Abstract
Background/Aim: Hepatitis A virus (HAV) infection is still one of the serious health problems worldwide, despite the existence of effective vaccines for HAV. Zinc compounds have antiviral activities against various DNA and RNA viruses. Therefore, we investigated the effects of zinc compounds on the antiviral activity of interferon against HAV. Materials and Methods: The effects of zinc compounds with or without interferon on HAV genotype IIIA HA11-1299 replication were examined in human hepatoma Huh7 cells. Cell viability was examined by the MTS assay. Inflammasome associated gene expression was examined by real-time reverse transcription-polymerase chain reaction. Results: Both zinc sulfate and zinc chloride had an inhibitory effect on HAV replication. Zinc sulfate tended to enhance while zinc chloride significantly enhanced the anti-HAV effect induced by interferon-alpha-2a. Zinc chloride significantly up-regulated mitogen-activated protein kinase 12 (MAPK12) and down-regulated 6 related genes [baculoviral IAP repeat containing 3 (BIRC3), interleukin 1 beta (IL1B), proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1), prostaglandin-endoperoxide synthase 2 (PTGS2), PYD and CARD domain containing (PYCARD), and tumor necrosis factor (TNF)]. Conclusion: Zinc chloride inhibits HAV replication and has additive effects on the anti-HAV activities of interferon.
Keywords: Antivirals, HAV, inflammasomes, interferon, zinc
Hepatitis A virus (HAV) is a single-stranded, positive sense RNA virus of ~7,600 nt in length. HAV is transmitted through the fecal-oral route and usually causes self-limited acute hepatitis, however, it occasionally leads to acute liver failure, causing death or the requirement of liver transplantation (1). Higher seroprevalence of anti-HAV antibodies reflects poorer water sanitation and increased exposure during childhood in developing countries (2). Sexual contact among men who have sex with men (MSM), and blood transfusion are other known transmission routes (3,4).
HAV infection is the leading cause of pediatric acute liver failure in Asian countries (5), although the use of the HAV vaccine can prevent symptomatic hepatitis A, acute liver failure is still caused by HAV infection, and liver transplantation is required in many cases (6). Therefore, anti-HAV drugs should be developed.
Zinc is the second most abundant trace metal, after iron, in the human body (7). The global prevalence of zinc deficiency is ~20%, with the majority occurring in developing countries (8). Zinc deficiency is common in developing countries as well as in Japan, which is a developed country (7). We have previously demonstrated that zinc sulfate inhibits HAV replication and upregulates expression of glucose-regulated protein 78 (GRP78) (9).
In the human myelomonocytic lymphoma cell line U937, zinc chloride increased interferon-α/β receptor 2 mRNA levels by approximately 30%, compared to the control levels (10). Zinc seems to directly inhibit picornaviral protease and have an inhibitory effect on its polyprotein processing (6). In the present study, we examined the effects of zinc compounds on HAV replication and whether zinc compounds could enhance the inhibitory effects of interferon on HAV replication. The effects of zinc chloride on the inflammasome associated signaling pathway in hepatocytes were also investigated.
Materials and Methods
Cells and virus. The human hepatoma cell lines Huh7 and HepG2 were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, Saint Louis, MO, USA) containing 10% fetal calf serum (FCS), 100 U/ml penicillin G and 200 μg/ml streptomycin at 37˚C in an atmosphere of 5% CO2 (9). The HAV genotype IIIA HA11-1299 was established by Okamoto et al. and grown in cell culture as described previously (11).
HAV infection. Approximately 1.0×105 Huh7 cells were placed on 6-well tissue culture plates (Iwaki Glass, Tokyo, Japan) 24 h prior to infection. Huh7 cells were infected with 0.1 multiplicity of infection (MOI) or 0.01 MOI HAV genotype IIIA HA11-1299 after cells were washed with phosphate-buffered saline (PBS) twice. After 24 h of infection, cells were washed with PBS and maintained in 5% FCS DMEM with or without zinc chloride (Wako Pure Chemical, Tokyo, Japan), zinc sulfate (Wako) and/or interferon-alpha-2a (Sigma-Aldrich). After 96 h of infection, cellular RNA was extracted for the determination of HAV RNA (Figure 1).
Figure 1. Illustration of the protocols for hepatitis A virus (HAV) infection, treatment with zinc compounds and cellular RNA extraction. PBS: Phosphate-buffered saline; FCS: fetal calf serum; DMEM: Dulbecco’s Modified Eagle Medium; IFN: interferon.
