Abstract
Background/Aim: The association between ejection fraction (EF) and mortality in TTS patients as compared to ACS is limited. This study aims to investigate the association between EF and clinical outcomes in patients with TTS as compared to ACS. Patients and Methods: This study compared in-hospital, and long-term incidence of clinical outcomes for 5 years in patients with TTS and ACS. The study was composed of two groups EF≥35% and EF<35%. Results: The long-term mortality of the EF≥35% for 5 years was significantly higher in TTS patients as compared to ACS (18.1% vs. 7.7%, log-Rank; p<0.01). Irrespective of EF, a non-cardiovascular death was significantly higher in TTS as compared to ACS patients with EF≥35 (6.4% vs. 2.1%; p=0.02) and with EF<35% (21.4% vs. 7.5%; p=0.03). Conclusion: The long-term mortality is significantly higher in TTS as compared to ACS dominated by a non-cardiovascular cause of death at 5-years-follow-up.
Keywords: Takotsubo, ACS, ejection fraction
The Takotsubo Syndrome (TTS) is generally accepted as a malignancy (1,2). The initial appearance of TTS resembles acute coronary syndrome (ACS), where the acute deterioration of the left ventricular (LV) systolic function can lead to severe complications, including congestive heart failure, thromboembolic events, cardiogenic shock and lethal arrhythmias, as well as an in-hospital mortality of 2.0%-8.7% for both conditions (2-7).
A transient reduced LV ejection fraction (EF) is a known phenomenon in patients with TTS. Admittedly, the range of EF has been estimated to be lower in patients with TTS compared to ACS at time of event (2,3). In-hospital and adverse cardiac events during long-term follow-up are higher in TTS patients with EF<35% as compared to patients with EF≥35% (8-12). Data on in-hospital and long-term outcomes comparing EF grade between TTS and ACS patients are limited (3,13,14).
This study sought to compare in-hospital and long-term mortality in patients with TTS versus ACS depending on EF. Moreover, predictors of mortality were evaluated.
Patients and Methods
A total of 648 patients presenting with TTS and ACS were included in the study retrospectively. TTS was defined by the Mayo clinic criteria (15). To evaluate the diagnosis of TTS, the electrocardiograms (ECGs), echocardiograms and angiograms were inspected by two independent experienced cardiologists. Other possible differential diagnoses as valvular disease, myocarditis, coronary spasm or chemotherapy-related cardiac dysfunction were excluded. Data of patients who were suffering from ACS between 2007 and 2008 (non-ST-elevation or ST-elevation myocardial infarction or unstable angina (NSTEMI or STEMI or UA)) in the Clinic for Cardiology, University Hospital Mannheim, and afterwards underwent treatment with percutaneous coronary intervention (PCI) involving a stent implantation, were examined and assessed using the department’s database. The study was composed of two groups. The first group had an EF≥35% and consisted of 94 patients with TTS and 432 patients with ACS (241 NSTEMI, 185 STEMI, 6 UA). The second group (EF<35%) included 42 TTS and 80 ACS patients (40 NSTEMI, 32 STEMI, 2 UA). EF was measured using the Simpson method particularly from apical in TTS and ACS patients (16). Data were used for index EF from initial echocardiography when accessible. Follow-up EF was considered up to 6 months after the index event. Baseline characteristics were assessed using a chart review. Mitral regurgitation (MR) and tricuspid regurgitation (TR) were measured using echocardiography (17). In-hospital events: i) atrial and ventricular arrhythmias, ii) cardiac rupture, iii) thromboembolic events, iv) pulmonary congestion with use of non-invasive positive-pressure ventilation, v) intubation, vi) use of a temporary pacemaker, vii) of inotropic agents, and viii) death, as well as long-term events: i) stroke, ii) life-threatening arrhythmia as asystole or ventricular arrhythmia (VT), iii) heart failure, iv) recurrence, v) thromboembolic events, and vi) mortality, were documented. Clinical outcomes, including adverse events, were observed during follow-up. Follow-up was conducted at 30 days, and once annually for 5 years. This study was organized in compliance with the announcement of Helsinki. The study protocol was recently approved by the Ethics Committee of the University Medical Centre Mannheim.
