Abstract
Background/Aim: Cocaine is a widely used recreational drug and is known for its nasal complications including epithelial, cartilage and bone damage. The aim of the study was to analyze the impact of cocaine on ciliary beat frequency (CBF) of human nasal epithelial cells and therefore better understand its side effects on nasal mucosa. Materials and Methods: Nasal epithelial cells of 21 healthy subjects were harvested and exposed in vitro to cocaine hydrochloride solutions ranging from 0.875% to 7%. High-speed video footage was acquired with phase contrast microscopy and CBF was analyzed with Sissons-Ammons Video Analysis (SAVA) software. Results: All tested concentrations led to a significant reduction in CBF compared to the control. Effects increased over time and with concentration. A mechanical inhibition of cilia by cocaine crystals was also observed. Conclusion: We assume that CBF reduction is part of the pathomechanism leading to nasal complications in cocaine abuse. Considering these results, clinical usage of cocaine should be critically evaluated and restricted to select cases only.
Keywords: Cocaine, ciliary beat frequency (CBF), human nasal epithelial cells
The leaves of the Erythroxylum coca plant have been chewed by the indigenous people of South America long before the first isolation of cocaine in 1855 by Friedrich Gaedcke was successful (1). By using it, the native South Americans could work longer and harder on the fields due to its stimulating effects and endure hunger for longer periods of time. In 1884, Carl Koller introduced cocaine as a local anesthetic in ophthalmology (2). At present, more potent anesthetic derivatives like lidocaine mostly replaced cocaine in the medical field (3). Various commercial products containing cocaine like beverages and cigarettes have been sold until the early 20th century. Due to its addictive nature and long-term side-effects, cocaine became outlawed for public use in most countries. Still, it is predominantly used as a recreational drug until today.
The most common form is its water-soluble hydrochloride salt which can be swallowed, injected or sniffed. The effect of cocaine is based on the fact that 35-37% of applied cocaine is absorbed systemically by the nasal mucosa (4,5). In 2017, approximately 18 million people world-wide consumed an average amount of 28,6 g of cocaine per year. Global cocaine use is still increasing and is centered in North America and Central and Western Europe (6).
Cocaine causes a local anesthesia by blocking sodium-ion channels in peripheral nerves and leads to euphoria and psychological addiction by blocking the reuptake of dopamin, serotonin and noradrenaline in the central nervous system (CNS) (7,8). Its sympathomimetic effects cause an elevated blood pressure and can promote arrhythmia and sudden cardiac death (9). In approximately 5% of cocaine users, the nasal application leads to cocaine induced midline destructive lesions (CIMDL) including hyposmia, crusting, ulcers, nasal septal perforation and bone erosion of the palate, skull base, orbita and paranasal sinuses (10).
Different local and systemic factors are attributed to these complications: Locally, vasoconstriction, as well as mechanical and chemical trauma play their role by promoting bacterial infection and necrosis. Other systemic factors like osteoblast inhibition, immunosuppression and auto-antibody formation are suspected (8,11,12). By influencing ciliary function, local harmful factors could be potentiated.
Mucociliary clearance (MC) ensures that the airways stay free of inhaled particles and detritus. A synchronous and fast ciliary beat, as well as the right amount and consistency of mucous is essential for a functioning MC (13-15). Disruption of ciliary function like in primary ciliary dyskinesia or change in mucous consistency like in cystic fibrosis lead to impaired MC and are associated with chronic, recurrent and more severe upper airway infections (16,17). There are many factors influencing CBF. CBF naturally is reduced during sleep and with increasing age (18). Long-term alcohol and nicotine abuse lead to CBF reduction (19,20).
Local defensins secreted by the mucosa provide an additional defense mechanism against pathogens. Prerequisite for sufficient defensin function is a specific environment provided by the mucous. Acidity leads to dysfunction (21). Cocaine hydrochloride (CHCL) has a pH of 3.44-5.37 (22).
There are only a few studies on the influence of cocaine on CBF. Photoelectrical CBF-analysis has shown CBF reduction for cocaine and other local anesthetics (23-25). Up to date, there is no study directly visualizing the effect of cocaine on the ciliary beat of nasal epithelial cells, which is the aim of this study.