Isolation of RNA and real-time reverse transcription (RT)-PCR. Total cellular RNA was extracted after HAV infection, using a RNeasy Mini kit (Qiagen, Hilden, North Rhine-Westphalia, Germany). To detect HAV genome sequences, RNA, oligo dT primers and random hexamers were used for cDNA synthesis using a PrimeScript RT reagent kit (Perfect Real Time; Takara BIO, Kusatsu, Shiga, Japan). HAV-specific RNA was quantitated by real-time PCR using a sense primer (5’-AGGCTACGGGTGAAACCTCTTAG-3’) and an antisense primer (5’-GCCGCTGTTACCCTATCCAA-3’) with Power SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA), following the manufacturer’s protocol on a 7500 Fast real-time PCR system (Applied Biosystems). The housekeeping gene β-actin-specific RNA was also quantitated by real-time PCR using a sense primer (5’-CAGCCATGTACGTTGCTATCCAGG-3’) and an antisense primer (5’-AGGTCCAGACGCAGGATGGCATG-3’). Each real-time PCR assay was performed in triplicate. PCR reaction was performed as follows: 95˚C for 10 min, followed by 40 cycles at 95˚C for 15 sec and 60˚C for 1 min. These data were analyzed by the ddCt method.
MTS assay. Approximately 1.0×103 cells were plated on 96 well tissue culture plates (Iwaki Glass) with or without zinc chloride, zinc sulfate or interferon-alpha-2a. After 72 h, MTS assays were performed with the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA). In brief, 20 μl MTS solution were added per well to 100 μl of media containing cells and left for 4 h at 37˚C in an atmosphere of 5% CO2. The absorbance at 490 nm was recorded using an iMark Microplate Absorbance Reader (Bio-Rad, Tokyo, Japan). Each assay was performed at least in triplicate.
Examination of the effects of zinc chloride on inflammasome associated signaling pathways in Huh7 cells infected with HAV. Human hepatoma Huh7 cells were infected with 0.1 MOI HAV genotype IIIA HA11-1299, and treated with or without 5 μM zinc chloride at the same time. Medium with or without 5 μM zinc chloride was exchanged on days 1, 3, 5. After 120 h of HAV infection and treatment with or without 5 μM zinc chloride, cellular RNA was extracted using the RNeasy Mini kit (Qiagen), and cDNA synthesis was performed using RT2 First Strand kit (Qiagen), on a GeneAmp PCR system 5700 (Applied Biosystems).
Inflammasome associated signaling pathways were analyzed by an RT2 Profiler PCR array (Qiagen) on a 7500 Fast real-time PCR system (Applied Biosystems), according to the manufacturer’s instruction. Data were analyzed by the RT2 Profiler PCR array Data Analysis software (Qiagen). Relative gene expression levels were normalized to housekeeping genes by ddCt method (12). We considered significant gene expression changes when the difference of gene expression was more than 2-fold and p-Value was less than 0.05.
Statistical analysis. All data are shown as the mean±standard deviation. Statistical analysis was performed by Student’s t-test. p<0.05 was considered as statistically significant.
Results
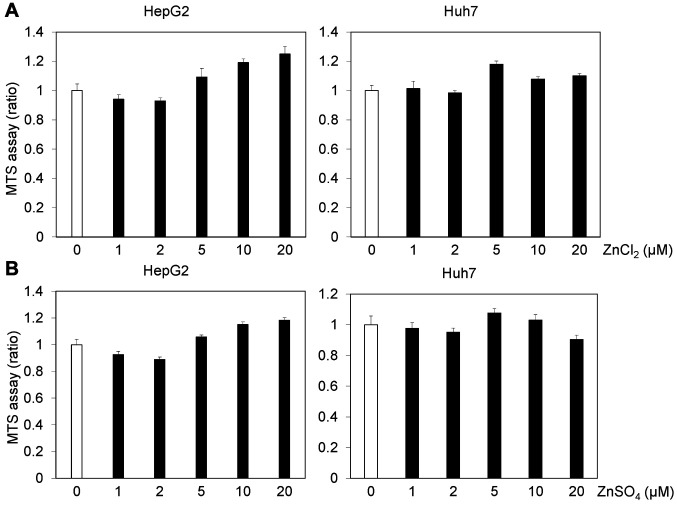
Zinc chloride inhibits HAV replication. To examine the cytotoxicity of zinc chloride and zinc sulfate, we performed MTS assays after 72 h of treatment of Huh7 or HepG2 cells with 0-20 μM zinc chloride or 0-20 μM zinc sulfate. Treatment with 5 or 10 μM zinc chloride or zinc sulfate did not reduce the viability of human hepatoma cell lines (Figure 2).
Figure 2. Effects of zinc compounds on the viability of human hepatocytes. The viability of HepG2 and Huh7 cells treated with zinc compounds for 72 h. (A) Zinc chloride (ZnCl2), (B) zinc sulfate (ZnSO4). Cell viability was measured by MTS assay (see Materials and Methods section). Data are expressed as means±standard deviations of triplicate determinations from 3 independent experiments.
