Abstract
Background/Aim: Periostin (POSTN) has a significant role in proliferation and migration of tumour cells as well as tumour progression. This study aimed to determinate POSTN expression in cancer cells in malignant and benign tumours of the mammary gland in female dogs. Materials and Methods: All together 83 cancers, 24 adenomas and 7 unchanged fragments of the mammary glands of bitches were investigated. Immunohistochemistry was performed using anti-POSTN, Ki-67 and HER2 antibodies. Results: POSTN expression was observed in cancer cells in 31.3% of malignancies and 12.5% of benign tumours. A significantly positive correlation between expression of POSTN in cancer cells and the degree of histological malignancy, expression of Ki-67 antigen and expression of POSTN in CAFs was found. Conclusion: The obtained results suggest the possible participation of POSTN in the process of carcinogenesis and progression of mammary tumors in bitches.
Keywords: Periostin, carcinoma, adenoma, bitches, dog
Periostin (POSTN) formerly known as “osteoblast-specific factor-2” was first discovered in the mouse osteoblast cell line (mouse osteoblastic MC3T3-E1) in 1993 (1). POSTN is a 93-kDa glycoprotein composed of 836 amino acids and produced by both osteoblasts and mesenchymal stromal cells located in the bone marrow and fibroblasts belonging to extracellular matrix proteins (ECM) (1-3). In POSTN we distinguish the N-terminal signal sequence, a domain with a high cysteine content called Emilin (EMI) and four Fasciclin domains (FAS1) (4-6). The FAS1 domain consists of 150 amino acids and acts as an integrin of the cell membrane. In the POSTN structure, there is also a C-terminal hydrophilic domain (CRT) which mainly performs the regulatory function of integrin-binding (3,7-9). POSTN due to its structure has the ability to bind to many integrin receptors, including αvβ3, αvβ5, α6β4, affecting the regulation of intracellular signaling pathways involving protein kinases PI3-K/Akt and FAK (focal adhesion kinase) (8,10). Activation of these pathways plays a significant role in the process of carcinogenesis, by increasing cell migration, intensifying angiogenesis and invasion of cancer cells (8,10). The result of these processes is an increase in the metastatic potential of the tumour and its increased growth. It has also been shown that POSTN participates in the regulation of mechanisms associated with the process of epithelial-mesenchymal transition (EMT) and remodelling of the ECM, thanks to which it plays an important role in oncogenesis (8,10).
POSTN is involved in many physiological and pathological processes. Among other things, POSTN plays an important role in wound healing and collagen production (8,11). Expression of the protein in question occurs mainly in fibrous connective tissue with high collagen content, among others in periodontal ligaments, periosteum, cornea and heart valves, as well as in many other organs, including the lungs, skin, thyroid, ovaries, placenta and pituitary gland (2,3,6,8). POSTN’s participation in carcinogenesis processes has been described in numerous studies (6,8,9,12-14). Increased expression of this protein has been found in various types of human cancers, including breast cancer, ovarian cancer, lung cancer, colorectal cancer and pancreatic cancer (6,8,9,12-14). In invasive ductal breast carcinomas (IDCs) in women, POSTN expression was observed in both tumour cells and cancer-associated fibroblasts (CAFs) (8,9,12-14).
Mammary gland tumours are common tumours found in bitches and their incidence is 3 times higher compared to breast tumours found in women (15-20). In male dogs, mammary gland cancers represent only 1% of all diagnosed cancers (15). Statistics show that the majority of oncological changes in the mammary gland in bitches are classified as malignant forms with epithelial origin - cancers, which constitute about 70% of all diagnosed tumours in female dogs in Lower Silesia (Poland) (16,17). Benign forms of breast cancer are much less common (16,17). Metastasis of mammary gland cancers are most commonly seen in nearby lymph nodes and lungs (15-17,21,22). Many authors show similarity in the pathogenesis of breast cancer in women and breast cancer in female dogs, including hormonal aetiology, age of onset, course of disease and status of metastases. Therefore, many researchers have suggested a dog’s mammary gland as a good model for researching breast cancer in women (19,20,23,24).
This work aimed to analyze POSTN expression in cancer cells in mammary carcinomas and adenomas in bitches and assess the relationship between clinical and pathological factors, i.e. age, race and histological grade of the tumour (G). In addition, the correlation of POSTN expression in cancer cells was assessed in relation to the expression of other cell markers associated with the carcinogenesis process, i.e. Ki-67 and human epidermal growth factor receptor 2 (HER2).