Statistical analysis. Data of continuous variables are presented as mean±standard deviation with a normal distribution, median (interquartile range IQR) with a non-normal distribution, while categorial variables are reported as frequencies and percentages (%). The Kolmogorov-Smirnov-Test was used to test normal distribution. To compare normal or non-normal distributions of continuous variables, we used Mann-Whitney-U-test and Student’s t-test, respectively. The exact Fischer test or chi-squared-test was used for distribution analysis of categorial variables. We applied two-tailed Fisher’s exact test in tests with sample size of n=5 or below. The relative risk for appearance of events was calculated using Fisher’s Exact Ratio test. Results are presented with 95% confidence intervals (CI). We estimated the differences in both groups using Kaplan Meier and applied Log-Rank statistics. Independent predictors of increased mortality were identified by a univariate analysis. Predictors with p<0.10 were analyzed using the Cox multivariate regression. Results were characterized as hazard ratios (HRs) with 95%CI. Statistical analysis was performed using IBM SPSS Statistics version 23.0. Values of p<0.05 were recognized as statistically significant.
Results
Patients with EF≥35%. Four hundred and thirty-two consecutive patients were included in this study with ACS and 94 consecutive patients with TTS. As expected, more women than men were affected significantly by TTS (86.2% vs. 35.0%, p<0.01. The heart rate was significantly higher in TTS patients 98±26 beats/min (bpm) versus 91±26 bpm compared to ACS patients (p<0.01). Furthermore, inverted T-waves were significantly more often present in TTS patients compared to ACS patients (91.0% vs. 47.0%, p<0.01), similar to corrected QT Interval being longer in TTS as compared to ACS patients (478 ms vs. 444 ms, p<0.01). LVEF was lower in TTS as compared to ACS at index event (44±6% vs. 54±9%, p<0.01). Nonetheless, follow-up LVEF was similar between TTS and ACS patients (55±8% vs. 54±9%, p=0.22). Interestingly, mitral and tricuspid regurgitation were more often observed in TTS patients as compared to ACS patients (50.0% vs. 28.9%, p<0.01, and 37.2% vs. 13.7%, p<0.01, respectively). Drugs on admission and discharge are listed in (Table I).
Table I. Baseline characteristics of 94 Takotsubo Syndrome (TTS) patients and 432 acute coronary syndrome (ACS) patients with EF≥35%.
*p-Values for the comparison between TTS and ACS. Significant p-Values are shown in bold. TTS: Takotsubo syndrome; ACS: acute coronary syndrome; SD: standard deviation; ECG: electrocardiogram; IQR: interquartile range; BP: blood pressure; LV: left ventricular; EF: ejection fraction; BMI: body-mass-index; COPD: chronic obstructive pulmonary disease; ACE: angiotensin-converting-enzyme; ASA: acetylsalicylic acid.
Life-threatening arrhythmias (LTAs), inotropic agents, resuscitation, in-hospital death and cardiogenic shock were demonstrated equivalent between TTS and ACS patients with EF≥35%. However, the necessity of noninvasive positive pressure ventilation (NPPV) or mechanical ventilation was significantly higher in TTS as compared to ACS patients (51.1% vs. 5.8%, p<0.01) (Table II).
Table II. Baseline characteristics of 42 Takotsubo Syndrome (TTS) patients and 80 acute coronary syndrome (ACS) patients with EF<35%.