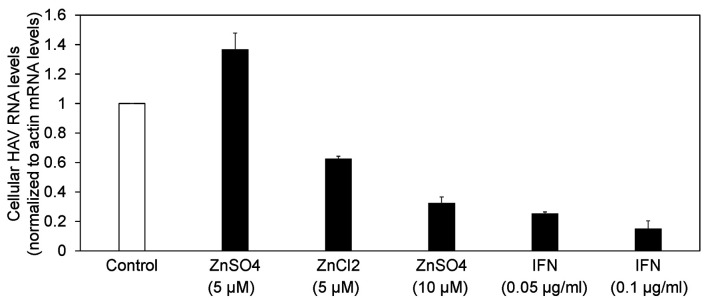
Next, we compared the antiviral activities of zinc chloride, zinc sulfate and interferon-alpha-2a against HAV in Huh7 cells infected with 0.01 MOI HAV (Figure 1 and Figure 3). Seventy-two hours of treatment with 5 μM zinc chloride suppressed HAV replication to 62.2% compared with the untreated control. Of note, we did not observe an inhibitory effect of 5 μM zinc sulfate on HAV replication (at 136%), although we have previously reported that 10 μM zinc sulfate had an inhibitory effect on HAV replication (reduced to 32.2%) (13). However, 72 h of treatment with 0.05 or 0.1 μg/ml interferon-alpha-2a suppressed HAV replication to 25.1 or 14.8%, respectively, compared with the untreated control. We determined that zinc chloride inhibits HAV replication.
Figure 3. Zinc compounds and interferon inhibit hepatitis A virus (HAV) replication. Drugs were added 24h post infection with 0.01 MOI HAV genotype IIIA HA11-1299. HAV RNA was measured by real-time reverse transcription-polymerase chain reaction. IFN: Interferon. Data are expressed as means±standard deviations of triplicate determinations from 3 independent experiments.
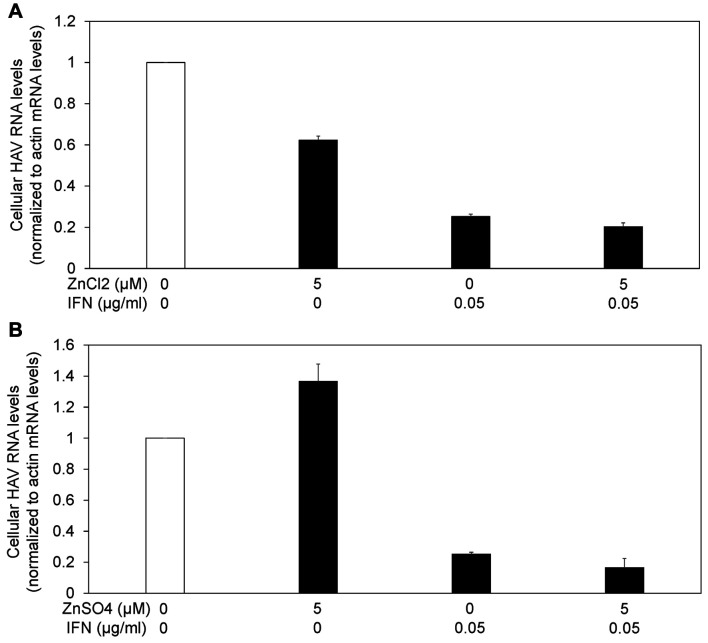
Suppression of HAV replication was stronger with the combination of interferon plus zinc compounds than with interferon alone. As interferon has also an antiviral effect through the induction of interferon stimulated genes (ISG) and the degradation of RNA viruses (14), we evaluated the anti-HAV activity of the combination of interferon plus zinc compounds (Figure 4). We infected Huh7 cells with 0.01 MOI HAV, and the drugs were added 24 h later. After 72 h of treatment with the drugs, cellular RNA was isolated, and HAV RNA levels were measured by real-time reverse transcription-polymerase chain reaction (RT-PCR).
Figure 4. Zinc compounds enhanced the antiviral activity of interferon against hepatitis A virus (HAV) replication. (A) Zinc chloride (ZnCl2), (B) zinc sulfate (ZnSO4). Drugs were added 24h post infection with 0.01 MOI HAV genotype IIIA HA11-1299. HAV RNA was measured by real-time reverse transcription-polymerase chain reaction. IFN: Interferon. Data are expressed as means±standard deviations of triplicate determinations from 3 independent experiments.
