. 48, 3035–3043. ( 10.1016/j.jbiomech.2015.07.028) [DOI] [PubMed] [Google Scholar]
- 35.Sunyer R, Jin AJ, Nossal R, Sackett DL. 2012. Fabrication of hydrogels with steep stiffness gradients for studying cell mechanical response. PLoS ONE 7, e46107 ( 10.1371/journal.pone.0046107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X, Sarvestani SK, Moeinzadeh S, He X, Jabbari E. 2012. Three-dimensional-engineered matrix to study cancer stem cells and tumorsphere formation: effect of matrix modulus. Tissue Eng. Part A 19, 669–684. ( 10.1089/ten.tea.2012.0333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng G, Tse J, Jain RK, Munn LL. 2009. Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS ONE 4, e4632 ( 10.1371/journal.pone.0004632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruppender NS, Merkel AR, Martin TJ, Mundy GR, Sterling JA, Guelcher SA. 2010. Matrix rigidity induces osteolytic gene expression of metastatic breast cancer cells. PLoS ONE 5, e15451 ( 10.1371/journal.pone.0015451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philbrick WM, et al. 1996. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol. Rev. 76, 127–173. ( 10.1152/physrev.1996.76.1.127) [DOI] [PubMed] [Google Scholar]
- 40.Henderson M, Danks J, Moseley J, Slavin J, Harris T, McKinlay M, Hopper J, Martin T. 2001. Parathyroid hormone-related protein production by breast cancers, improved survival, and reduced bone metastases. J. Natl Cancer Inst. 93, 234–237. ( 10.1093/jnci/93.3.234) [DOI] [PubMed] [Google Scholar]
- 41.Henderson MA, Danks JA, Slavin JL, Byrnes GB, Choong PFM, Spillane JB, Hopper JL, Martin TJ. 2006. Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res. 66, 2250–2256. ( 10.1158/0008-5472.CAN-05-2814) [DOI] [PubMed] [Google Scholar]
- 42.Fleming NI, Trivett MK, George J, Slavin JL, Murray WK, Moseley JM, Anderson RL, Thomas DM. 2009. Parathyroid hormone-related protein protects against mammary tumor emergence and is associated with monocyte infiltration in ductal carcinoma in situ. Cancer Res. 69, 7473–7479. ( 10.1158/0008-5472.CAN-09-0194) [DOI] [PubMed] [Google Scholar]
- 43.Sterling JA, Edwards JR, Martin TJ, Mundy GR. 2011. Advances in the biology of bone metastasis: how the skeleton affects tumor behavior. Bone. Skeletal Complications Cancer 48, 6–15. ( 10.1016/j.bone.2010.07.015) [DOI] [PubMed] [Google Scholar]
- 44.Hotte SJ, Winquist EW, Stitt L, Wilson SM, Chambers AF. 2002. Plasma osteopontin: associations with survival and metastasis to bone in men with hormone-refractory prostate carcinoma. Cancer 95, 506–512. ( 10.1002/cncr.10709) [DOI] [PubMed] [Google Scholar]
- 45.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, Guise TA, Massagué J. 2003. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549. ( 10.1016/S1535-6108(03)00132-6) [DOI] [PubMed] [Google Scholar]
- 46.Fernandez-Garcia B, Eiró N, Marín L, González-Reyes S, González LO, Lamelas ML, Vizoso FJ. 2014. Expression and prognostic significance of fibronectin and matrix metalloproteases in breast cancer metastasis. Histopathology 64, 512–522. ( 10.1111/his.12300) [DOI] [PubMed] [Google Scholar]
- 47.Kaplan RN, Rafii S, Lyden D. 2006. Preparing the ‘soil’: the premetastatic niche. Cancer Res. 66, 11 089–11 093. ( 10.1158/0008-5472.CAN-06-2407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stefanelli VL, et al. 2019. Citrullination of fibronectin alters integrin clustering and focal adhesion stability promoting stromal cell invasion. J. Int. Soc. Matrix Biol. 82, 86–104. ( 10.1016/j.matbio.2019.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamiche C, et al. 2012. The gap junction protein Cx43 is involved in the bone-targeted metastatic behaviour of human prostate cancer cells. Clin. Exp. Metastasis 29, 111–122. ( 10.1007/s10585-011-9434-4) [DOI] [PubMed] [Google Scholar]
- 50.Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. 2004. Pathology: cancer cells compress intratumour vessels. Nature 427, 695 ( 10.1038/427695a) [DOI] [PubMed] [Google Scholar]
- 51.Fahy N, Menzel U, Alini M, Stoddart MJ. 2019. Shear and dynamic compression modulates the inflammatory phenotype of human monocytes in vitro. Front. Immunol. 10, 383 ( 10.3389/fimmu.2019.00383) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are publicly available at doi:10.7274/r0-ng8g-4b75.