Materials and Methods
The study was carried out in the Department of Otorhinolaryngology, Head and Neck surgery at the University Medical Centre Mannheim, Germany after approval was given by the ethics committee of the Medical Faculty Mannheim, University of Heidelberg (reference number: 2010-267n-MA). 21 subjects (11 female, 10 male) with an average age of 31.5 years ranging from 19 to 51 years have been included in the study. Subjects with rhinosinusitis, using topical nasal drugs and smokers were excluded. Written informed consent was obtained from all participants.
Samples of the nasal mucosa were harvested by brushing the middle nasal meatus with a cytology brush (Gynobrush Plus, Heinz Herenz, Germany).
Before harvesting the samples, the subjects were asked to blow their nose and anterior rhinoscopy was performed. Subjects with residual mucous and any signs of rhinitis where ruled out. After that, the brush was moistened with 0.9% saline solution and inserted in the wider middle nasal meatus where the epithelium was scraped by rotating and moving the brush back and forth. Immediately after brushing, the harvested cells were transferred into 5 ml of RPMI medium (RPMI 1640, cell culture tested, standard, L-glutamine: 300 mg/l, PromoCell, Heidelberg, Germany) and stored at room temperature.
Tests were performed 3-9 h after obtaining the cells, utilizing the plateau phase of the ciliary beat frequency in RPMI medium (26).
Solutions where created to achieve test concentrations of 7%, 3.5%, 1.75% and 0.875% in the test medium by dissolving CHCL in RPMI medium. Unaltered RPMI medium was used as control (0%).
The medium containing the harvested cells was split into five Petri dishes and put under a phase contrast microscope (Leica Microsystems GmbH, Wetzlar, Germany) with 400-fold magnification.
After ensuring that each dish contained vital cell clusters with beating nasal cilia, the test medium was added. Immediately afterwards, a cell cluster was focused and videos were recorded every 60 s for 15 min using a high-speed video camera and the Sissons-Ammons Video Analysis (SAVA) software (19). Each sequence had a length of 2s with a frame rate of 100/s. The starting point t=0 was set at the beginning of the first video. Only intact cell clusters were selected, due to the fact that disrupted ciliated epithelial edges show significantly lower CBF (27).
Since the cell clusters were moved by the added media, it could take up to 30 s until video recording could be started. Because of this shift, baseline measurements before adding the test media were not comparable and a negative control was used instead. Using SAVA on the acquired videos, CBF was determined by selecting rectangular regions of interests (ROIs) containing ciliated cells. For each cell cluster, three different ROIs where specified and tracked over time.
Results
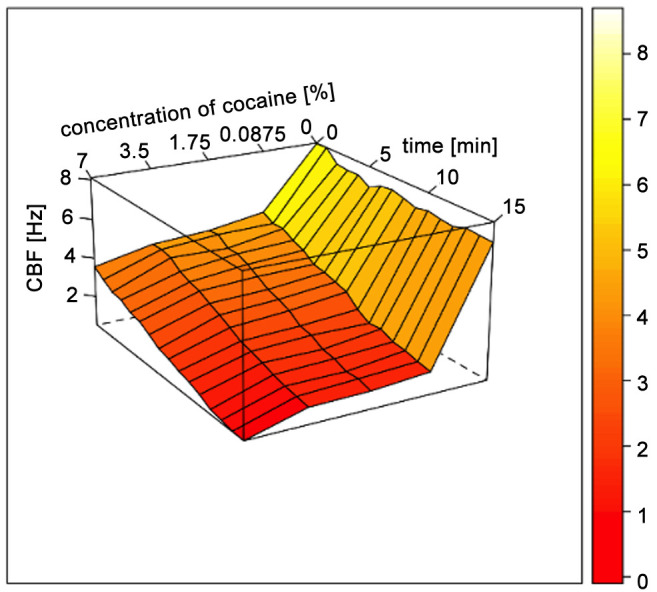
After analyzing all samples (n=21), an instant inhibiting effect of CHCL on CBF could be shown. The effect increased with time and slightly with concentration. After 1 min, median CBF in 0.875% CHCL/RPMI solution was significantly lower than in RPMI only (4.52±1.17 Hz vs. 8.09±1.85 Hz; p=0.001). Further reduction of CBF over time was observed with an endpoint of 1.65±1.17 Hz at t=15 min. CBF reduction at 5, 10 and 15 min was significantly lower compared to CBF in RPMI only (p=0.001). Similar observations were made for 1.75%, 3.5% and 7% CHCL/RPMI solutions, showing a slight increase of overall CBF reduction with increasing concentration of CHCL. Crystal formation was observed in all test solutions, increasing with time and concentration, leading to a mechanical obstruction of the cilia (Figure 1). All Results are listed in Table I and a graphical visualization is provided in Figure 2.