*p-Values for the comparison between female TTS and female ACS. Significant p-Values are shown in bold. TTS: Takotsubo syndrome; ACS: acute coronary syndrome; SD: standard deviation; ECG: electrocardiogram; IQR: interquartile range; BP: blood pressure; LV: left ventricular; EF: ejection fraction; BMI: body-mass-index; COPD: chronic obstructive pulmonary disease; ACE: angiotensin-converting-enzyme; ASA: acetylsalicylic acid
Patients with EF<35%. Eighty consecutive patients were included in this study with ACS and 42 consecutive patients with TTS. EF was presented higher in TTS as compared to ACS patients per event (27±6% vs. 23±6%, p<0.01). The observations were sustained over 5-year-follow-up with significantly more increased EF in TTS compared to ACS (42±15 vs. 23±6, p<0.01). However, tricuspid regurgitation was more documented in TTS patients as compared to ACS patients (45.2% vs. 12.5%, p<0.01). Drugs on admission were similar between ACS and TTS patients. However, TTS patients were numerically more strongly medicated with beta-blockers at discharge as compared to ACS patients (71.4% vs. 56.3%, p=0.10) (Table III). Additionally, TTS patients were significantly more often treated with therapeutic anticoagulants at discharge compared to ACS patients (31.0% vs. 10.0%, p<0.01).
Table III. In-hospital events and treatment strategy in Takotsubo Syndrome and acute coronary syndrome (ACS patients) with EF≥35% and EF<35.
*p-Values for the comparison between TTS and ACS. Significant p-Values are shown in bold. NPPV: Non-invasive positive pressure ventilation; ICU: intermediate care unit; IQR: interquartile range; EF: ejection fraction
In patients with EF<35%, the rates of in-hospital events and treatment strategy were identical in comparison to patients with EF ≥35% (Table II). Admittedly, the rate of resuscitation (32.5% vs. 11.9%, p=0.01) and device-implantation (28.7% vs. 7.1%, p<0.01) as well as in-hospital deaths (36.3% vs. 11.9%, p<0.01) were significantly higher in ACS as compared to TTS patients, respectively (p<0.01).
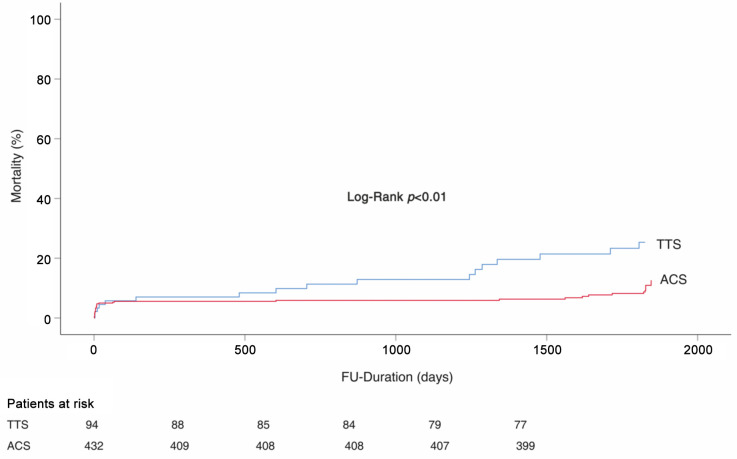
Short- and long-term mortality. The in-hospital mortality rates were comparable between TTS and ACS patients with EF≥35% (Table IV). However, the long-term mortality was significantly more declined in TTS patients as compared to ACS patients log-rank; p<0.01 (Figure 1). This difference was dominated by non-cardiovascular death (6.4% vs. 2.1%, p=0.02). These were chronic obstructive pulmonary dieses (COPD), anal cancer, lung cancer, and acute kidney injury.
Table IV. Outcome in 94 Takotsubo Syndrome (TTS) patients and 432 acute coronary syndrome (ACS) patients with EF≥35.
*p-Values for the comparison between TTS and ACS patients. Significant p-Values are shown in bold. **Cardiovascular cause of death: cardiac decompensation, cardiac shock, asystole, sudden cardiac arrest, acute myocardial infarction, ventricular fibrillation, myocardial perforation. ***Noncardiovascular cause of death: COPD, lung cancer, acute kidney injury, metastatic anal cancer or unknown. EF: Ejection fraction
Figure 1. Kaplan-Meier survival analysis in patients with EF≥35%; TTS: Takotsubo syndrome; ACS: acute coronary syndrome; EF: ejection fraction; FU: follow-up.