Suppression of HAV replication was stronger with the combination of 0.05 μg/ml interferon-alpha-2a plus 5 μM zinc chloride than with 0.05 μg/ml interferon-alpha-2a alone [25.2±1.2 (%) vs. 20.2±1.9 (%); n=3, p<0.05] (Figure 4A). Suppression of HAV replication tended to be stronger with the combination of 0.05 μg/ml interferon-alpha-2a plus 5 μM zinc sulfate than with 0.05 μg/ml interferon-alpha-2a alone [25.2±1.2 (%) vs. 16.5±5.9 (%); n=3, p=0.067] (Figure 4B). Thus, we demonstrated that zinc chloride could enhance the inhibitory effect of interferon on HAV replication.
Zinc chloride affects inflammasome associated signaling pathways in Huh7 cells infected with HAV. It has been reported that nucleotide organization domain (NOD)-like receptors (NLRs) can play a role in the host response to HAV infection (15,16). Therefore, we examined the effects of 5 μM zinc chloride on the replication of HAV genotype IIIA HA11-1299. We infected Huh7 cells with 0.1 MOI HAV and added 5 μM zinc chloride at the same time. After 120 h of HAV infection and treatment with or without 5 μM zinc chloride, cellular RNA was extracted, and HAV RNA was measured by real-time RT-PCR. HAV RNA levels were reduced to 75% in zinc chloride-treated Huh7 cells, compared with those in zinc chloride-untreated Huh7 cells.
It has recently been reported that chaperon-mediated autophagy (CMA) is involved in hepatic immunity and liver diseases, including acute-on-chronic liver failure (17-19). Therefore, we examined the effects of zinc chloride on human hepatic inflammasome associated signaling pathways. We collected total RNAs from HAV-infected Huh7 cells that were treated with or without zinc chloride to examine the influence of zinc chloride on human inflammasome associated pathways by using a real-time RT-PCR-based array. Among 84 genes that were examined, one and six genes, respectively, were significantly up-regulated and down-regulated (2-fold more) by zinc chloride in HAV-infected Huh7 cells (Figure 5 and Table I). Among them, tumor necrosis factor (TNF) and interleukin 1 beta (IL1B) were up-regulated in inflammatory cells of the liver after 3 weeks of HAV infection (20). HAV has been shown to up-regulate TNF, leading to the induction of Th1 cytokines and cytotoxic T lymphocyte (CTL) response (21). TNF-producing regulatory T cells (Treg) have been associated with severe hepatitis A (22).
Figure 5. Clustergram of gene expression analysis of inflammasome associated signaling pathways in human hepatoma Huh7 cells treated with or without zinc chloride (Zn Cl2). C: Untreated control.
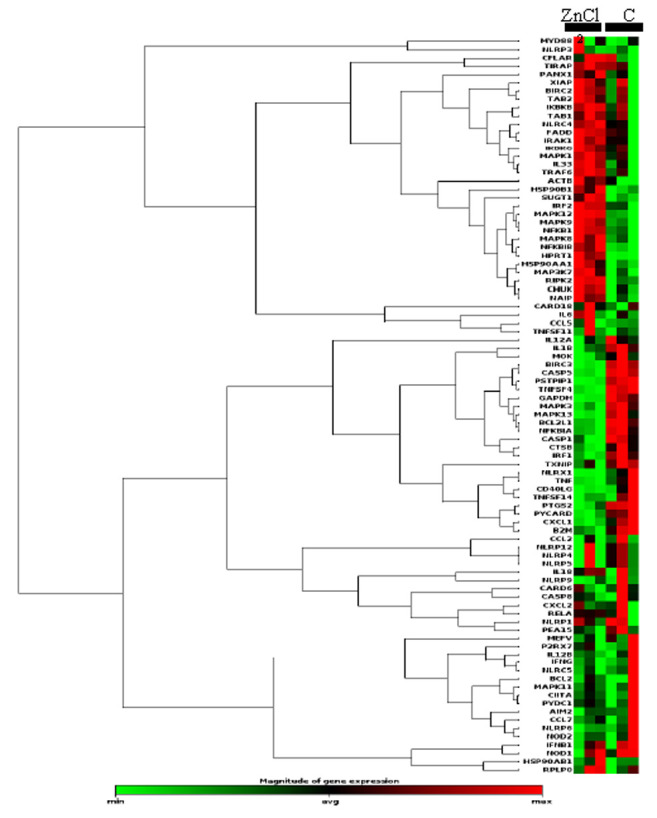
Table I. Significantly changed inflammasome associated gene expressions by zinc chloride treatment of Huh7 cells infected with hepatitis A virus.
