Abstract
Backround: Due to extensive surgical intervention for macroscopic complete cytoreduction in epithelial ovarian cancer (EOC) patients, severe complications in the postoperative course are possible. Patients and Methods: A total of 345 EOC patients who underwent cytoreductive surgery were retrospectively evaluated regarding risk factors for an unfavorable postoperative course. Possible pre-, intra- and postoperative risk factors were statistically analyzed performing multivariate ordinal logistic regression. Results: A total of 345 EOC patients underwent cytoreductive surgery. There were no complications in 114 patients, mild complications in 114 patients and severe complications in 117 patients. The risk factor evaluation identified age (p=0.049), smoking (p=0.032) and duration of surgery (p<0.0001) as significant factors for severe postoperative morbidity. Conclusion: In EOC patients age, smoking and the duration of surgery have significant impact on the postoperative course. Only the duration of surgery can be positively influenced by a well-trained EOC team.
Keywords: Ovarian cancer, postoperative complications, Postoperative period, surgical oncology
In epithelial ovarian cancer (EOC) the prognostic impact of macroscopic complete cytoreduction has been shown unequivocally. Often, complex surgery will be needed harbouring the risk of severe postoperative complications (1). These postoperative complications may delay adjuvant therapy and therefore have a negative impact on survival (2). Using the Memorial Sloan Kettering Cancer Center secondary surgical event score we classified all postoperative complications within the first 60 days after surgery from grade 1 to 5. The aim of this study was the identification of possible risk factors for an unfavorable postoperative course (3).
Patients and Methods
This retrospective study was conducted in accordance with the standards of the local ethics committe of the medical faculty of the Rheinische Friedrich Wilhelms University, Bonn, Germany (Nr: 314/20). All 345 patients who underwent cytoreductive surgery for EOC between 2004 and 2016 were included in this study and data were collected and analyzed. Postoperative complications were assessed