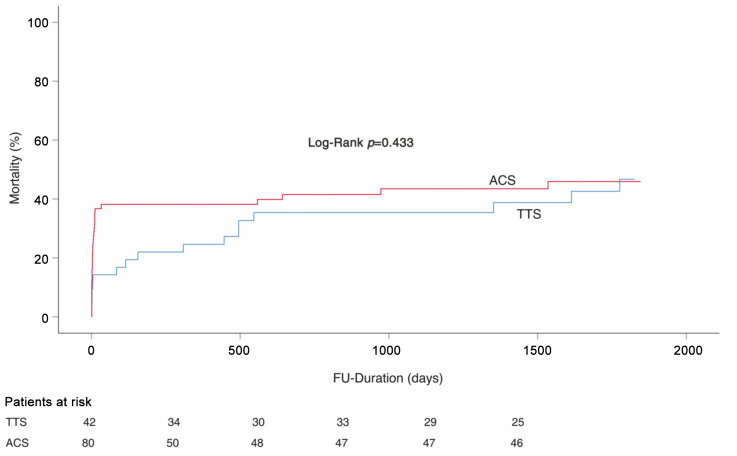
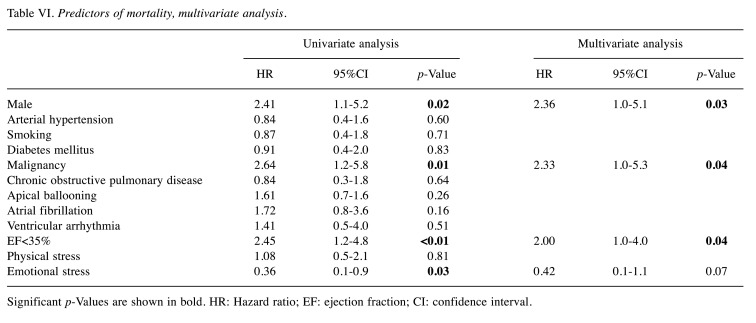
In patients with EF<35%, in-hospital mortality was significantly higher in ACS patients compared to TTS patients (36.3% vs. 11.9%, p<0.01), (Table V). However, the long-term mortality rate was similar in both groups long-rank; p=0.83, (Figure 2). In Cox univariate analysis, male gender (HR=2.41, 95%CI=1.1-5.2; p=0.02), malignancy (HR=2.64, 95%CI=1.2-5.8; p=0.01), EF<35% (HR=2.45, 95%CI=1.2-4.8; p<0.01), and emotional stress (HR=0.36, 95%CI=0.1-0.9; p=0.03) were associated with a high rate of mortality. In multivariate Cox regression analysis, male gender (HR=2.36, 95%CI=1.0-5.1, p=0.03), malignancy (HR=2.33, 95%Cl=1.0-5.3%, p=0.04) and EF<35% (HR=2.00, 95%CI=1.0-4.0, p=0.04) were determined as independent predictors of mortality (Table VI).
Table V. Outcome in 42 Takotsubo Syndrome (TTS) patients and 80 acute coronary syndrome (ACS) patients with EF<35%.
*p-Values for the comparison between TTS and ACS patients. Significant p-Values are shown in bold. **Cardiovascular cause of death: cardiac decompensation, cardiac shock, asystole, sudden cardiac arrest, acute myocardial infarction, ventricular fibrillation, myocardial perforation. ***Noncardiovascular of death: COPD, respiratory failure, lung cancer, acute kidney injury, breast cancer, hematological cancer, blast crisis, subarachnoid hemorrhage, hypoxic brain damage
Figure 2. Kaplan-Meier survival analysis in patients with EF<35%; TTS: Takotsubo syndrome; ACS: acute coronary syndrome; EF: ejection fraction; FU: follow-up.