MAPK12: Mitogen-activated protein kinase 12; BIRC3: baculoviral IAP repeat containing 3; IL1B: interleukin 1 beta; PSTPIP1: proline-serinethreonine phosphatase interacting protein 1; PTGS2: prostaglandinendoperoxidesynthase 2; PYCARD: PYD and CARD domain containing; TNF: tumor necrosis factor. Gene expression changes were considered statistically significant at p<0.05 and the difference of gene expression was more than 2-fold. Each experiment was performed at least in triplicate.
Discussion
In the present study, we demonstrated that both zinc sulfate and zinc chloride have an inhibitory effect on HAV replication. We also showed that zinc sulfate tended to enhance the anti-HAV effect induced by interferon-alpha-2a and that zinc chloride enhanced the anti-HAV effect induced by interferon-alpha-2a. Our results suggest that zinc chloride may affect the inflammasome associated signaling pathway in hepatocytes.
Activation of the interferon pathway promotes the transcriptional induction of many interferon-stimulated genes (ISGs), resulting in antiviral, anticancer and immunostimulatory status (23). Zinc chloride has been shown to up-regulate interferon alpha/beta receptor 2 mRNA levels (10), and to induce 2-5A synthetase (24). It has also been shown to regulate double-stranded RNA-activated protein kinase (PKR) (25) and to inhibit TNF-mediated apoptosis through heat shock protein 70 (26). We also demonstrated that zinc chloride inhibits the expression of TNF mRNA in Huh7 cells (Table I). Zinc chloride has also been shown to induce interferon-gamma-inducing factors (27). In the present study, zinc chloride induced interferon regulatory factor 2 (IRF2) (1.35-fold, p=0.005459), compared with untreated Huh7 control. Thus, there may be an association between zinc chloride and activation of interferon signaling pathways.
Zinc compounds may have potential therapeutic effects on patients infected with picornaviruses, including HAV (9,28), other positive sense RNA viruses, such as Sindbis virus, human immunodeficiency virus (HIV) and hepatitis E virus (HEV) (13,29,30), and DNA viruses, such as vaccinia virus (31). Among picornaviruses, zinc chloride has been shown to inhibit the replication of rhinovirus 2 (28) and destabilize poliovirus capsid (32). Zinc chloride has also been shown to inhibit HIV reverse transcriptase and HEV RNA-dependent RNA polymerase (RdRp) (13,30). These antiviral effects of zinc chloride may be through direct antiviral mechanisms or indirect mechanisms through the interferon system.
Wei et al. have reported that zinc compounds do not interfere with transmissible gastroenteritis virus (TGEV) cell binding but that zinc compounds mediate antiviral effects through inhibition of viral entry or egress or the intracellular phase of the viral life-cycle (33). In vitro experiments have demonstrated that Zn2+ possesses antiviral activity through inhibition of SARS-CoV-2 RNA polymerase (34). As HAV also infects humans through the fecal-oral route, zinc may prevent HAV infection (9).
In general, human blood concentration of zinc is 80-130 μg/dl and zinc and zinc acetate are prescribed at concentrations of 34 mg daily and 150-250 mg daily, respectively, for zinc deficiency (7,35). We have previously reported that 50-100 μM zinc sulfate only inhibited HAV replication in a dose-dependent manner, and 500-1000 μM zinc sulfate treatment for 48 h reduced viability of Huh7-derived cell lines (9). In the present study, to examine the effects of zinc compounds on antiviral activities against HAV by interferon-alpha-2a, we used lower concentrations of zinc chloride or zinc sulfate at ~20 μM or ~20 μM, respectively.
However, human blood concentration of interferon-alpha is 10 pg/ml and pegylated interferon-alpha-2a are prescribed at concentrations of 90-180 μg/body weekly, for chronic hepatitis B (36). In the present study, to examine the effects of zinc chloride on antiviral activities of interferon-alpha-2a against HAV, we used 0.05 μg/ml interferon-alpha-2a in cell culture medium for 96 h (Figure 2).
We also examined the effects of 0.05 μg/ml interferon-alpha-2a with or without 5 μM zinc chloride on the replication of HAV genotype IIIA HA11-1299 after serial passages in Huh7 cells. In this experiment, we infected Huh7 cells with 0.1 MOI HAV and added the drugs at the same time. Serial passages were performed as previously described (37). After 3 serial passages, HAV RNA disappeared from Huh7 cells treated with interferon-alpha-2a alone or in combination.
It has been reported that the supernatant of Huh7-derived cell line Huh-7.5 cells infected with noncytopathic HAV HM175/p16 contain two populations of virus particles: membrane-cloaked quasi-enveloped virus (eHAV) and non-enveloped virus (38). HAV cellular receptor 1 (HAVcr-1), integrinβ and gangliosides have been candidates as HAV cell surface or endosomal receptors (39,40). One of the limitations of our study is that we did not examine the effect of zinc chloride on HAV RNA levels in conditioned medium. Further studies are needed in this regard. The other limitation of our study is that FCS contained zinc in the present study although we used zinc-free medium.