by the Memorial Sloan Kettering Cancer Center secondary surgical event score (3). Histologies and surgical reports were used for a retrospective determination of the peritoneal cancer index (4). For statistical analysis, patients were divided into two groups; those with no or mild complications (secondary surgical events grade 1 and 2) and those with severe complications (secondary surgical events grade 3 to 5).
Possible risk factors for severe secondary surgical events were analyzed by multivariate ordinal logistic regression. The quality review was performed by Pearson and Deviance tests. Further data were analyzed by binary ordinal logistic regression. Differences were defined to be significant when p≤0.05. All statistical analyses were performed using Minitab Version 18.
Results
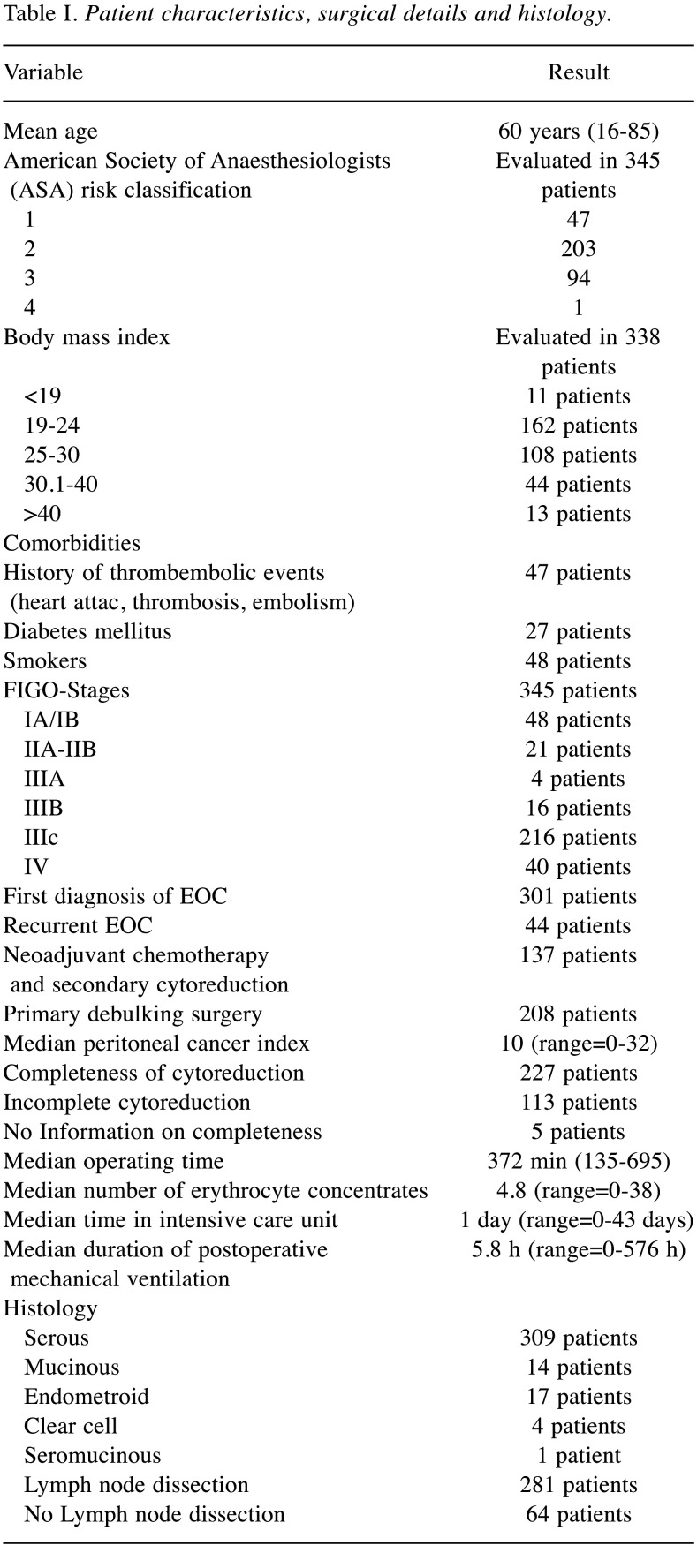
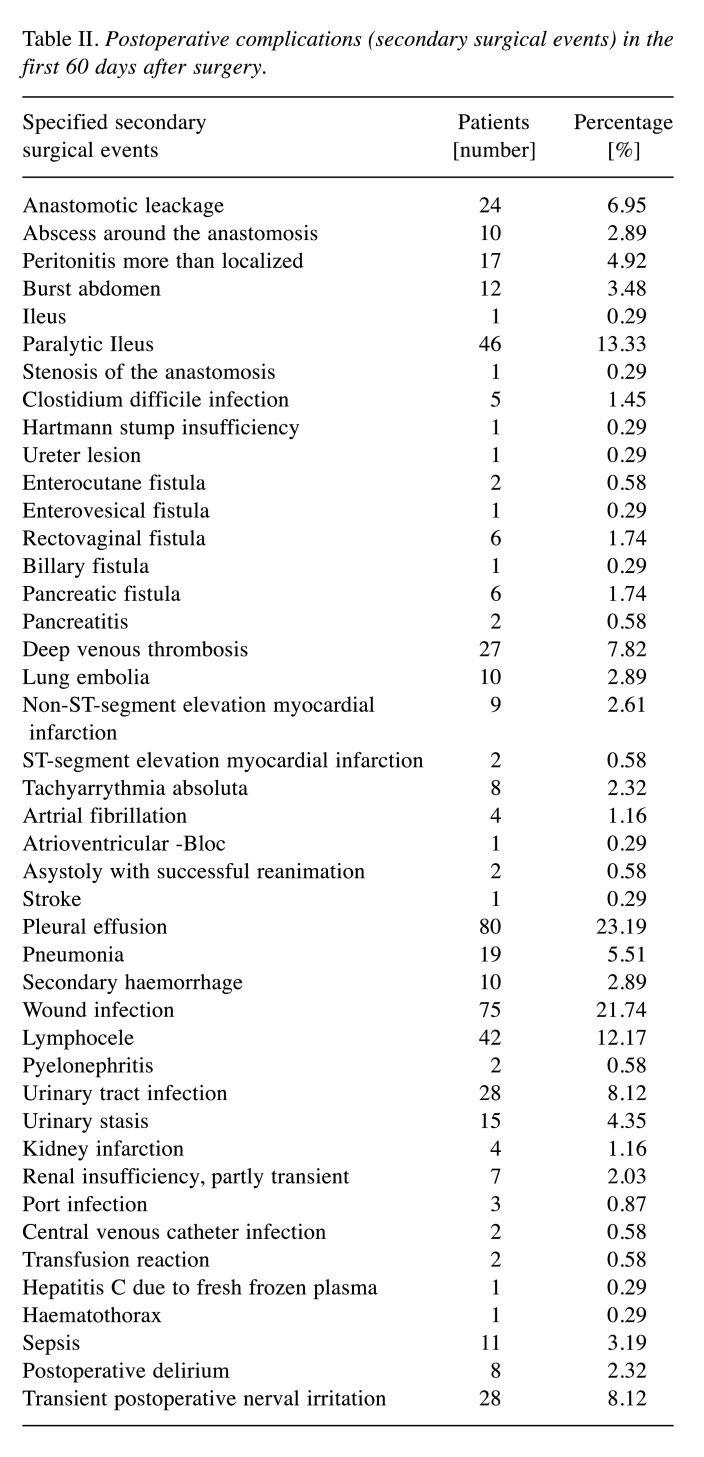
A total of 345 patients underwent cytoreductive surgery for EOC. Patient variables analyzed in the multivariate analysis are listed in Table I. Table II shows all secondary surgical events in detail while Table III shows all graded secondary surgical events and the number of patients affected. 7 patients died in the postoperative course within the first 60 days after surgery. 1 patient suffered a heart attack, 1 patient with a known aortic stenosis experienced postoperative cardiac decompensation while 2 patients died of septic pneumonia in the late postoperative course. 3 patients experienced a septic shock, 2 due to an anastomotic leckage and 1 due to an abscess followed by an enterocutaneous fistula. The most common secondary surgical events were pleural effusion, wound infection, and paralytic ileus.
Table I. Patient characteristics, surgical details and histology.
Table II. Postoperative complications (secondary surgical events) in the first 60 days after surgery.
Table III. Postoperative complications graded according to the MSKCC secondary surgical event score.