Table VI. Predictors of mortality, multivariate analysis.
Significant p-Values are shown in bold. HR: Hazard ratio; EF: ejection fraction; CI: confidence interval
Discussion
This study analyzed and compared baseline characteristics and clinical outcomes involving the in-hospital and long-term mortality of 5 years depending on EF between TTS and ACS patients. We revealed that: i) the short-term mortality was more declined in ACS patients as compared to TTS patients with EF<35, dominated by a cardiovascular cause of death with a similar long-term outcome, and ii) whereas the short-term mortality rate in patients with EF≥35% was similar in both groups, the long-term mortality was significantly higher in TTS as compared to ACS patients, dominated by a non-cardiovascular cause of death.
The rate of in-hospital mortality in TTS patients with EF≥35% was 5.3% compared to 4.7% in the ACS group. Recently published data showed that an in-hospital mortality rate in TTS is comparable to ACS (2); however, these data were not related and divided according to EF. In addition, the rate of respiratory failure and consecutive need of respiratory support was significantly higher in TTS compared to ACS patients with EF≥35% (51.1% vs. 5.8%). Requiring respiratory support was more observed as compared to other published data in patients with TTS (18,19). Of note, our clinical outcome data showed that a long-term mortality rate in TTS patients with EF≥35% as compared to ACS patients was significantly higher (18.1% vs. 7.7%). Most patients died due to noncardiac reasons, such as progressive cancer or chronic obstructive pulmonary diseases (COPD). It is, therefore, considered that some patients may require respiratory support due to COPD and not because of presentation of TTS. Looi et al. have described worse long-term mortality in ACS patients as compared to TTS (19). On the other hand, Stiermaier et al. have reported more worse clinical outcomes in TTS patients as compared to ACS patients (20).
In addition, a malignancy rate was described to be high and may be affected the non-cardiovascular mortality in TTS patients. This finding was presented in some clinical trials and may be responsible for increasing the mortality rate in TTS patients as well as the presentation of leukemia, lymphoma, solid cancer or metastatic solid cancer, which certainly does not improve the prognosis (21-25). TTS patients might require cardiac rehabilitation to improve the poor prognosis, particularly those with EF≥35%. It has been observed that cardiac rehabilitation may help with these patients’ outcome, using exercise and weight reduction (26).
Regardless of EF, the rate of thromboembolic in-hospital events and during follow-up time is higher in TTS as compared to ACS patients. Templin et al. have reported a high risk for thromboembolic events as ventricular thrombi and stroke or transient ischemic attacks in TTS patients (2). The slow blood flow in the LV may be one of the reasons for the development of ventricular thrombi in TTS patients. In the course of TTS, EF increased, and the wall motion abnormalities disappeared completely. Presented thrombi could leave left chamber into the peripheral arteries which can increase the risk of an embolic event and stroke. Other explanations for thromboembolic events in TTS patients with a moderated EF≥35% might point towards malignancy. Our data present a higher cancer rate in TTS patients with EF≥35% as compared to ACS patients. Several other studies have shown an association between TTS and cancer (21,23,27). A high rate of breast cancer has also been observed in TTS patients following gastrointestinal and gynecological malignancies (28).
In-hospital mortality in TTS patients with EF<35% was observed in 11.9%. Previously published data on TTS have shown that a reduced EF<35% is more associated with a poor outcome as compared to EF>35%. Our data showed a higher rate than the reported rate in the literature (1-4.5%) (2,14,29,30). This rate is significantly lower as compared to ACS patients with EF<35% (36.3%). Consequently, a cardiopulmonary resuscitation rate was significantly higher in ACS patients as compared to TTS patients with EF<35% (11.9% vs. 32.5%). This might be related to the higher rate of ventricular arrhythmias in ACS patients as compared to TTS patients (16.7% vs. 28.7%). Citro et al. have reported a higher incidence of cardiogenic shock, intra-aortic balloon pump use, and acute heart failure in TTS patients with EF<35% as compared to EF>35% (8). Our data present a similar cardiogenic shock rate in TTS in comparison to ACS patients with EF<35%.