Materials and Methods
Patients and tumours. The study included 107 cases of mammary tumors (83 cancers and 24 adenomas) taken from bitches of different breeds aged from 7 to 14 years who underwent mastectomy procedures performed in the University Environmental and Life Sciences in Wrocław in 2017-2019. A group of adenomas has been used in our previous studies (25). In addition, 7 normal mammary glands were posthumously collected from healthy female dogs that suffered traffic accidents. Haematoxylin and eosin (H&E) staining. Tumour sections were fixed for 24 h in 4% buffered formalin. The material was dehydrated and embedded in paraffin blocks and cut into 4 μm thick sections. The material was stained using the H&E method and IHC reactions were performed on it. To assess the histological type according to Goldschmidt et al. (26) was used, while the histological grade (G-grading) of tumours was determined according to the Pena et al. scale taking into account the number of coils formed, testicular pleomorphism and mitotic index (27).
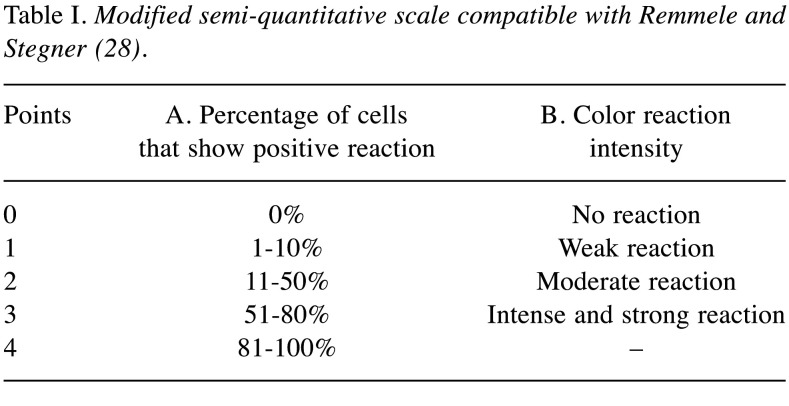
IHC and IHC assessment. For the immunohistochemical reactions the following antibodies were used: polyclonal rabbit antibodies: Periostin, HER-2 and monoclonal mouse antibodies: Ki-67 clone MIB-1. Expression of the tested markers was assessed using an Olympus BX53 light microscope at 400x magnification. The degree of POSTN expression in cancer cells and CAFs was assessed on the basis of a modified “semi-quantitative” scale (IRS-immunoreactive score) according to Remmele and Stegner (Table I). The assessment took into account both the percentage of cells showing a positive response (A) and the intensity of the IHC reaction (B). The final result is the product of both results obtained (A * B) and its value ranges from 1 to 12 points. Rated according to the following scale: no reaction=0 points (–); weak reaction intensity=1-2 points (+); moderate reaction intensity=3-4 points (++); strong reaction intensity=6-12 points (+++) (28). Ki-67 expression was analyzed on the basis of a scale including the percentage of positive stained nuclei of cancer cells to all cancer cells in 5 randomly selected fields of view: 0-5% no reaction (–), 6-25% weak reaction (+), 26-50% average response (++), above 50% cells intensive reaction (+++) (25). The severity of HER2 receptor expression was assessed using a scale that takes into account the percentage of cancer cells showing a positive membrane response to all cancer cells (29). Detailed description of the IHC methods and IHC assessment were featured in our previous work (25).
Table I. Modified semi-quantitative scale compatible with Remmele and Stegner (28).
Statistical analysis. Statistical analysis was performed using the Statistica 12.0 software (StatSoft, Krakow, Poland). Data normality was tested using the W Shapiro-Wilk test. The correlation was assessed using the Spearman correlation test. Differences between the results in tumours of varying grade and between adenomas and cancers were compared using the Kruskal-Wallis test and the Mann-Whitney U-test. Significance level was assumed for p<0.05.
Ethical approval. According to the Polish law, standard diagnostic procedures and studies conducted on animal tissue and archival material do not require permission from the Ethical Board.