Interferon and broad-spectrum non-specific antiviral agents have been shown to be effective for the inhibition of HAV replication (14). In this study, we showed that zinc chloride inhibited HAV replication and enhanced the antiviral activity induced by interferon. Zinc chloride may affect the inflammasome-associated signaling pathway in hepatocytes. Further studies are needed in order to explore the effect of zinc chloride on inflammasome-associated signaling.
Conflicts of Interest
No competing interests exist in relation to this study.
Authors’ Contributions
T.K., R.S. and R.M. designed, and T.K. and R.S. performed the experiments, analyzed data and performed statistical analyses. H.T, F.M, N.M., H.O. and M.M. commented on the article. T.K. and R.S. conceived the study. T.K., R.S. and R.M. wrote the article. All Authors read and approved the final article.
Acknowledgements
This work was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Numbers JP19fk0210043 (T.K., H.O.) and JP20fk0210075 (T.K.).
References
- 1.Abutaleb A, Kottilil S. Hepatitis A: Epidemiology, natural history, unusual clinical manifestations, and prevention. Gastroenterol Clin North Am. 2020;49(2):191–199. doi: 10.1016/j.gtc.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khounvisith V, Xaiyaphet X, Chanthavilay P, Nouanthong P, Vongphachanh B, Reinharz D, Muller CP, Black AP. Hepatitis A virus in Lao People’s Democratic Republic: Seroprevalence and risk factors. Am J Trop Med Hyg. 2020;103(1):164–168. doi: 10.4269/ajtmh.19-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallian P, Barlet V, Mouna L, Gross S, Morel P, Le Cam S, Ricard C, Maugard C, Pouchol E, Flan B, Visse C, Djoudi R, Figoni J, De Valk H, Tiberghien P, Roque-Afonso AM. Persisting higher prevalence of hepatitis A virus RNA in blood donors, France, 2018. Euro Surveill. 2019;24(47):1900685. doi: 10.2807/1560-7917.ES.2019.24.47.1900695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koga M, Lim LA, Ogishi M, Satoh H, Kikuchi T, Adachi E, Sugiyama R, Kiyohara T, Suzuki R, Muramatsu M, Koibuchi T, Tsutsumi T, Yotsuyanagi H. Comparison of the clinical features of hepatitis A in people living with HIV between pandemics in 1999-2000 and 2017-2018 in the Metropolitan area of Japan. Jpn J Infect Dis. 2020;73(2):89–95. doi: 10.7883/yoken.JJID.2019.275. [DOI] [PubMed] [Google Scholar]
- 5.Lal BB, Sood V, Snehavardhan P, Khanna R, Pasupuleti SSR, Siloliya M, Kumar G, Alam S. A novel, bedside, etiology specific prognostic model (Peds-HAV) in Hepatitis A induced pediatric acute liver failure. Hepatol Int. 2020;4(4):483–490. doi: 10.1007/s12072-020-10050-0. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal R, Goel A. Hepatitis A: Epidemiology in resource-poor countries. Curr Opin Infect Dis. 2015;28(5):488–496. doi: 10.1097/QCO.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 7.Kodama H, Tanaka M, Naito Y, Katayama K, Moriyama M. Japan’s practical guidelines for zinc deficiency with a particular focus on taste disorders, inflammatory bowel disease, and liver cirrhosis. Int J Mol Sci. 2020;21(8):2941. doi: 10.3390/ijms21082941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa M, Kanda T, Suganami A, Nakamoto S, Win NN, Tamura Y, Nakamura M, Matsuoka S, Yokosuka O, Kato N, Ohara O, Okamoto H, Moriyama M, Shirasawa H. Antiviral activity of zinc sulfate against hepatitis A virus replication. Future Virol. 2019;14(6):399–406. doi: 10.2217/fvl-2019-0031. [DOI] [Google Scholar]
- 10.Nagamine T, Nakajima K, Takada H, Sekine Y, Suzuki K. Induction of type 1 interferon receptor by zinc in U937 cells. Cytokine. 2009;46(3):346–350. doi: 10.1016/j.cyto.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Jiang X, Kanda T, Wu S, Nakamoto S, Saito K, Shirasawa H, Kiyohara T, Ishii K, Wakita T, Okamoto H, Yokosuka O. Suppression of La antigen exerts potential antiviral effects against hepatitis A virus. PLoS One. 2014;9(7):e101993. doi: 10.1371/journal.pone.0101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu S, Kanda T, Imazeki F, Nakamoto S, Tanaka T, Arai M, Roger T, Shirasawa H, Nomura F, Yokosuka O. Hepatitis B virus e antigen physically associates with receptor-interacting serine/threonine protein kinase 2 and regulates IL-6 gene expression. J Infect Dis. 2012;206(3):415–420. doi: 10.1093/infdis/jis363. [DOI] [PubMed] [Google Scholar]