There are some limitations to this study. This study is monocentric and the number of patients with TTS was relatively small compared to other studies. A bias due to unknown or unmeasured cofounders cannot be excluded due to the retrospective character of the study. However, EF measurement using the Simpson method is not always measurable, especially in patients with obesity, COPD or under ICU management. Finally, in some patients, data are missing about cause of death.
Despite these limitations, our data show that male gender, malignancy and EF<35% can be estimated as independent predictors of the mortality in TTS patients. A reduced EF has been proposed by numerous studies as an independent predictor of an in-hospital complication in TTS patients and ACS patients (8-10). Additional independent predictors of in-hospital complications in TTS have been previously noticed, such as an existence of physical stressor, old age, male as gender, elevated troponin level and acute neurologic or psychiatric illness (2,8,31).
Considering these data, it might be recommended to order a frequent in-hospital follow-up and long-term follow-up in TTS patients with an initial EF<35% and EF≥35%. TTS may present a catch-up phenomenon.
In conclusion, an in-hospital mortality rate in patients with EF<35% is significantly lower in TTS compared to ACS patients dominated by cardiovascular deaths, but the long-term outcome is comparable in both groups during a 5-year follow-up. Even though the in-hospital mortality of TTS and ACS with EF≥35% is similar, the long-term mortality rate is significantly higher in TTS compared to ACS patients with a major impact on a non-cardiovascular cause of death at 5-years of follow-up. Respectively, TTS patients with EF≥35% require longer follow-up and post-rehabilitation support compared to ACS patients to reduce the long-time mortality.
Conflicts of Interest
The Authors report no conflicts of interest.
Authors’ Contributions
Data were gathered by MA, IE, TG and MK. MA analyzed all data. XZ and SL supported the descriptive statistics. Echocardiographic assessment was performed by IE and MB. IA and MB performed coronary angiography. IA and IE designed the study and approved the statistical analysis. MA and IA prepared the manuscript. All Authors approved the final version.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Sheppard MN. Takotsubo syndrome - stress-induced heart failure syndrome. Eur Cardiol. 2015;10(2):83–88. doi: 10.15420/ecr.2015.10.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KE, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 3.Citro R, Rigo F, D’Andrea A, Ciampi Q, Parodi G, Provenza G, Piccolo R, Mirra M, Zito C, Giudice R, Patella MM, Antonini-Canterin F, Bossone E, Piscione F, Salerno-Uriarte J, Tako-Tsubo Italian Network Investigators Echocardiographic correlates of acute heart failure, cardiogenic shock, and in-hospital mortality in tako-tsubo cardiomyopathy. JACC Cardiovasc Imaging. 2014;7(2):119–129. doi: 10.1016/j.jcmg.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Schneider B, Athanasiadis A, Schwab J, Pistner W, Gottwald U, Schoeller R, Toepel W, Winter KD, Stellbrink C, Muller-Honold T, Wegner C, Sechtem U. Complications in the clinical course of tako-tsubo cardiomyopathy. Int J Cardiol. 2014;176(1):199–205. doi: 10.1016/j.ijcard.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Isogai T, Yasunaga H, Matsui H, Tanaka H, Ueda T, Horiguchi H, Fushimi K. Out-of-hospital versus in-hospital takotsubo cardiomyopathy: Analysis of 3719 patients in the diagnosis procedure combination database in japan. Int J Cardiol. 2014;176(2):413–417. doi: 10.1016/j.ijcard.2014.07.110. [DOI] [PubMed] [Google Scholar]