Figure 1. Phase contrast microscopy of cocaine crystals showing after 1 min in 3.5% cocaine hydrochloride solution and settling on nasal epithelial cell cluster (400-fold magnification).
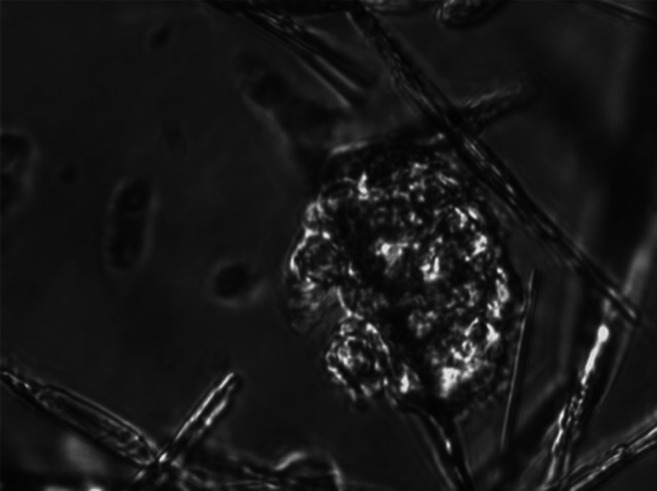
Table I. Mean ciliary beat frequency (CBF) for the control group (0%) and tested cocaine hydrochloride (CHCL) solutions with standard deviation (SD).
All CBF values acquired in the test solutions showed a significant reduction compared to the control group (p-value=0.001). Time in min. CBF in Hz.
Figure 2. Wireframe surface plot showing changes of ciliary beat frequency (CBF) depending on time and concentration of cocaine hydrochloride (CHCL) solution.
Discussion
Cocaine is a well-known stimulant and local anesthetic. Anesthesia is achieved by binding to sodium channels in peripheral nerve fibers, making them unable to depolarize and form action potentials and thus disrupting signal conduction (28). Its medical use is limited and it has mostly been replaced by its more potent derivates like lidocaine (3). Central effects are caused by blockage of presynaptic monoamine transporters in the CNS, leading to elevated dopamin, serotonin and noradrenaline levels. This results in a short-term awareness increase and loss of impulse control (29). Long-term abuse leads to addiction via various neurobehavioral mechanisms (30).
Today, cocaine is one of the most commonly used recreational drugs (6). Nasal long-term use can lead to damage of local epithelial cells, cartilage and bone (10). Exposing nasal epithelial cells to cocaine was shown to lead to a significant reduction of CBF. This CBF reducing effect could be attributed to pharmaceutical properties and the above-mentioned mechanical obstruction of cilia by CHCL crystals. Although CHCL has a solubility in water of 714 g/l at 25˚C, we found CHCL crystals settling on monitored cell colonies increasing with time and concentration. Crystals were found in samples containing all tested concentrations. This is probably caused by dilution with RPMI instead of water, since RPMI is already saturated with the contained nutrient components.
Since nasal doses in recreational use of cocaine far surpass its solubility (20-100 mg per nostril), mechanical inhibition of the nasal cilia by CHCL crystals is very likely in those cases. Anesthetic solutions for topical application can contain 4 or 10% CHCL, making a mechanical inhibition less likely but still possible. At the cellular level, the regulation of cilia motility is not fully understood yet. An increase of intracellular calcium (Ca2+) levels leads to stimulation of CBF and is the common endpoint of all known signal pathways. Ca2+ can be mobilized from intracellular stores or transported from outside the cell (31). In vitro exposure of airway epithelia to Ca2+ and Acetylcholin (ACH) leads to an increase in CBF (32). Ca2+ can be transported directly into the cell or activate transmembrane adenylyl cyclases leading to cAMP-pathway activation and CBF increase by Dynein-arm phosphorylation via PKA (33). ACH triggers muscarinergic ACH receptors (mACH-R) on the cell membrane causing hydrolyzation and activation of inositol-trisphosphate (IP3) which in turn leads to Ca2+ release from intracellular stores (13). Cocaine interferes in this pathway by antagonizing mACH-R (34).