The multivariate ordinal logistic regression analysis of 344 patient data (the data of 1 patient was missing for the duration of surgery) identified smoking (p=0.032) as the strongest preoperative factor for the development of severe complications. The age of the patients (p=0.049) and the duration of surgery (p<0.001) were the two other risk factors significantly associated with severe complications. Table IV shows the results of the mulitvariate analysis. By performing binary logistic regression with 340 patient data (data on the completeness of cytoreduction was missing in 5 surgery reports), we found a significant decrease in complete cytoreduction with increasing age of patients (p=0.010). The risk of leaving surgery with a residual disease increased 1.29-fold every ten years with increasing patient age. The age of the patients was not related to a prolonged hospital stay (p=0.244).
Table IV. Multivariate analysis of potential risk factors (age, preoperative anemia, ASA, BMI, history of thrombembolic events, diabetes, elevated creatinin levles, neoadjuvant chemotherapy, smoking, recurrent disease, primary disease, number of transfused erythrocytes, complete or incomplete resection, duration of postoperative mechanical ventilation, duration of intensive care).
The body mass index, available in 338 patients, was no predictor for residual disease (p=0.820; OR=1.03) or the duration of surgery (p=0.383). The duration of surgery had no impact on postoperative thrombembolic events (p=0.197) or the number of erythrocyte concentrates received (p=0.770). The risk of thrombembolic events was reduced in the group of patients with a history of thrombembolic events (OR=0.715).
The available data on the peritoneal cancer index in 330 patients demonstrated no significant correlation with a preoperative anemia or the duration of the postoperative intensive care. However, a peritoneal cancer index of more than 15 correlated with a higher incidence of a postoperative pancreatic fistula (OR=0.083). The peritoneal cancer index had significant impact on the duration of surgery (p<0.001) and the completeness of cytoreduction (p<0.001; OR=0.800). The significant correlation between the peritoneal cancer index and Ca125 (p=0.034) was negligible in the metric set (Figure 1).
Figure 1. Duration of surgery and PCI.

The risk of a preoperative anemia was 1.57-fold higher in the group of patients with neoadjuvant chemotherapy than in the group of patients without neoadjuvant chemotherapy.
