Abstract
Background/Aim: Thoracic neurogenic tumors are most frequently located in the posterior part of the mediastinum or on the chest wall, along the intercostal nerves. Schwannomas are very well tolerated for a long period, until the tumor reaches a large size and compression of the neighbouring mediastinal organs, chest wall or spine appears. The purpose of this article was to present a case of a giant right forth intercostal nerve Schwannoma, completely resected by a right antero-lateral thoracotomy. In addition, intrathoracic giant neurogenic tumors are a rarity. Case Report: The patient presented with only diminished tolerance to physical activity with no other obvious symptoms. Standard chest radiography revealed a well-defined opacity of subcostal intensity, occupying two thirds of the right hemithorax, forming a common body with the mediastinal shadow. Thoracic computed tomography (CT) identified a 21/11 cm solid mass that compresses the right lung and the right main bronchus with both a solid component and a central liquid area. Open surgery was performed in order to remove the tumor, which was 20.5/12.5/9 cm in size and weighed 1,830 g, well defined, with no invasion of the adjacent organs, having a solid-fibromatous aspect as well as a central necrotic area. The origin of the tumor was confirmed from the posterolateral part of the forth intercostal nerve. Pathology examination and immunohistochemistry confirmed the diagnosis of a benign Schwannoma. Conclusion: Benign intrathoracic Schwannomas are asymptomatic for long periods and the main therapeutic option is complete surgical resection. The surgical approach, either open or video-assisted is dictated by the localisation of the tumor, local extension and most importantly the size of the neurogenic mass.
Keywords: Schwannoma, anterolateral thoracotomy, giant neurogenic tumors, dumbbell tumor
The incidence of neurogenic mediastinal tumors is between 12-21% of the total mediastinal tumors (1), being most of the time benign lesions (2). Neurogenic tumors are frequently found in the posterior mediastinum, intercostal space, thoracic sympathetic nerve or ganglion, thoracic vagus nerve and very rarely developed in the lung (3). Of all the posterior mediastinal masses, over 75% of cases are neurogenic tumors (4). Overall, benign neurogenic tumors represent 93.5% of cases and the malignant ones only 6.5% (5).
Benign neurogenic tumors are most often found on the intercostal or vagus nerves. Alongside neurofibromatosis, ganglioneuromas or paragangliomas, Schwannomas are the most common, found in 48% of cases (6). Schwannoma has several names in the literature such as neurilemmoma, neurilenoma or neuroma, with its origin in the Schwann cells. The first person to describe these cells was Theodor Schwann, a German physiologist, biologist and histologist (7).
Malignant neurogenic tumors are more often found in children or young adults and represent 67.2% of cases and rarely discovered at an adult age (8,9). On the other hand, mediastinal localisation of a peripheral nerve cyst-tumor is extremely rare, 0.5-7% of cases, and malignant Schwannoma in 5% of cases, usually associated with Recklinghausen disease (10-12).
Case Report
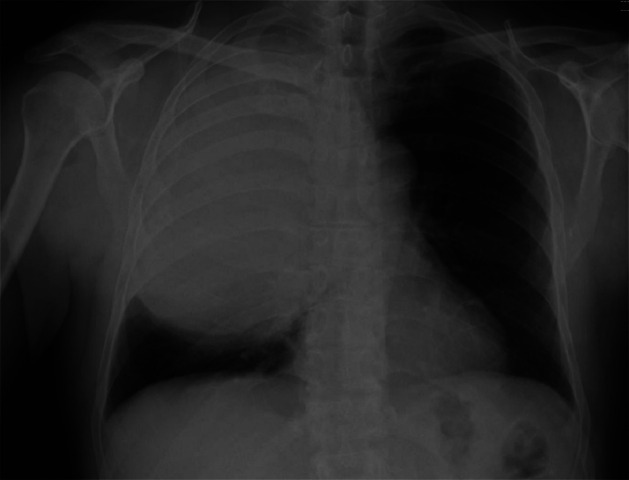
A 60-year-old male presented in our clinic for a diminished tolerance to physical activity in the last few weeks and mild dyspnea, with no other thoracic symptoms and associated cardiac or respiratory pathology. Standard chest radiography (Figure 1) revealed a well-defined, homogenous mass occupying two thirds of the right hemithorax forming a common body with the mediastinal shadow. Blood tests show a slightly elevated pCO2. Respiratory volume tests revealed a restrictive ventilator defect with significantly lower forced expiratory volume (FEV 1 – 0.98 l/58%) and forced vital capacity (FVC – 1.71 l/64%) then the predicted values.