There are also studies showing a benefit to mucociliary clearance after blocking epithelial sodium channels (ENaC). This effect is accredited to an increased airway hydration (35,36). While inhibiting voltage-dependent sodium-ion channels in peripheral nerves (37), it is not known whether cocaine blocks ENaC. Overall, this study shows another possible additional mechanism of nasal mucosa damaging by cocaine leading to CIMDL. The role of this mechanism in the pathogenesis of CIMDL remains partly unclear, but it can be assumed that disruption of mucociliary clearance via the signal paths described above promotes local toxicity, increases drug exposure time and weakens local immunodefense mechanisms.
This is the first study analyzing the impact of cocaine on CBF with highspeed video-microscopy. Our results help to better understand the pathophysiology and the damage mechanism of intranasal consumption of cocaine. But there are a few limitations which must be considered. Due to the time gap between adding of the CHCL solution and acquiring the first video sequences, there is already a significant reduction of CBF for all CHCL concentrations compared to the control group at t=0.
Cocaine obtained from street vendors for recreational use is rarely pure. Besides pharmacological inactive “fillers” like sugars (e.g. innositol, mannitol, glucose), there is a wide variety of drugs added to mimic the effect of cocaine (e.g. caffeine, lidocaine, levamisole) or alleviate unwanted effects like increased blood pressure, tachycardia and anxiety (e.g. diltiazem, hydroxyzine) (38). This study cannot differentiate if occurrence of CIMDL is promoted by cocaine alone. Additional effects of the cutting agents must be taken into account. A calcium antagonist like diltiazem, for example, is also very likely to reduce CBF even more than CHCL alone.
It also has to be considered that the setup does not allow analysis of the irreversibility of the observed effect. Since the cell clusters where not fixed in the test solutions, it was not possible to wash out the CHCL without moving the cells. Previous studies have shown reversible CBF reduction with 1.5-20% CHCL solutions and irreversible CBF reduction between 7-20% CHCL solutions (23-25). All studies where limited to in vitro testing. It is not known, how long CBF reduction by cocaine lasts in vivo. Presuming an irreversible CBF reduction, the effects may last for at least 5 days, which is the observed regeneration time of nasal mucosa after mechanical injury with intact basal cells and basement membrane (39).
Conclusion
CHCL is a potent inhibitor of CBF in nasal epithelial cells. Beside the other negative effects of cocaine, the associated reduction of mucociliary clearance might contribute to development of the cocaine induced midline destructive lesions including hyposmia, crusting, ulcers, nasal septal perforation and bone erosion.
Conflicts of Interest
The Authors declare that they have no conflicts of interest.
Authors’ Contributions
Alexander Nastev and Richard Birk conceived and planned the experimental design. Alexander Nastev, Richard Birk, C. Emika Mueller and Daniel Haeussler carried out the experiments. Richard Birk and Alexander Nastev took lead in writing the article. J. Ulrich Sommer and Richard Birk performed statistical analysis. Alexander Nastev, J. U. Sommer, Wieland Behr, Boris A. Stuck, Emika Mueller, Angela Schell, Benedikt Kramer, Daniel Haeussler and Karl Hoermann contributed to the interpretation of the results, provided critical feedback and improved the article. All Authors carefully read and commented on the article.
Acknowledgements
The Authors of the present study would like to thank Petra Prohaska for technical support.
References
- 1.Gaedcke F. Ueber das erythroxylin, dargestellt aus den blättern des in südamerika cultivirten strauches erythroxylon coca lam. Archiv der Pharmazie. 1855;132(2):141–150. [Google Scholar]
- 2.Koller C. Vorläufige mittheilung über locale anästhesirung am auge. Bericht über die sechzehnte versammlung der ophthalmologischen gesellschaft. Rostock: Adler. 1884:60–63. [Google Scholar]
- 3.Calatayud J, González Á. History of the development and evolution of local anesthesia since the coca leaf. Anesthesiology. 2003;98(6):1503–1508. doi: 10.1097/00000542-200306000-00031. [DOI] [PubMed] [Google Scholar]
- 4.Greinwald JH Jr., Holtel MR. Absorption of topical cocaine in rhinologic procedures. Laryngoscope. 1996;106(10):1223–1225. doi: 10.1097/00005537-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Liao BS, Hilsinger RL Jr, Rasgon BM, Matsuoka K, Adour KK. A preliminary study of cocaine absorption from the nasal mucosa. Laryngoscope. 1999;109(1):98–102. doi: 10.1097/00005537-199901000-00019. [DOI] [PubMed] [Google Scholar]
- 6.United Nations publication Sales United nations office on drugs and crime, world drug report. United Nations publication Sales No. E.19.XI.8. 2019. Available at: https://wdr.unodc.org/wdr2019/prelaunch/WDR19_Booklet_4_STIMULANTS.pdf.