Discussion
Optimal cytoreduction in EOC patients aims to increase overall survival but harbours the risk of increased morbidity and a prolonged recovery which in turn decreases survival (5). The preoperative prediction of postoperative complications would be ideal to define high-risk patients. Despite intensive efforts in the preoperative work up, this remains difficult. In the present study, smoking was identified as the strongest predictor for severe complications. The age of the patients and the duration of surgery were two other significant predictors of severe complications. Smoking in general shows an increased overall morbidity with wound complications, infections, pulmonary and neurological complications (6). The analysis of the database of the National Surgical Quality Improvement Program (NASQIP) identified smoking, elevated preoperative creatinine levels (above 1.5 mg/dl) and insulin-dependent and non-insulin-dependent diabetes as significant risk factors for a re-operation. Reoperation shortly after initial surgery increases morbidity and mortality (7-10). We did not find a correlation between diabetes and morbidity or an elevated creatinin and morbidity. But creatinin levels were elevated in only 3 patients. In line with the above, the evaluation of more than 500,000 patients data from the American College of Surgeons in NSQIP identified smoking as a significant promoter of pneumonia, cardiac arrest, myocardial infarction, stroke, sepsis, septic shock, organ space infection and increased postoperative mortality (10).
An age above 75 years has been determined as a risk factor for higher postoperative morbidity and mortality. In general surgery in the elderly is feasible but the risk for increased severe morbidity and increased mortality is almost 40%. In addition, adjuvant chemotherapy may be delayed or not administered at all. Even when complete cytoreduction is dispensed due to the age of the patient, incomplete surgery also bears the risk of increased perioperative morbidity. Especially in patients over 80 years of age, FIGO stage IV, low albumin levels, and higher risk classification according to the American Society of Anaesthesiologists (ASA), morbidity and mortality increases rapidly (11). Data analysis of the NSQIP demonstrated a linear progression of morbidity and an exponential progression of mortality with increasing age. The increase in morbidity and mortality was seen despite the absence of further risk factors at the age of 70 and over. Predictive factors for morbidity and mortality in the elderly patients were urgent surgery, unintentional weight loss, preoperative anemia, and blood transfusions. Preoperative anemia and blood transfusions had no significant impact on severe morbidity in our cohort. Furthermore, an age over 80 years was associated with the highest risk for wound infections without any correlation to other risk factors (12). The frailty of aging itself without additional specific comorbidities is not usually noticeable in the normal state. But the stress of surgery requires reserve capacities which may not be available in older patients. Therefore, age may sometimes be the only assessable risk factor in this patient cohort (13).
In addition to the experience of the surgeon, the complexity of the surgical intervention significantly determines the duration of the surgery and the postoperative morbidity, especially in the older patients (12). An increase in surgery duration of only 30 minutes in patients with EOC results in 17% more cardiovascular events, 7% more respiratory events, and 15% more wound infections (12). Surgery duration of more than 10 hours and an ASA risk classification of 3 or more were the only significant factors for grade 3 and 4 secondary surgical events in patients with pseudomyxoma peritonei (14). The Tennesse Surgical Quality Collaborative showed that the risk for secondary surgical events increases significantly after 2.1 hours of surgery. A significant risk of deep infections at the surgical site begins only after 42 minutes of surgery. Each additional hour of surgery time supports the development of a postoperative sepsis (15). Even in laparoscopic procedures, the duration of surgery represents a significant risk factor for postoperative complications (16). Well trained surgeons minimize the duration of surgery which results in a reduction in mortality of 69% (17).
The ASA risk classification was irrelevant in our study for severe secondary surgical events. This could be due to the previous policy of the clinic to administer neoadjuvant chemotherapy whenever aszites more than 500 cc was present. Therefore, severely and critically affected EOC patients may already have been excluded. Other studies have indeed shown that a higher ASA risk classification correlates with the grade of secondary surgical events. Especially in older patients, the ASA risk classification is significantly associated with severe secondary surgical events (11,12,14,18). In a retrospective study even a 72% correct identification of high-risk patients was suggested based on the combination of ASA risk classification, low albumin levels, aszites, bleeding disorders, urgency of surgery, higher extended procedure score, and age of the patients (18).
Obesity is a known risk factor for decreased overall survival in patients with EOC (19). In some studies, morbid obesity, indicated by a body mass index of more than 39.9, has been associated with severe secondary surgical events (20). Other studies show that obese patients are only at risk for impaired wound healing (21). In our study the body mass index had no significant influence on the postoperative course, incomplete cytoreduction, or a prolonged duration of surgery. Nevertheless, it should be noted that morbid obesity with a body mass index of more than 39.9 was present in only 13 patients.