- 6.Singh K, Carson K, Shah R, Sawhney G, Singh B, Parsaik A, Gilutz H, Usmani Z, Horowitz J. Meta-analysis of clinical correlates of acute mortality in takotsubo cardiomyopathy. Am J Cardiol. 2014;113(8):1420–1428. doi: 10.1016/j.amjcard.2014.01.419. [DOI] [PubMed] [Google Scholar]
- 7.El-Battrawy I, Borggrefe M, Akin I. Takotsubo syndrome and embolic events. Heart Fail Clin. 2016;12(4):543–550. doi: 10.1016/j.hfc.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Citro R, Radano I, Parodi G, Di Vece D, Zito C, Novo G, Provenza G, Bellino M, Prota C, Silverio A, Antonini-Canterin F, Rigo F, Vriz O, Galasso G, Bossone E, Salerno-Uriarte J, Piscione F. Long-term outcome in patients with takotsubo syndrome presenting with severely reduced left ventricular ejection fraction. Eur J Heart Fail. 2019 doi: 10.1002/ejhf.1373. [DOI] [PubMed] [Google Scholar]
- 9.El-Battrawy I, Ansari U, Lang S, Behnes M, Schramm K, Fastner C, Zhou X, Kuschyk J, Tulumen E, Roger S, Borggrefe M, Akin I. Impact and management of left ventricular function on the prognosis of takotsubo syndrome. Eur J Clin Invest. 2017;47(7):477–485. doi: 10.1111/eci.12768. [DOI] [PubMed] [Google Scholar]
- 10.Perelshtein Brezinov O, Klempfner R, Zekry SB, Goldenberg I, Kuperstein R. Prognostic value of ejection fraction in patients admitted with acute coronary syndrome: A real world study. Medicine (Baltimore) 2017;96(9):e6226. doi: 10.1097/MD.0000000000006226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eyuboglu M, Akdeniz B. Left ventricular ejection fraction in the prognosis of acute coronary syndromes. Int J Cardiol. 2017;234:137. doi: 10.1016/j.ijcard.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 12.Dopheide JF, Knopf P, Zeller GC, Vosseler M, Abegunewardene N, Munzel T, Espinola-Klein C. Low il-10/tnfalpha ratio in patients with coronary artery disease and reduced left ventricular ejection fraction with a poor prognosis after 10 years. Inflammation. 2015;38(2):911–922. doi: 10.1007/s10753-014-0053-5. [DOI] [PubMed] [Google Scholar]
- 13.Previtali M, Repetto A, Panigada S, Camporotondo R, Tavazzi L. Left ventricular apical ballooning syndrome: Prevalence, clinical characteristics and pathogenetic mechanisms in a european population. Int J Cardiol. 2009;134(1):91–96. doi: 10.1016/j.ijcard.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 14.Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, Haas TS, Hodges JS, Maron BJ. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55(4):333–341. doi: 10.1016/j.jacc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 15.Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, YH S, Migliore F, Horowitz JD, Shimokawa H, Luscher TF, Templin C. International expert consensus document on takotsubo syndrome (part I): Clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032–2046. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otterstad JE, Froeland G, St John Sutton M, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997;18(3):507–513. doi: 10.1093/oxfordjournals.eurheartj.a015273. [DOI] [PubMed] [Google Scholar]
- 17.Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL, Scientific Document Committee of the European Association of Cardiovascular Imaging Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the european association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2013;14(7):611–644. doi: 10.1093/ehjci/jet105. [DOI] [PubMed] [Google Scholar]