Results
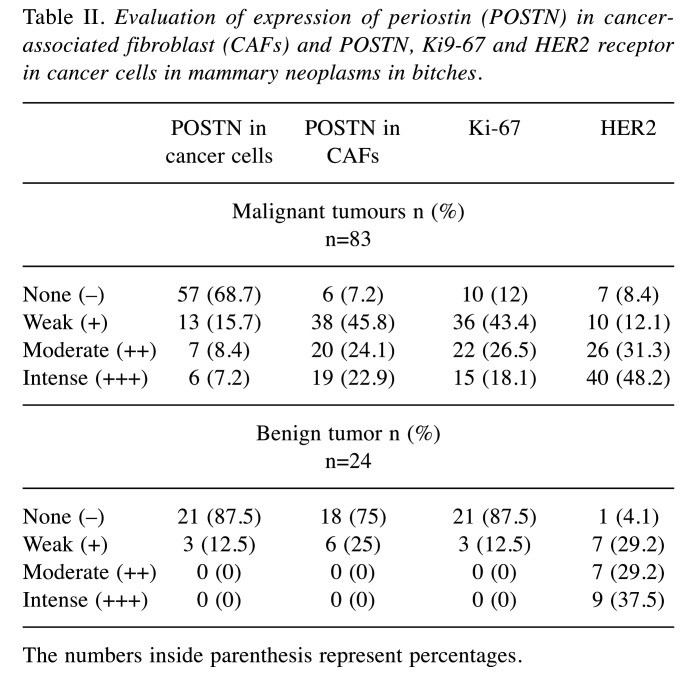
The average age of all patients was approximately 9 years. There were no statistically significant correlations between the expression of POSTN in cancer cells and the age and breed of dogs. Using IHC studies, the expression of POSTN, Ki-67 and HER2 in tumour cells was assessed in malignant and benign lesions of the milk line in bitches (Table II). POSTN expression was observed in the tumour cell cytoplasm in 31.3% of cases (n=26) cancers, of which 7.2% of changes (n=6) were characterized by high intensity of POSTN expression at the level of 6-12 points on the Remmele scale, 8, 4% of changes (n=7) with medium expression intensity, i.e. 3-5 points, and 15.7% of changes (n=13), low expression intensity, i.e. 1-2 points. (Table II, Figure 1).
Table II. Evaluation of expression of periostin (POSTN) in cancerassociated fibroblast (CAFs) and POSTN, Ki9-67 and HER2 receptor in cancer cells in mammary neoplasms in bitches.
The numbers inside parenthesis represent percentages.
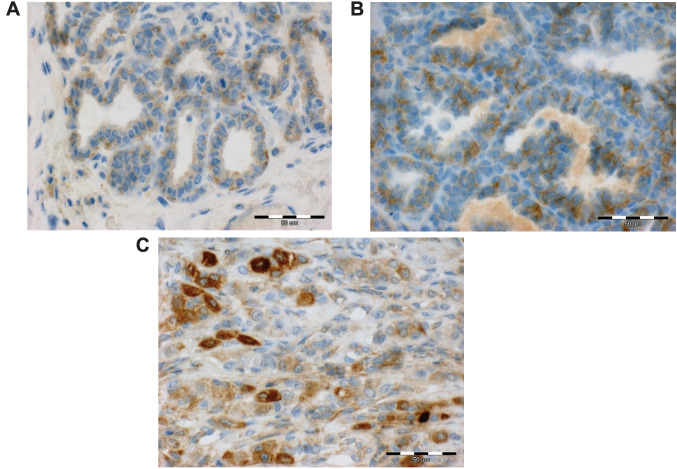
Figure 1. Expression of periostin (POSTN) in the cytoplasm of cancer cells in mammary cancers in female dogs. (A) Weak POSTN reaction in cancer cells in simple adenoma. (B) Moderate POSTN reaction in cancer cells in simple tubulopapillary carcinoma. (C) Intense reaction in cancer cells in solid carcinoma. Scale bar=50 μm.
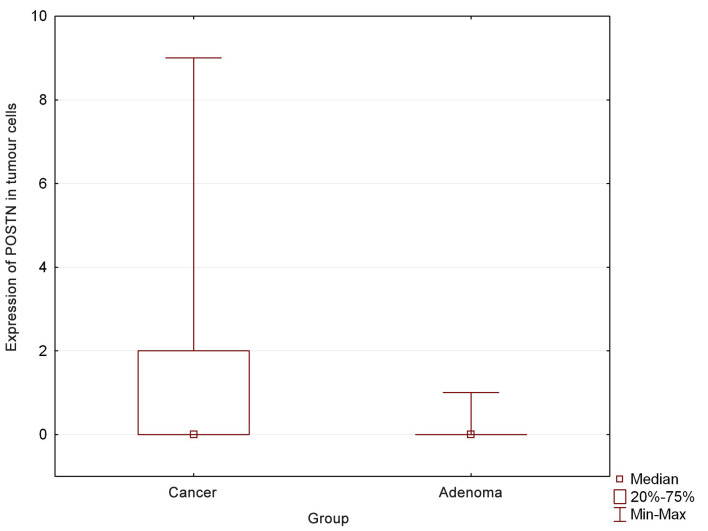
In tumour cell, POSTN expression was also observed in 12.5% of benign tumours (n=3), of which all tumours showed low POSTN expression levels (1 Remmele scale) (Table III) (Figure 1). Statistically significant differences were found in POSTN expression levels in cancer cells compared to adenomas (p=0.04) (Figure 2). There was no expression of POSTN in the cells in normal mammary gland tissue.