- 13.Kaushik N, Subramani C, Anang S, Muthumohan R, Shalimar, Nayak B, Ranjith-Kumar CT, Surjit M. Zinc salts block Hepatitis E virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase. J Virol. 2017;91(21):e00754-17. doi: 10.1128/JVI.00754-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, Kiyohara T, Kanda T, Imazeki F, Fujiwara K, Gauss-Müller V, Ishii K, Wakita T, Yokosuka O. Inhibitory effects on HAV IRES-mediated translation and replication by a combination of amantadine and interferon-alpha. Virol J. 2010;7:212. doi: 10.1186/1743-422X-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanda T, Sasaki R, Masuzaki R, Takahashi H, Mizutani T, Matsumoto N, Nirei K, Moriyama M. Co-occurrence of hepatitis A infection and chronic liver disease. Int J Mol Sci. 2020;21(17):6384. doi: 10.3390/ijms21176384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Z, Lemon SM. Innate immunity to enteric hepatitis viruses. Cold Spring Harb Perspect Med. 2019;9(3):a033464. doi: 10.1101/cshperspect.a033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angelini G, Castagneto Gissey L, Del Corpo G, Giordano C, Cerbelli B, Severino A, Manco M, Basso N, Birkenfeld AL, Bornstein SR, Genco A, Mingrone G, Casella G. New insight into the mechanisms of ectopic fat deposition improvement after bariatric surgery. Sci Rep. 2019;9(1):17315. doi: 10.1038/s41598-019-53702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dash S, Aydin Y, Moroz K. Chaperone-mediated autophagy in the liver: Good or bad. Cells. 2019;8(11):1308. doi: 10.3390/cells8111308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You Y, Li WZ, Zhang S, Hu B, Li YX, Li HD, Tang HH, Li QW, Guan YY, Liu LX, Bao WL, Shen X. SNX10 mediates alcohol-induced liver injury and steatosis by regulating the activation of chaperone-mediated autophagy. J Hepatol. 2018;69(1):129–141. doi: 10.1016/j.jhep.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Polotsky YE, Vassell RA, Binn LN, Asher LV. Immuno-histochemical detection of cytokines in tissues of Aotus monkeys infected with hepatitis A virus. Ann N Y Acad Sci. 2019;730:318–321. doi: 10.1111/j.1749-6632.1994.tb44279.x. [DOI] [PubMed] [Google Scholar]
- 21.Locarnini S. A virological perspective on the need for vaccination. J Viral Hepat. 2000;7 (Suppl 1):5–6. doi: 10.1046/j.1365-2893.2000.00020.x. [DOI] [PubMed] [Google Scholar]
- 22.Choi YS, Jung MK, Lee J, Choi SJ, Choi SH, Lee HW, Lee JJ, Kim HJ, Ahn SH, Lee DH, Kim W, Park SH, Huh JR, Kim HP, Park JY, Shin EC. Tumor necrosis factor-producing T-regulatory cells are associated with severe liver injury in patients with acute hepatitis A. Gastroenterology. 2018;154(4):1047–1060. doi: 10.1053/j.gastro.2017.11.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somerville TDD, Xu Y, Wu XS, Maia-Silva D, Hur SK, de Almeida LMN, Preall JB, Koo PK, Vakoc CR. ZBED2 is an antagonist of interferon regulatory factor 1 and modifies cell identity in pancreatic cancer. Proc Natl Acad Sci USA. 2020;117(21):11471–11482. doi: 10.1073/pnas.1921484117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salzberg S, Hyman T, Turm H, Kinar Y, Schwartz Y, Nir U, Lejbkowicz F, Huberman E. Ectopic expression of 2-5A synthetase in myeloid cells induces growth arrest and facilitates the appearance of a myeloid differentiation marker. Cancer Res. 1997;57(13):2732–2740. [PubMed] [Google Scholar]
- 25.Kronfeld-Kinar Y, Vilchik S, Hyman T, Leibkowicz F, Salzberg S. Involvement of PKR in the regulation of myogenesis. Cell Growth Differ. 1999;10(3):201–212. [PubMed] [Google Scholar]
- 26.Van Molle W, Van Roy M, Van Bogaert T, Dejager L, Van Lint P, Vanlaere I, Sekikawa K, Kollias G, Libert C. Protection of zinc against tumor necrosis factor induced lethal inflammation depends on heat shock protein 70 and allows safe antitumor therapy. Cancer Res. 2007;67(15):7301–7307. doi: 10.1158/0008-5472.CAN-06-4010. [DOI] [PubMed] [Google Scholar]