Figure 1. Standard thoracic radiography – mass in the right hemithorax.
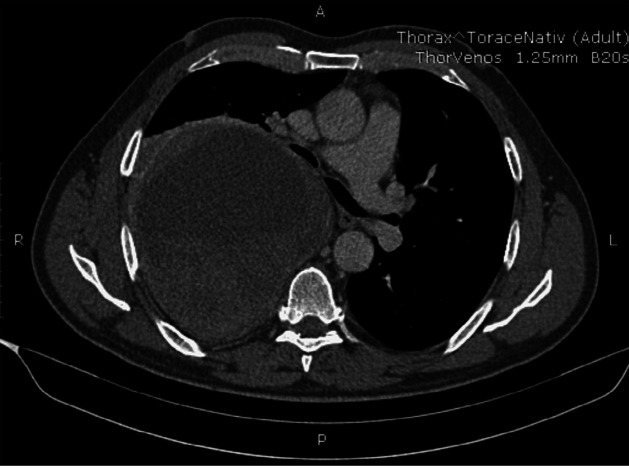
Bronchoscopy studies identified compression of the posterior wall of the right main bronchus, which continued throughout the bronchial tree. Standard and contrast thoracic CT (Figure 2) confirmed the presence of a giant, 21/11 cm mass, occupying the entire right hemithorax, compressing the right lung and bronchial tree and moving the mediastinal organs towards the left side. The mass was mostly solid, homogenous, with a central liquid area suggesting necrosis, with no invasion of the neighbouring organs or chest wall.
Figure 2. Thoracic computed tomography – mass in right hemithorax.
Open surgery was performed by right anterolateral thoracotomy with access through the fifth intercostal space. Intraoperatively we discovered a well-defined tumor, adherent to the upper and middle lobe of the lung, compressing it. We identified a wide base for the tumor from the forth-intercostal space, with no local invasion, being relatively easy to detach from the adjacent lung with complete resection of the tumor. The mass was 20.5/12.5/9 cm in size and weighed 1,830 g. After removal of the tumor, complete expansion of the lung was obtained.
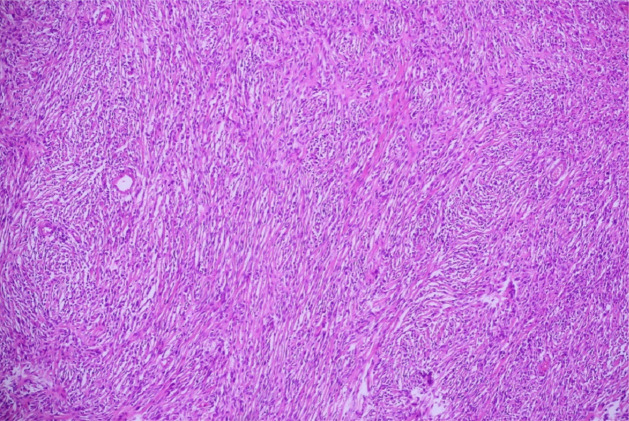
Postoperative evolution of the patient was favourable, with no complications, with the patient being discharged after 7 days. Histopathological examination (Figure 3) macroscopically described a giant encapsulated tumor, with a yellow, macronodular structure and a large central cystic degeneration, with hematic contents and white areas with sclerotic aspect. Microscopically the tumor was surrounded by a thick, collagenous capsule that contains numerous capillary vessels and lymphoid follicles. Tumor contained fusiform Schwann cells, with no atypical cells or mitosis. Cells were distributed as fascicles in hypercelular areas (Antony A) with palisade aspect, alternating with hypocellular areas with myxoid or cystic degeneration (Antony B), with no signs of capsular invasion.
Figure 3. Histopathological microscopic view. Magnification ×20.