- 7.Purdy RE, Julien RM, Fairhurst AS, Terry MD. Effect of carbamazepine on the in vitro uptake and release of norepinephrine in adrenergic nerves of rabbit aorta and in whole brain synaptosomes. Epilepsia. 1977;18(2):251–257. doi: 10.1111/j.1528-1157.1977.tb04474.x. [DOI] [PubMed] [Google Scholar]
- 8.Tikhonov DB, Zhorov BS. Mechanism of sodium channel block by local anesthetics, antiarrhythmics, and anticonvulsants. J Gen Physiol. 2017;149(4):465–481. doi: 10.1085/jgp.201611668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Leary ME, Hancox JC. Role of voltage-gated sodium, potassium and calcium channels in the development of cocaine-associated cardiac arrhythmias. Br J Clin Pharmacol. 2010;69(5):427–442. doi: 10.1111/j.1365-2125.2010.03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trimarchi M, Bertazzoni G, Bussi M. Cocaine induced midline destructive lesions. Rhinology. 2014;52(2):104–111. doi: 10.4193/Rhin13.112. [DOI] [PubMed] [Google Scholar]
- 11.Singh MK, Elefteriou F, Karsenty G. Cocaine and amphetamine-regulated transcript may regulate bone remodeling as a circulating molecule. Endocrinology. 2008;149(8):3933–3941. doi: 10.1210/en.2008-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiesner O, Russell KA, Lee AS, Jenne DE, Trimarchi M, Gregorini G, Specks U. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum. 2004;50(9):2954–2965. doi: 10.1002/art.20479. [DOI] [PubMed] [Google Scholar]
- 13.Braiman A, Priel Z. Efficient mucociliary transport relies on efficient regulation of ciliary beating. Respir Physiol Neurobiol. 2008;163(1-3):202–207. doi: 10.1016/j.resp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Satir P, Sleigh MA. The physiology of cilia and mucociliary interactions. Annu Rev Physiol. 1990;52:137–155. doi: 10.1146/annurev.ph.52.030190.001033. [DOI] [PubMed] [Google Scholar]
- 15.Stannard W, O’Callaghan C. Ciliary function and the role of cilia in clearance. J Aerosol Med. 2006;19(1):110–115. doi: 10.1089/jam.2006.19.110. [DOI] [PubMed] [Google Scholar]
- 16.Munkholm M, Mortensen J. Mucociliary clearance: Pathophysiological aspects. Clin Physiol Funct Imaging. 2014;34(3):171–177. doi: 10.1111/cpf.12085. [DOI] [PubMed] [Google Scholar]
- 17.Robinson M, Bye PTB. Mucociliary clearance in cystic fibrosis. Pediatr Pulmonol. 2002;33(4):293–306. doi: 10.1002/ppul.10079. [DOI] [PubMed] [Google Scholar]
- 18.Houtmeyers E, Gosselink R, Gayan-Ramirez G, Decramer M. Regulation of mucociliary clearance in health and disease. Eur Respir J. 1999;13(5):1177–1188. doi: 10.1034/j.1399-3003.1999.13e39.x. [DOI] [PubMed] [Google Scholar]
- 19.Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41(5):293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Utiyama DM, Yoshida CT, Goto DM, de Santana Carvalho T, de Paula Santos U, Koczulla AR, Saldiva PH, Nakagawa NK. The effects of smoking and smoking cessation on nasal mucociliary clearance, mucus properties and inflammation. Clinics (Sao Paulo) 2016;71(6):344–350. doi: 10.6061/clinics/2016(06)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abou Alaiwa MH, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, Zabner J, Welsh MJ. Ph modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and ll-37. Proc Natl Acad Sci USA. 2014;111(52):18703–18708. doi: 10.1073/pnas.1422091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewulf J, Hecq JD, Huvelle S, Godet M, Gillet P, Jamart J, Galanti LM. Long-term stability of cocaine hydrochloride aqueous solution 50 mg/ml (5%) at room temperature and at 5 degrees c +/- 3 degrees c in glass bottles. Int J Pharm Compd. 2015;19(3):268–270. [PubMed] [Google Scholar]
- 23.Coressen G, Allen C. Cultured human respiratory epithelium: Its use in the comparison of the cytotoxic properties of local anesthetics. Anesthesiology. 1960;21(3):237–243. [PubMed] [Google Scholar]