- 27.Poleganov MA, Pfeilschifter J, Mühl H. Expanding extracellular zinc beyond levels reflecting the albumin-bound plasma zinc pool potentiates the capability of IL-1beta, IL-18, and IL-12 to act as IFN-gamma-inducing factors on PBMC. J Interferon Cytokine Res. 2007;27(12):997–1001. doi: 10.1089/jir.2007.0037. [DOI] [PubMed] [Google Scholar]
- 28.Merluzzi VJ, Cipriano D, McNeil D, Fuchs V, Supeau C, Rosenthal AS, Skiles JW. Evaluation of zinc complexes on the replication of rhinovirus 2 in vitro. Res Commun Chem Pathol Pharmacol. 1989;66(3):425–440. [PubMed] [Google Scholar]
- 29.Bracha M, Schlesinger MJ. Inhibition of sindbis virus replication by zinc ions. Virology. 1976;72(1):272–277. doi: 10.1016/0042-6822(76)90330-5. [DOI] [PubMed] [Google Scholar]
- 30.Fenstermacher KJ, DeStefano JJ. Mechanism of HIV reverse transcriptase inhibition by zinc: formation of a highly stable enzyme-(Primer-Template) complex with profoundly diminished catalytic activity. J Biol Chem. 2011;286(47):40433–40442. doi: 10.1074/jbc.M111.289850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz E, Margalith E. Inhibition of vaccinia virus maturation by zinc chloride. Antimicrob Agents Chemother. 1981;19(2):213–217. doi: 10.1128/aac.19.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratka M, Lackmann M, Ueckermann C, Karlins U, Koch G. Poliovirus-associated protein kinase: destabilization of the virus capsid and stimulation of the phosphorylation reaction by Zn2+ J Virol. 1989;63(9):3954–3960. doi: 10.1128/jvi.63.9.3954-3960.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei Z, Burwinkel M, Palissa C, Ephraim E, Schmidt MF. Antiviral activity of zinc salts against transmissible gastroenteritis virus in vitro. Vet Microbiol. 2012;160(3-4):468–472. doi: 10.1016/j.vetmic.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, Svistunov AA, Petrakis D, Spandidos DA, Aaseth J, Tsatsakis A, Tinkov AA. Zinc and respiratory tract infections: perspectives for COVID-19 (Review) Int J Mol Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moriyama M, Matsumura H, Fukushima A, Ohkido K, Arakawa Y, Nirei K, Yamagami H, Kaneko M, Tanaka N, Arakawa Y. Clinical significance of evaluation of serum zinc concentrations in C-viral chronic liver disease. Dig Dis Sci. 2006;51(11):1967–1977. doi: 10.1007/s10620-005-9051-7. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto A, Nishiguchi S, Enomoto H, Tanaka Y, Shinkai N, Okuse C, Kang JH, Matsui T, Miyase S, Yatsuhashi H, Nagaoka S, Kanda T, Enomoto M, Yamada R, Hiramatsu N, Saito S, Takaguchi K, Ito K, Masaki T, Morihara D, Tsuge M, Chayama K, Ikeda F, Kagawa T, Kondo Y, Murata K, Tanaka E. Pilot study of tenofovir disoproxil fumarate and pegylated interferonalpha 2a add-on therapy in Japanese patients with chronic hepatitis. B. J Gastroenterol. 2020 doi: 10.1007/s00535-020-01707-6. [DOI] [PubMed] [Google Scholar]
- 37.Ait-Goughoulte M, Kanda T, Meyer K, Ryerse JS, Ray RB, Ray R. Hepatitis C virus genotype 1a growth and induction of autophagy. J Virol. 2008;82(5):2241–2249. doi: 10.1128/JVI.02093-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, Jeong SH, Walker C, Lanford RE, Lemon SM. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496(7445):367–371. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das A, Barrientos R, Shiota T, Madigan V, Misumi I, McKnight KL, Sun L, Li Z, Meganck RM, Li Y, Kaluzna E, Asokan A, Whitmire JK, Kapustina M, Zhang Q, Lemon SM. Gangliosides are essential endosomal receptors for quasi-enveloped and naked hepatitis A virus. Nat Microbiol. 2020;5(9):1069–1078. doi: 10.1038/s41564-020-0727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanda T, Sasaki R, Masuzaki R, Matsumoto N, Ogawa M, Moriyama M. Cell culture systems and drug targets for hepatitis A virus infection. Viruses. 2020;12(5):533. doi: 10.3390/v12050533. [DOI] [PMC free article] [PubMed] [Google Scholar]