The strength of our study lies in its homogenous data set, since only those EOC patients who were actually assigned to cytoreductive surgery with the intention of complete resection were included in our analysis. However, due to the retrospective nature of the study, a possible bias in patient selection cannot be excluded.
Conclusion
In EOC patients the risk factors of age, smoking and the duration of surgery have a significant impact on the postoperative morbidity and mortality. Only the duration of surgery may be positively influenced by a well-trained EOC team. Therefore, detailed preoperative assessment of the expected surgical complexity in EOC patients is essential. The expertise of the EOC surgeon should be appropriate for the level of complexity of EOC surgery in order to reduce postoperative morbidity and mortality due to shorter duration of surgery.
Conflicts of Interest
The Authors declare no conflicts of interest.
Authors’ Contributions
Conception and design: E.K. Egger, A. Mustea; Data analysis and interpretation: E.K. Egger, M. Condic, M.D. Keyver-Paik; Investigation: E.K. Egger, N. Kohls, D. Könsgen, S. Klaschik, T. Hilbert; Writing–original draft preparation: E.K. Egger, D. Exner; Writing–review and editing: T. Vilz, M.B. Stope; Supervision: A. Mustea.
References
- 1.Koscielny A, Ko A, Egger EK, Kuhn W, Kalff JC, Keyver-Paik MD. Prevention of anastomotic leakage in ovarian cancer debulking surgery and its impact on overall survival. Anticancer Res. 2019;39(9):5209–5218. doi: 10.21873/anticanres.13718. [DOI] [PubMed] [Google Scholar]
- 2.Grimm C, Harter P, Alesina PF, Prader S, Schneider S, Ataseven B, Meier B, Brunkhorst V, Hinrichs J, Kurzeder C, Heitz F, Kahl A, Traut A, Groeben HT, Walz M, du Bois A. The impact of type and number of bowel resections on anastomotic leakage risk in advanced ovarian cancer surgery. Gynecol Oncol. 2017;146(3):498–503. doi: 10.1016/j.ygyno.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Strong VE, Selby LV, Sovel M, Disa JJ, Hoskins W, Dematteo R, Scardino P, Jaques DP. Development and assessment of memorial sloan kettering cancer center’s surgical secondary events grading system. Ann Surg Oncol. 2015;22(4):1061–1067. doi: 10.1245/s10434-014-4141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lampe B, Kroll N, Piso P, Forner DM, Mallmann P. Prognostic significance of sugarbaker’s peritoneal cancer index for the operability of ovarian carcinoma. Int J Gynecol Cancer. 2015;25(1):135–144. doi: 10.1097/IGC.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 5.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2007;197(6):676.e1–676.e7. doi: 10.1016/j.ajog.2007.10.495. [DOI] [PubMed] [Google Scholar]
- 6.Grønkjær M, Eliasen M, Skov-Ettrup LS, Tolstrup JS, Christiansen AH, Mikkelsen SS, Becker U, Flensborg-Madsen T. Preoperative smoking status and postoperative complications: A systematic review and meta-analysis. Ann Surg. 2014;259(1):52–71. doi: 10.1097/SLA.0b013e3182911913. [DOI] [PubMed] [Google Scholar]
- 7.Birkmeyer JD, Hamby LS, Birkmeyer CM, Decker Mv, Karon NM, Dow RW. Is unplanned return to the operating room a useful quality indicator in general surgery. Arch Surg. 2001;136(4):405–411. doi: 10.1001/archsurg.136.4.405. [DOI] [PubMed] [Google Scholar]
- 8.Rama-Maceiras P, Rey-Rilo T, Moreno-Lopez E, Molins-Gauna N, Sanduende-Otero Y, Pensado-Castiñeiras A. Unplanned surgical reoperations in a tertiary hospital: perioperative mortality and associated risk factors. Eur J Anaesthesiol. 2011;28(1):10–15. doi: 10.1097/eja.0b013e32833e33b0. [DOI] [PubMed] [Google Scholar]