- 18.El-Battrawy I, Gietzen T, Ansari U, Behnes M, Lang S, Zhou X, Borggrefe M, Akin I. Short-term and long-term incidence of stroke in takotsubo syndrome. ESC Heart Fail. 2018;5(6):1191–1194. doi: 10.1002/ehf2.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looi JL, Lee M, Webster MWI, To ACY, Kerr AJ. Postdischarge outcome after takotsubo syndrome compared with patients post-acs and those without prior cvd: Anzacs-qi 19. Open Heart. 2018;5(2):e000918. doi: 10.1136/openhrt-2018-000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stiermaier T, Moeller C, Oehler K, Desch S, Graf T, Eitel C, Vonthein R, Schuler G, Thiele H, Eitel I. Long-term excess mortality in takotsubo cardiomyopathy: Predictors, causes and clinical consequences. Eur J Heart Fail. 2016;18(6):650–656. doi: 10.1002/ejhf.494. [DOI] [PubMed] [Google Scholar]
- 21.Girardey M, Jesel L, Campia U, Messas N, Hess S, Imperiale A, Blondet C, Trinh A, Ohlmann P, Morel O. Impact of malignancies in the early and late time course of takotsubo cardiomyopathy. Circ J. 2016;80(10):2192–2198. doi: 10.1253/circj.CJ-16-0388. [DOI] [PubMed] [Google Scholar]
- 22.Zaghlol R, Kashyap K, Al-Shbool G, Basyal B, Desale S, Campia U, Barac A. Usefulness of malignancy as a predictor of worsein-hospital outcomes in patients with takotsubo cardiomyopathy. Am J Cardiol. 2019;123(6):995–1001. doi: 10.1016/j.amjcard.2018.11.054. [DOI] [PubMed] [Google Scholar]
- 23.Burgdorf C, Kurowski V, Bonnemeier H, Schunkert H, Radke PW. Long-term prognosis of the transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): Focus on malignancies. Eur J Heart Fail. 2008;10(10):1015–1019. doi: 10.1016/j.ejheart.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Sattler K, El-Battrawy I, Lang S, Zhou X, Schramm K, Tulumen E, Kronbach F, Roger S, Behnes M, Kuschyk J, Borggrefe M, Akin I. Prevalence of cancer in takotsubo cardiomyopathy: Short and long-term outcome. Int J Cardiol. 2017;238:159–165. doi: 10.1016/j.ijcard.2017.02.093. [DOI] [PubMed] [Google Scholar]
- 25.Joy PS, Guddati AK, Shapira I. Outcomes of takotsubo cardiomyopathy in hospitalized cancer patients. J Cancer Res Clin Oncol. 2018;144(8):1539–1545. doi: 10.1007/s00432-018-2661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu CM, McKeon J, Abbott JD, Jiang L, Wu WC. Referral to cardiac rehabilitation and outcomes for patients with takotsubo cardiomyopathy. J Cardiopulm Rehabil Prev. 2019;39(3):E8–E11. doi: 10.1097/HCR.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 27.Parkkonen O, Mustonen P, Puurunen M, Valkonen K, Nieminen M, Sinisalo J. Coagulation changes in takotsubo cardiomyopathy support acute phase reaction and catecholamine excess, but not thrombus production. Int J Cardiol. 2014;177(3):1063–1065. doi: 10.1016/j.ijcard.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Kim H, Senecal C, Lewis B, Prasad A, Rajiv G, Lerman LO, Lerman A. Natural history and predictors of mortality of patients with takotsubo syndrome. Int J Cardiol. 2018;267:22–27. doi: 10.1016/j.ijcard.2018.04.139. [DOI] [PubMed] [Google Scholar]
- 29.Kow K, Watson TJ, Foo D, Ho HH. Clinical characteristics and outcomes of south-east asian patients with takotsubo (stress-induced) cardiomyopathy. Int J Cardiol Heart Vasc. 2018;21:29–31. doi: 10.1016/j.ijcha.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ, Ruschitzka F, Filippatos G, Mebazaa A, Omerovic E. Current state of knowledge on takotsubo syndrome: A position statement from the taskforce on takotsubo syndrome of the heart failure association of the european society of cardiology. Eur J Heart Fail. 2016;18(1):8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 31.Madhavan M, Rihal CS, Lerman A, Prasad A. Acute heart failure in apical ballooning syndrome (takotsubo/stress cardiomyopathy): Clinical correlates and mayo clinic risk score. J Am Coll Cardiol. 2011;57(12):1400–1401. doi: 10.1016/j.jacc.2010.10.038. [DOI] [PubMed] [Google Scholar]