- 24.Ingels KJ, Nijziel MR, Graamans K, Huizing EH. Influence of cocaine and lidocaine on human nasal cilia. Beat frequency and harmony in vitro. Arch Otolaryngol Head Neck Surg. 1994;120(2):197–201. doi: 10.1001/archotol.1994.01880260067012. [DOI] [PubMed] [Google Scholar]
- 25.van de Donk HJM, van Egmond ALM, van den Heuvel AGM, Zuidema J, Merkus FWHM. The effects of drugs on ciliary motility iii. Local anaesthetics and anti-allergic drugs. Int J Pharm. 1982;12(1):77–85. doi: 10.1016/0378-5173(82)90135-1. [DOI] [Google Scholar]
- 26.Sommer JU, Gross S, Hörmann K, Stuck BA. Time-dependent changes in nasal ciliary beat frequency. Eur Arch Otorhinolaryngol. 2010;267(9):1383–1387. doi: 10.1007/s00405-010-1211-5. [DOI] [PubMed] [Google Scholar]
- 27.Thomas B, Rutman A, O’Callaghan C. Disrupted ciliated epithelium shows slower ciliary beat frequency and increased dyskinesia. Eur Respir J. 2009;34(2):401–404. doi: 10.1183/09031936.00153308. [DOI] [PubMed] [Google Scholar]
- 28.Covino BG. Physiology and pharmacology of local anesthetic agents. Anesth Prog. 1981;28(4):98–104. [PMC free article] [PubMed] [Google Scholar]
- 29.Spronk DB, van Wel JH, Ramaekers JG, Verkes RJ. Characterizing the cognitive effects of cocaine: A comprehensive review. Neurosci Biobehav Rev. 2013;37(8):1838–1859. doi: 10.1016/j.neubiorev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Bickel WK, Snider SE, Quisenberry AJ, Stein JS, Hanlon CA. Competing neurobehavioral decision systems theory of cocaine addiction: From mechanisms to therapeutic opportunities. Prog Brain Res. 2016;223:269–293. doi: 10.1016/bs.pbr.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmid A, Salathe M. Ciliary beat co-ordination by calcium. Biol Cell. 2011;103(4):159–169. doi: 10.1042/BC20100120. [DOI] [PubMed] [Google Scholar]
- 32.Do BH, Ohbuchi T, Wakasugi T, Koizumi H, Yokoyama M, Hohchi N, Suzuki H. Acetylcholine-induced ciliary beat of the human nasal mucosa is regulated by the pannexin-1 channel and purinergic p2x receptor. Am J Rhinol Allergy. 2018 doi: 10.1177/1945892418770292. [DOI] [PubMed] [Google Scholar]
- 33.Nlend MC, Schmid A, Sutto Z, Ransford GA, Conner GE, Fregien N, Salathe M. Calcium-mediated, purinergic stimulation and polarized localization of calcium-sensitive adenylyl cyclase isoforms in human airway epithelia. FEBS Lett. 2007;581(17):3241–3246. doi: 10.1016/j.febslet.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrera MR, Meijler MM, Janda KD. Cocaine pharmacology and current pharmacotherapies for its abuse. Bioorg Med Chem. 2004;12(19):5019–5030. doi: 10.1016/j.bmc.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Astrand AB, Hemmerling M, Root J, Wingren C, Pesic J, Johansson E, Garland AL, Ghosh A, Tarran R. Linking increased airway hydration, ciliary beating, and mucociliary clearance through enac inhibition. Am J Physiol Lung Cell Mol Physiol. 2015;308(1):L22–L32. doi: 10.1152/ajplung.00163.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mall MA. Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: Lessons from mouse models. J Aerosol Med Pulm Drug Deliv. 2008;21(1):13–24. doi: 10.1089/jamp.2007.0659. [DOI] [PubMed] [Google Scholar]
- 37.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there. Nat Rev Drug Discov. 2006;5(12):993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 38.Broseus J, Gentile N, Esseiva P. The cutting of cocaine and heroin: A critical review. Forensic Sci Int. 2016;262:73–83. doi: 10.1016/j.forsciint.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 39.Ohashi Y, Nakai Y, Ikeoka H, Furuya H. Regeneration of nasal mucosa following mechanical injury. Acta Otolaryngol Suppl. 1991;486:193–201. doi: 10.3109/00016489109134996. [DOI] [PubMed] [Google Scholar]