- 9.Kazaure HS, Chandra V, Mell MW. Unplanned reoperations after vascular surgery. J Vasc Surg. 2016;63(3):730–736. doi: 10.1016/j.jvs.2015.09.046. [DOI] [PubMed] [Google Scholar]
- 10.Turan A, Mascha EJ, Roberman D, Turner PL, You J, Kurz A, Sessler DI, Saager L. Smoking and perioperative outcomes. Anesthesiology. 2011;114(4):837–846. doi: 10.1097/ALN.0b013e318210f560. [DOI] [PubMed] [Google Scholar]
- 11.Langstraat C, Aletti GD, Cliby WA. Morbidity, mortality and overall survival in elderly women undergoing primary surgical debulking for ovarian cancer: A delicate balance requiring individualization. Gynecol Oncol. 2011;123(2):187–191. doi: 10.1016/j.ygyno.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203(6):865–877. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Evers BM, Townsend CM, Thompson JC. Organ physiology of aging. Surg Clin North Am. 1994;74(1):23–39. doi: 10.1016/S0039-6109(16)46226-2. [DOI] [PubMed] [Google Scholar]
- 14.Saxena A, Yan TD, Chua TC, Morris DL. Critical assessment of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2010;17(5):1291–1301. doi: 10.1245/s10434-009-0875-9. [DOI] [PubMed] [Google Scholar]
- 15.Daley BJ, Cecil W, Clarke PC, Cofer JB, Guillamondegui OD. How slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Am Coll Surg. 2015;220(4):550–558. doi: 10.1016/j.jamcollsurg.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 16.Jackson TD, Wannares JJ, Lancaster RT, Rattner DW, Hutter MM. Does speed matter? The impact of operative time on outcome in laparoscopic surgery. Surg Endosc. 2011;25(7):2288–2295. doi: 10.1007/s00464-010-1550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bristow RE, Zahurak ML, Diaz-Montes TP, Giuntoli RL, Armstrong DK. Impact of surgeon and hospital ovarian cancer surgical case volume on in-hospital mortality and related short-term outcomes. Gynecol Oncol. 2009;115(3):334–338. doi: 10.1016/j.ygyno.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Cham S, Chen L, St. Clair CM, Hou JY, Tergas A I, Melamed A, Ananth CV, Neugut AI, Hershman DL, Wright JD. Development and validation of a risk-calculator for adverse perioperative outcomes for women with ovarian cancer. Am J Obstet Gynecol. 2019;220(6):571.e1–571.e8. doi: 10.1016/j.ajog.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagle CM, Dixon SC, Jensen A, Kjaer SK, Modugno F, deFazio A, Fereday S, Hung J, Johnatty SE, Australian Ovarian Cancer Study Group, Fasching PA, Beckmann MW, Lambrechts D, Vergote I, Van Nieuwenhuysen E, Lambrechts S, Risch H A, Rossing MA, Doherty JA, Wicklund KG, Chang-Claude J, Goodman MT, Ness RB, Moysich K, Heitz F, du Bois A, Harter P, Schwaab I, Matsuo K, Hosono S, Goode EL, Vierkant RA, Larson MC, Fridley BL, Høgdall C, Schildkraut JM, Weber RP, Cramer DW, Terry KL, Bandera EV, Paddock L, Rodriguez-Rodriguez L, Wentzensen N, Yang HP, Brinton LA, Lissowska J, Høgdall E, Lundvall L, Whittemore A, McGuire V, Sieh W, Rothstein J, Sutphen R, Anton-Culver H, Ziogas A, Pearce CL, Wu AH, Webb PM. Ovarian Cancer Association Consortium Obesity and survival among women with Ovarian cancer: results from the Ovarian Cancer Association Consortium. Br J Cancer. 2015;113(5):817–826. doi: 10.1038/bjc.2015.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Bakkum-Gamez JN, Weaver AL, McGree ME, Cliby WA. Impact of obesity on surgical and oncologic outcomes in ovarian cancer. Gynecol Oncol. 2014;135(1):19–24. doi: 10.1016/j.ygyno.2014.07.103. [DOI] [PubMed] [Google Scholar]
- 21.Smits A, Lopes A, Das N, Kumar A, Cliby W, Smits E, Bekkers R, Massuger L, Galaal K. Surgical morbidity and clinical outcomes in ovarian cancer - the role of obesity. BJOG. 2016;123(2):300–308. doi: 10.1111/1471-0528.13585. [DOI] [PubMed] [Google Scholar]