The final histopathological diagnosis was that of benign giant thoracic schwannoma. Immunohistochemistry tests for cytokeratin, vimentin and epidermal growth factor receptor (EGFR) were negative; however, tests were positive for S100 protein. These tests also confirmed our diagnosis of a benign intrathoracic schwannoma. Follow-up was made after 6 months and 1 year with excellent results, lung volume tests improved dramatically (FEV1 – 1.72 L-90%; FVC – 2.48 L-92%) and standard chest radiography was normal, with no sign of local recurrence.
Discussion
Neurogenic tumors represent the most common mediastinal neoplastic disease in children, found in 46.2% of cases, where as in adults in 11.2% of cases. Schwannomas make up a 25-34% of the total cases of neurogenic tumors (13). Some authors consider that benign schwannomas represent 48.3% of the total number of thoracic neurogenic tumors and that 6.71% of them go through malignant transformation (14). An intrathoracic neurogenic tumor is considered giant if it occupies more than half of the hemithorax, which is a rare occurrence (3). In the presented case the tumor occupied two thirds of the right hemithorax and was removed using an anterolateral thoracotomy, only large enough to excise the tumor but with some sacrifice to intraoperative visibility.
Regarding symptoms, most benign neurogenic tumors are asymptomatic (15). In the cases of giant neurogenic tumors there is no consensus regarding clinical diagnosis (3). Most adult patients (84% of cases) and children (60% of cases) with neurogenic tumors are asymptomatic (13). Other authors consider that malignant neurogenic tumors are symptomatic in most cases in children (76.4% of cases) and much less in adults (36.7% of cases) (16). In addition, other authors consider that while malignant neurogenic tumors are mostly symptomatic, the benign ones are not, being well tolerated over long periods. (17).
According to some authors, there is no clinically significant difference between malignant or benign neurogenic tumors (18). However, due to the large size of the tumor, mediastinal compression can occur, especially the compression of the vena cava with secondary superior vena cava syndrome (19). In our case, the patient presented with only a decreased tolerance to physical activity with no other significant clinical signs. Even so, due to the large size of the tumor, differential diagnosis was between a benign schwannoma and a possible giant intercostal nerve neurofibroma.
Schwannomas appear especially between 20-50 years of age and has an equal distribution between genders. Microscopically there are two types of cell patterns: Anthony A (Schwann cells as palisades or the presence of Verocay bodies) and Anthony B (polymorphic Schwann cells with a free myxoid component) (20). Both patterns can be present at the same time in the same Schwannoma, as was the case with our patient. Schwannomas are lobulated tumors and sometimes present with central degeneration, and are formed outward from the nerve axis. Microscopically, solitary neurofibromas have a structure formed of spindle cells with wavy nuclei, with no cellular pleomorphism, with various degrees of myxoid degeneration and the tumor is formed within the nerve axis (20).
Immunohistochemical differentiation between schwannomas and neurofibromas is extremely useful and based on the following markers: CD56, CD34, S100 protein, calretinin. Calretinin is much more specific to Schwannomas and is not found in neurofibromas, CD56 appears in 77% of cases of Schwannomas whereas CD34 appears in 80% of cases of neurofibromas (21). On the other hand, immunoreactivity towards S100 protein seems to be the same in both schwannomas and neurofibromas. In our case, although very rare, given the size of the tumor, we considered a malignant transformation of the schwannoma, which usually presents as an epithelioid or angiosarcomatous transformation (22). Malignant Schwannomas are part of peripheral nerve sheath tumors. Some authors consider that 50% of malignant peripheral nerve sheath tumors appear in patients with type I Recklinghausen disease; however, there have been reported cases of malignant schwannomas in patients without the disease (23).
Some authors consider that the lifetime risk for developing malignant peripheral nerve sheath tumors in patients with type I neurofibromatosis is between 8-13% (24). Malignant peripheral nerve sheath tumors do not present a specific or sensitive marker for immunohistochemistry, most of the times being classified as a sarcoma, due to its origin and behaviour (25). In our case there were no histological elements or immunohistochemistry results to confirm a malignant transformation of the resected tumor.