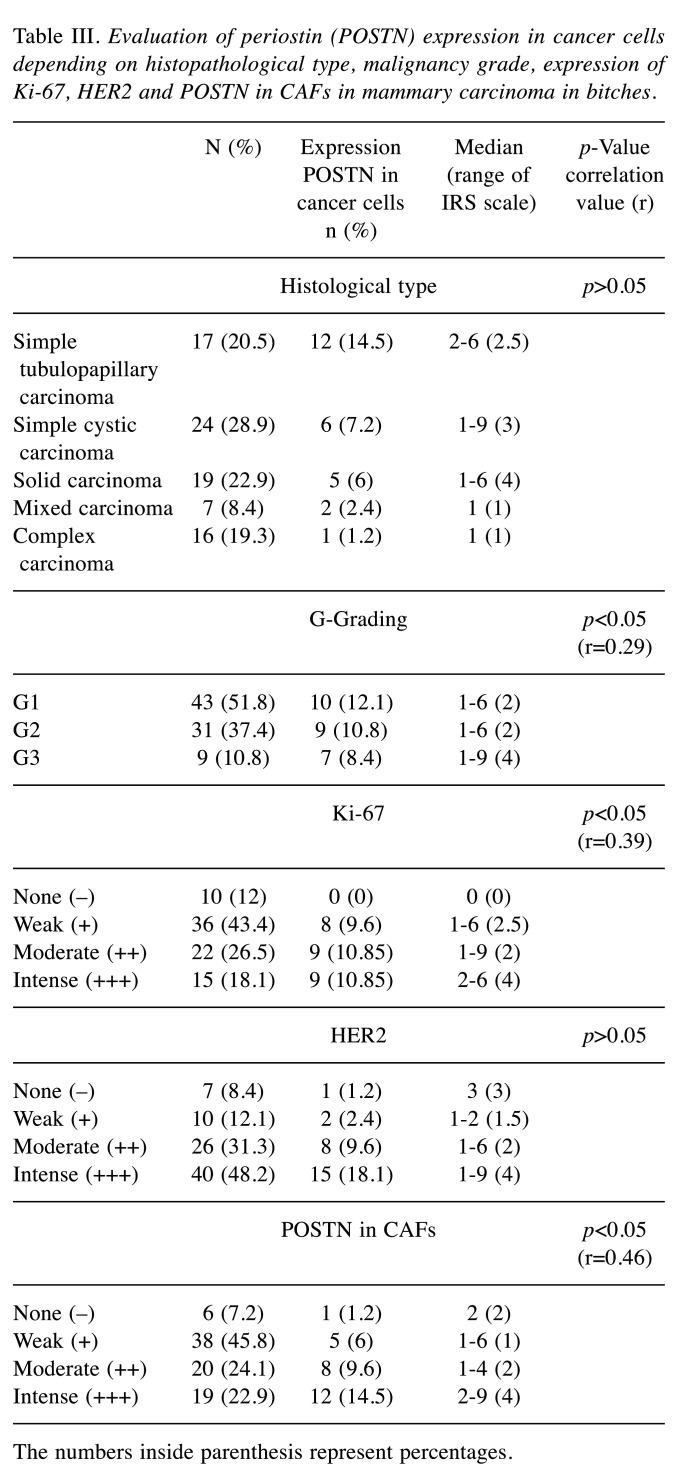
Table III. Evaluation of periostin (POSTN) expression in cancer cells depending on histopathological type, malignancy grade, expression of Ki-67, HER2 and POSTN in CAFs in mammary carcinoma in bitches.
The numbers inside parenthesis represent percentages.
Figure 2. Periostin (POSTN) expression in cancer cells was higher in mammary cancers compared to adenomas in female dogs.
Tested tumours were assessed using H&E staining in accordance with WHO guidelines, and the results are shown in Table III. The highest levels of POSTN expression in the cytoplasm of tumour cells were found in the following types of cancer: simple tubular-papillary carcinoma 14.5% (n=12); followed by simple cystic cancer 7.2% (n=6), solid cancer 6% (n=5); mixed cancer 2.4% (n=2); 1.2% complex cancer (n=1) among all tumours examined (Table III).
In the group of malignant tumours, the grade of histological malignancy (G-Grading) was assessed, and the results are presented in Table III. POSTN expression in the cytoplasm of tumour cells was found in 12.1% of cancers (n=10) in histological grade 1 (G1), in 10, 8% of cases (n=9) with histological grade 2 (G2) and in 8.4% of cases (n=7) with histological grade 3 (G3) (Table III). Statistical analysis showed a positive correlation between the expression of POSTN in cancer cells and tumour histological grade (p<0.05; r=0.29). In addition, higher POSTN expression was observed in tumour cells in G3 cancers compared to G2 (p=0.02), as well as in G3 cancers relative to G1 (p=0.008).
The relationship between POSTN expression in cancer cells and expression of Ki-67 antigen and HER2 receptor is presented in Table III. Statistical analysis showed a positive correlation between expression of POSTN and Ki-67 in cancer cells (p<0.05; r=0.39). There was no correlation between POSTN expression and HER2 receptor expression in cancer cells.
Expression of POSTN was also found in the tumour stroma in CAFs in 92.7% of cancers and at a lower level in 25% of adenomas in bitches. Expression of the test protein in both CAFs and tumour cells was observed in cancers. Only one of the cases of malignant tumours examined that showed POSTN expression in tumour cells did not express POSTN in CAFs (Table III). In addition, a positive correlation was found between POSTN expression in cancer cells and POSTN expression in CAFs for both the group of malignant neoplasms (p<0.05; r=0.46) and for the group of benign neoplasms (p<0.05; r=0.45).
Discussion
Extracellular matrix proteins are a large group of proteins, including POSTN (10,30). These proteins increase angiogenesis, cell proliferation, increase cell migration and increase tumour metastatic potential. POSTN as an ECM protein may play a similar role in oncogenesis processes in both humans and animals. Literature analysis indicates the role of POSTN as a potential prognostic factor in many types of cancers in humans, including breast cancer in women (6,8,9,12).
High POSTN expression is mainly observed in CAFs, and to a lesser extent in cancer cells, including breast cancer in women (12,14). On the other hand, there are also studies in which expression of POSTN is described only in tumour cells, while there is no information regarding its expression in the stroma. The literature describes different levels of intensity of POSTN expression in cancer cells of breast tumours in women (8,9,12-14). For example, in studies of breast cancer in women, Kim et al. (14) observed intensified expression of POSTN in cancer cells in 57.7% of cases, similarly Puglisi et al. (31) in 57% of cases and Ratajczak-Wielgomas et al. (13) in 50% of cases. Nuzzo et al. (12) in 40% of cancers and Xu et al. (32) in 30% of breast cancers in women recorded slightly lower expression. Similarly to the results presented above, in our studies expression of POSTN in cancer cells was found in 31.3% of breast cancers, i.e. at a similar level compared to breast cancer in women. In our studies, POSTN expression levels in cancer cells (31.3%) were at a lower level in relation to POSTN expression in the stroma (92.7%) in breast cancer in female dogs. In our previous study, POSTN expression in the stroma was found in 92.2% of breast cancers (25). In addition, it is worth noting that POSTN expression is not found in normal breast tissue in women (6,30,33). We did not observe POSTN expression in normal mammary gland tissue from bitches in our studies. This result indicates that POSTN may be involved in the process of carcinogenesis in both cancer cells and stromal cells in malignant tumours.
Numerous interactions between tumour cells and the tumour microenvironment surrounding them have been described in the literature (34-36). Thus, cancer can both transform its microenvironment and can respond to factors produced by this microenvironment. These interactions have been described as tumour host-reaction (34). In addition, the microenvironment can intensify the transformation of normal cells into cancer cells and modify elements of their stroma (35,36). Both tumour cells and stromal cells interact, affecting angiogenesis, proliferation and invasion of tumour cells, e.g. by remodeling the tumour microenvironment (35-37). One of the elements of the tumour stroma are cancer-associated fibroblast (CAFs) cells that play an important role in the process of carcinogenesis in humans (8,9,12). In our previous studies using IHC, podoplanin (PDPN) and POSTN expression in CAFs was found in mammary tumours in bitches (25,38). Morover POSTN expression at mRNA level was found in mammary tumours in female dogs (25,39,40). In addition, cancer-associated stroma (CAS) in dogs has many similarities to the human stromal tumour of the mammary gland and may have an important role in tumour transformation (24,39-41). Probably POSTN is a similarly regulated gene of CAS between human breast cancer and mammary cancer in bitches (39). An interesting observation in our research is the finding of a statistically significant positive correlation between the expression of POSTN in cancer cells and CAFs in both cancers and adenomas. Other authors also indicated the importance of reciprocal interactions between cancer cells and stromal weaving. Similar results were obtained in the studies of Nuzzo et al. who found a positive correlation between expression of POSTN in cancer cells and expression of POSTN in the stroma in breast cancer in women (12). These results indicate that POSTN may promote cancer cell invasion. This relationship may also indicate a similar function of the protein in question in the process of carcinogenesis of both cancer cells and the stroma itself. It can be assumed that both processes are connected with each other, may have some common mechanisms and run simultaneously. In our work, we focused on understanding the relationship between POSTN expression in cancer cells in breast cancer and factors that may indicate the stage of cancerous changes and poor prognosis for the patient, which allowed us to better understand the function of POSTN in breast carcinogenesis in female dogs.