Thoracic computed tomography (CT) and magnetic resonance imagining studies are the main imaging tools used for this type of pathology. Even so, it is difficult to determine whether a tumor is benign or malignant (3,18). A series of authors attempted to differentiate between malignant and benign neurogenic tumors using a series of CT characteristics. Benign neurogenic tumors appear as round, well-defined masses of soft tissue with the presence of central cystic degeneration (20) whereas malignant tumors present with local invasion, bone tissue destruction, lung metastases or pleural effusions (26). However, in order to establish the extension of the lesion in the spine, magnetic resonance imaging studies are far superior to a thoracic CT (3).
Neurogenic tumors can be mistaken for solitary pleural fibrous tumors, chest wall tumors or other mediastinal masses (27,28). CT-guided biopsy is useful in obtaining a preoperative diagnosis however, due to the low cell count in benign tumors, malignant or benign traits are not always obvious (29,30).
The evolution of these tumors can lead to local complications such as compression of the neighbouring organs, bone destruction or growth in the spinal canal with associated medullar compression (1). A typical case of neurogenic tumor development is the one of a dumbbell tumor. Most authors agree that this type of tumor, especially if it is associated with bone destruction, requires a multidisciplinary team. For dumbbell tumors with spinal involvement, in order to prevent medullar lesions, some authors suggest using intraoperative spinal neurogenic motor evoked potential monitoring (31). For small dumbbell tumors a video-assisted approach is preferred and laminectomy is associated (32). For benign neurogenic tumors, complete surgical resection of the tumor is sufficient (17). The objectives of surgical removal are preventing local extension, obtaining a histopathological diagnosis, preventing malignant transformation and establishing the necessity of further treatment (14).
Minimal invasive surgery, according to some authors, is reserved for tumors that are smaller than 8 cm in diameter (33). Contraindications for this method are the involvement of the spinal artery or medullar extension of the tumor (16). However, a very large tumor, such as the one we presented, can be a major inconvenience, making minimally invasive surgery impossible to perform. The advantages of minimally invasive surgery compared to open surgery are obvious: reduces hospital stay, reduces postoperative pain, and reduces surgery duration and minimal postoperative blood loss (34-36).
According to some authors, video assisted surgery can be used even for malignant neurogenic tumors, with very satisfying results and minimal postoperative complications (37). Even so, a video assisted approach can present its own series of postoperative complications such as hoarseness, Claude Bernard Horner syndrome or brachial plexus lesions. Most specialists agree that minimally invasive surgery is reserved for cases with no local invasion or medullar extension (31,38). Postoperative survival for thoracic neurogenic tumors is directly correlated with tumor size, whether the tumor is malignant or benign and if the mass is completely removed.
Conclusion
In benign giant neurogenic tumors, using open surgery, the golden standard of treatment is the complete removal of the mass. Due to these tumors most often being asymptomatic, early discovery is based on routine chest radiographies. The surgical approach, open or video assisted, is dictated by tumor placement, local extension and most importantly, tumor size.
Conflicts of Interest
The Authors declare no conflicts of interest regarding this study.
Authors’ Contributions
CS, VG, AM performed the surgical procedure; NB, CS, MD, IB reviewed literature data, CD, LI preoperative investigation the patient, IB, NB CS prepared the draft of the manuscript, CS was advisor of the surgical procedures CS, NB reviewed the final version of the manuscript. The Authors read and approved the final version of the manuscript.