Literature analysis showed significant differences in the levels of POSTN expression in cancer cells between the group of malignant and benign tumours in breast tumours in women. For example, in their study, Zhang et al. found high POSTN levels in breast cancer in women in relation to normal tissue of this gland (33). Ratajczak-Wielgomas et al. showed a significantly higher level of POSTN expression in the tumour cell cytoplasm in IDC (invasive ductal carcinoma) compared to pre-invasive cancers (DIDC) and breast dysplasia (FC-fibrocystic breast change) in women (13). Similarly, Kim et al. showed higher levels of the tested protein in cancer cells compared to pre-invasive types and normal breast tissue in women (14). Similarly, in our studies statistically significant differences in POSTN expression levels in malignant tumour cells were found compared to benign mammary tumours in female dogs. Higher levels of the tested protein in malignant lesions in both humans and animals indicate the potential role and involvement of POSTN in the process of neoplastic transformation of epithelial cells in breast cancers in women, as well as in breast cancer in female dogs.
In routine histopathological diagnostics, the degree of histological malignancy is determined as an indicator of the aggressiveness of the examined tumours. The literature indicates the relationship between POSTN expression and factors important in determining the aggressiveness of neoplastic changes, e.g. the degree of histological malignancy. Xu et al. obtained a positive correlation between the intensity of POSTN expression in cancer cells and the degree of histological malignancy in breast cancer in women (32). A similar relationship was observed in the studies of Ratajczak-Wielgomas et al. who found an increase in the level of POSTN expression in cancer cells along with an increase in the histological grade (G) in IDC (13). In addition, Xu et al. obtained a positive correlation between the expression of POSTN in cancer cells and the grade of tumour malignancy, metastases to lymph nodes and other organs, and the size of the tumour (32). Similarly to the reports described above, in our studies we also found a positive correlation between the expression of POSTN in cancer cells and the degree of histological malignancy. These results indicate that POSTN may increase metastatic potential and participate in the increase of the invasiveness of the cancer in question in both humans and female dogs.
In our work, we analyzed the correlation between the intensity of POSTN expression in cancer cells and other factors indicating tumour aggressiveness. One way to determine the degree of tumour aggressiveness is by assessing cell proliferation of the antigen Ki-67 protein. The Ki-67 protein is used in diagnostics of skeletal tumours, and its high nuclear expression is observed in malignant lesions, which may indicate unfavourable prognosis for the patient. In the performed analyses, we showed a statistically positive correlation between the expression of POSTN and the expression of cell proliferation antigen Ki-67 in cancer cells in mammary cancer. The presented results are consistent with studies indicating a positive relationship between POSTN expression in cancer cells and the intensity of Ki-67 expression in breast cancer in women (38). The results of our research indicate that POSTN may be associated with the progression and increase of invasion of mammary glands in bitches. Based on the obtained results, it can be assumed that POSTN may be, as in the case of breast cancer, an important prognostic factor (11,31,32,42).
To sum up, our research is the first to report an evaluation of POSTN expression in cancer cells in malignant tumours of female dogs. Neoplasms with higher aggressiveness were characterized by higher levels of the studied protein, which may indicate the role of POSTN in the process of carcinogenesis of mammary glands cells in bitches. Perhaps in the future the intensification of POSTN expression in breast cancer of female dogs will contribute to a better prognostic assessment in this type of disease.
Conflicts of Interest
The Authors declare that they have no competing interests.
Authors’ Contributions
Designed the study and conducted the experiments were accomplish by PB, RC, MN, AB. IHC and IHC evaluation were performed by PB, KRW, AP. The statistical analysis were performed by IJ. Writing and editing the paper – PB, MPO, PD, MN. All the Authors have approved the final manuscript.
Acknowledgements
The Authors would like to appreciate the staff of the Department of Pathology at the Wroclaw University of Environmental and Life Sciences and the Department of Histology and Embryology of Wroclaw Medical University.
References
- 1.Takeshita S, Kikuno R, Tezuka K, Amman E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294 (Pt1):271–278. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101(3):695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14(7):1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 4.Hoersch S, Andrade-Navarro MA. Periostin shows increased evolutionary plasticity in its alternatively spliced region. BMC Evol Biol. 2010;10:30. doi: 10.1186/1471-2148-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Field S, Uyttenhove C, Stroobant V, Cheou P, Donckers D, Coutelier JP, Simpson PT, Cummings MC, Saunus JM, Reid LE, Kutasovic JR, McNicol AM, Kim BR, Kim JH, Lakhani SR, Neville AM, Van Snick J, Jat PS. Novel highly specific anti-periostin antibodies uncover the functional importance of the fascilin 1-1 domain and highlight preferential expression of periostin in aggressive breast cancer. Int J Cancer. 2016;138(8):1959–1970. doi: 10.1002/ijc.29946. [DOI] [PubMed] [Google Scholar]
- 6.Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci. 2009;66(14):2219–2230. doi: 10.1007/s00018-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuzzo PV, Buzzatti G, Ricci F, Rubagotti A, Argellati F, Zinoli L, Boccardo F. Periostin: a novel prognostic and therapeutic target for genitourinary cancer. Clin Genitourin Cancer. 2014;12(5):301–311. doi: 10.1016/j.clgc.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Ratajczak-Wielgomas K, Dziegiel P. The role of periostin in neoplastic processes. Folia Histochem Cytobiol. 2015;53(2):120–132. doi: 10.5603/FHC.a2015.0014. [DOI] [PubMed] [Google Scholar]
- 9.Ratajczak-Wielgomas K, Grzegrzolka J, Piotrowska A, Gomulkiewicz A, Witkiewicz W, Dziegiel P. Periostin expression in cancer-associated fibroblasts of invasive ductal breast carcinoma. Oncol Rep. 2016;36(5):2745–2754. doi: 10.3892/or.2016.5095. [DOI] [PubMed] [Google Scholar]
- 10.Morra L, Moch H. Periostin expression and epithelial-mezenchymal transition in cancer: a review and an update. Virchows Arch. 2011;459:465–475. doi: 10.1007/s00428-011-1151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal. 2008;2(1-2):9–17. doi: 10.1007/s12079-008-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuzzo PV, Rubagotti A, Zinoli L, Salvi S, Boccardo S, Boccardo F. The prognostic value of stromal and epithelial periostin expression in human breast cancer: correlation with clinical pathological features and mortality outcome. BCM Cancer. 2016;16:95. doi: 10.1186/s12885-016-2139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratajczak-Wielgomas K, Grzegrzolka J, Piotrowska A, Matkowski R, Wojnar A, Rys J, Ugorski M, Dziegiel P. Expression of periostin in breast cancer cells. Int J Oncol. 2017;51(4):1300–1310. doi: 10.3892/ijo.2017.4109. [DOI] [PubMed] [Google Scholar]
- 14.Kim GE, Lee JS, Park MH, Yoon JH. Epithelial periostin expression is correlated with poor survival in patients with invasive breast carcinoma. Plos One. 2017;12(11):e0187635. doi: 10.1371/journal.pone.0187635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saba CF, Rogers KS, Newman SJ, Mauldin GE, Vail DM. Mammary gland tumors in male dogs. J Vet Intern Med. 2007;21(5):1056–1059. doi: 10.1892/0891-6640(2007)21[1056:mgtimd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Ciaputa R, Kandefer- Gola M, Nowak M, Madej JA. Prevalence of tumours in domestic animals in the lower silesia (Poland) in 2009-2011. Bull Vet Inst Pulawy. 2015;57(1):53–59. doi: 10.2478/bvip-2013-0010. [DOI] [Google Scholar]
- 17.Ciaputa R, Madej JA, Łagodzki P, Pakuła J, Kandefer- Gola M, Janus I, Dzimira S, Nowak M. Prevalence of tumors in domestic and exotic animals in Lower Silesia between 2012 and 2013. Bull Vet Inst Pulawy. 2017;73(2):104–110. doi: 10.21521/mw.5637. [DOI] [Google Scholar]
- 18.Egenvall A, Bonnett BN, Ohagen P, Olson P, Hedhammar A, von Euler H. Incidence of and survival after mammary tumors in a population of over 80,000 insured female dogs in Sweden from 1995 to 2002. Prev Vet Med. 2005;69:109–127. doi: 10.1016/j.prevetmed.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Shafiee R, Javanbakht J, Atyabi N, Kheradmand P, Kheradmand D, Bahrami A, Daraei H, Khadivar F. Diagnosis, classification and grading of canine mammary tumours as a model to study human breast cancer: an Clinico-Cytohistopathological study with environmental factors influencing public health and medicine. Cancer Call Int. 2013;13:79. doi: 10.1186/1475-2867-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Abdelmegeed SM, Mohammed S. Canine mammary tumors as a model for human disease. Oncol Lett. 2018;15(6):8195. doi: 10.3892/ol.2018.8411. 8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez Alenza MD, Peña L, del Castillo N, Nieto AI. Factors influencing the incidence and prognosis of canine mammary tumours. J Small Anim Pract. 2000;41(7):287–291. doi: 10.1111/j.1748-5827.2000.tb03203.x. [DOI] [PubMed] [Google Scholar]
- 22.Szczubiał M, Dąbrowski R, Śmiech A, Łopuszański W, Gawron W, Kusy R, Iwonicki R. Efficacy of some clinical factors on prognosing the course of malignant mammary tumours in bitches. Medycyna Wet. 2004;60:160–164. [Google Scholar]
- 23.Vascellari M, Capello K, Carminato A, Zanardello C, Baioni E, Mutinelli F. Incidence of mammary tumors in the canine population living in the Veneto region (Northeastern Italy): Risk factors and similarities to human breast cancer. Prev Vet Med. 2016;126:183–189. doi: 10.1016/j.prevetmed.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Markkanen E. Know thy model: Charting molecular homology in stromal reprogramming between canine and human mammary tumors. Front Cell dev Biol. 2019;7:348. doi: 10.3389/fcell.2019.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borecka P, Ratajczak-Wielgomas K, Ciaputa R, Kandefer- Gola M, Janus I, Piotrowska A, Kmiecik A, Podhorska-Okolów M, Dzięgiel P, Nowak M. Expression of periostin in cancer-associated fibroblasts in mammary cancer in female dogs. In Vivo. 2020;34(3):1017–1026. doi: 10.21873/invivo.11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldschmidt M, Pena L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumours. Vet Pathol. 2011;48(1):117–131. doi: 10.1177/0300985810393258. [DOI] [PubMed] [Google Scholar]
- 27.Clemente M, Perez-Alenza MD, Illera JC, Pena L. Histologic, immunologic and ultrastructural description of vasculogenic mimicry in canine mammary cancer. Vet Pathol. 2010;47(2):265–274. doi: 10.1177/0300985809353167. [DOI] [PubMed] [Google Scholar]
- 28.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohisto-chemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8(3):138–140. [PubMed] [Google Scholar]
- 29.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical Oncology and College of American Pathologists Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Liu J, Wang Z, Huang Y, Liu W, Zhu X, Cai Y, Fang X, Lin S, Yuan L, Ouyang G. Periostin contributes to the acquisition of multipotent stem cell-like properties in human mammary epithelial cells and breast cancer cells. PLoS One. 2013;8(8):e72962. doi: 10.1371/journal.pone.0072962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puglisi F, Puppin C, Pegolo E, Andreetta C, Pascoletti G, D’Aurizio F, Pandolfi M, Fasola G, Piga A, Damante G, Di Loreto C. Expression of periostin in human breast cancer. J Clin Pathol. 2008;61(4):494–498. doi: 10.1136/jcp.2007.052506. [DOI] [PubMed] [Google Scholar]
- 32.Xu D, Xu H, Ren Y, Liu C, Wang X, Zhang H, Lu P. Cancer stem cell-related gene periostin: a novel prognostic marker for breast cancer. PLoS One. 2012;7(10):46670. doi: 10.1371/journal.pone.0046670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Zhang G, Li J, Tao Q, Tang W. The expression analysis of periostin in human breast cancer. J Surg Res. 2010;160(1):102–106. doi: 10.1016/j.jss.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 34.McAllister SS, Weinberg RA. Tumor-host-interaction: a far-reaching relationship. J Clin Oncol. 2010;28(26):4022–4028. doi: 10.1200/JCO.2010.28.4257. [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Goswami S, Sahai E, Wyckoff JB, Segall JE, Condeelis JS. Tumor cells caught in the act of invading: their strategy for enhanced cell motility. Trends Cell Biol. 2005;15(3):138–145. doi: 10.1016/j.tcb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Hunter KW. Host genetics and tumor metastasis. Br J Cancer. 2004;90(4):752–755. doi: 10.1038/sj.bjc.6601590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos AA, Lopes CC, Ribeiro JR, Martins LR, Santos JC, Amorim IF, Gärtner F, Matos AJ. Identification of prognostic factors in canine mammary malignant tumours: a multivariable survival study. BMC Vet Res. 2013;9:1. doi: 10.1186/1746-6148-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borecka P, Ciaputa R, Janus I, Piotrowska A, Ratajczak-Wielgomas K, Kmiecik A, Podhorska-Okolów M, Dzięgiel P, Nowak M. Expression of podoplanin in mammary cancers in female dogs. In Vivo. 2020;34(1):213–223. doi: 10.21873/invivo.11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amini P, Nassiri S, Ettlin J, Malbon A, Markkanen E. Next-generation RNA sequencing of FFPE subsections reveals highly conserved stromal reprogramming between canine and human mammary carcinoma. Dis Model Mech. 2019;12(8):dmm040444. doi: 10.1242/dmm.040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amini P, Nassiri S, Malbon A, Markkanen E. Differential stromal reprogramming in benign and malignant naturally occurring canine mammary tumours identifies disease-modulating stromal components. Sci Rep. 2020;10(1):5506. doi: 10.1038/s41598-020-62354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ettlin J, Clementi E, Amini P, Malbon A, Markkanen E. Analysis of gene expression signatures in cancer-associated stroma from canine mammary tumours reveals molecular homology to human breast carcinomas. Int J Mol Sci. 2017;18(5):1101. doi: 10.3390/ijms18051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li C, Xu J, Wang Q, Geng S, Yan Z, You J, Li Z, Zou X. Prognostic value of periostin in early-stage breast cancer treated with conservung surgery and radiotherapy. Oncol Lett. 2018;15:8072–8078. doi: 10.3892/ol.2018.8310. [DOI] [PMC free article] [PubMed] [Google Scholar]