References
- 1.Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest. 2005;128(4):2893–2909. doi: 10.1378/chest.128.4.2893. [DOI] [PubMed] [Google Scholar]
- 2.Strollo DC, Rosado-de-Christenson ML, Jett JR. Primary mediastinal tumors: part II. Tumors of the middle and posterior mediastinum. Chest. 1997;112(5):1344–1357. doi: 10.1378/chest.112.5.1344. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Yan J, Ren S, Guo Y, Gao Y, Zhou L. Giant neurogenic tumors of mediastinum: report of two cases and literature review. Chin J Cancer Res. 2013;25(2):259–262. doi: 10.3978/j.issn.1000-9604.2013.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi M, Yoshino I, Fukuyama S, Osoegawa A, Kameyama T, Tagawa T, Maehara Y. Surgical treatment of neurogenic tumors of the chest. Ann Thorac Cardiovasc Surg. 2004;10(3):148–151. [PubMed] [Google Scholar]
- 5.Ardissone F, Andrion A, D’Alessandro L, Borasio P, Maggi G. Neurogenic intrathoracic tumors. A clinicopathological review of 92 cases. Thorac Cardiovasc Surg. 1986;34(4):260–264. doi: 10.1055/s-2007-1020424. [DOI] [PubMed] [Google Scholar]
- 6.Akyildiz EU, Yalcinkaya U. Thoracic neurogenic tumors: A clinicopathologic evaluation of 42 cases. Neurology Asia. 2015;20(1):59–63. [Google Scholar]
- 7.Thomas TA. Theodor Schwann: A founding father of biology and medicine. In History of medicine. Curr Med Issues. 2017;15:299–301. doi: 10.4103/cmi.cmi_81_17. [DOI] [Google Scholar]
- 8.Ribet ME, Cardot GR. Neurogenic tumors of the thorax. Ann Thorac Surg. 1994;58(4):1091–1095. doi: 10.1016/0003-4975(94)90464-2. [DOI] [PubMed] [Google Scholar]
- 9.Hoover EL, Hsu HK, Dressler C, Fani K, Webb H, Ketosugbo A, Kharma B. Neuroblastoma: a rare primary intrathoracic neurogenic tumor in adults. Tex Heart Inst J. 1988;15(2):107–112. [PMC free article] [PubMed] [Google Scholar]
- 10.Reeder LB. Neurogenic tumors of the mediastinum. Semin Thorac Cardiovasc Surg. 2000;12(4):261–267. doi: 10.1053/stcs.2000.16738. [DOI] [PubMed] [Google Scholar]
- 11.Marchevsky AM. Mediastinal tumors of peripheral nervous system origin. Semin Diagn Pathol. 1999;16(1):65–78. [PubMed] [Google Scholar]
- 12.Grillo HC, Ojemann RG, Scannell JG, Zervas NT. Combined approach to "dumbbell" intrathoracic and intraspinal neurogenic tumors. Ann Thorac Surg. 1983;36(4):402–407. doi: 10.1016/s0003-4975(10)60477-8. [DOI] [PubMed] [Google Scholar]
- 13.Takeda S, Miyoshi S, Minami M, Matsuda H. Intrathoracic neurogenic tumors--50 years’ experience in a Japanese institution. Eur J Cardiothorac Surg. 2004;26(4):807–812. doi: 10.1016/j.ejcts.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Bicakcioglu P, Demirag F, Yazicioglu A, Aydogdu K, Kaya S, Karaoglanoglu N. Intrathoracic neurogenic tumors. Thorac Cardiovasc Surg. 2014;62(2):147–152. doi: 10.1055/s-0033-1343898. [DOI] [PubMed] [Google Scholar]
- 15.Schmezer A, Reinosch W, Laqua D, Bahr R. Thoracic neurinoma: a rare tumor of the posterior mediastinum. Chirurg. 1996;67(1):90–92. [PubMed] [Google Scholar]
- 16.Riquet M, Mouroux J, Pons F, Debrosse D, Dujon A, Dahan M, Jancovici R. Videothoracoscopic excision of thoracic neurogenic tumors. Ann Thorac Surg. 1995;60(4):943–946. doi: 10.1016/0003-4975(95)00487-6. [DOI] [PubMed] [Google Scholar]
- 17.Shields TW, Reynolds M. Neurogenic tumors of the thorax. Surg Clin North Am. 1988;68(3):645–668. doi: 10.1016/s0039-6109(16)44538-x. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi T, Kawai N, Watanabe T, Yasukawa M, Morita K, Ohbayashi C, Tojo T. Primary intrathoracic malignant neurogenic tumor: report of three cases and comparison with benign neurogenic tumors resected at our institution. Surg Case Rep. 2015;1(1):6. doi: 10.1186/s40792-014-0013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galie N, Vasile R, Savu C, Petreanu C, Grigorie V, Tabacu E. Superior vena cava syndrome – surgical solution--case report. Chirurgia (Bucur) 2010;105(6):835–838. [PubMed] [Google Scholar]
- 20.Pavlus JD, Carter BW, Tolley MD, Keung ES, Khorashadi L, Lichtenberger JP III. Imaging of thoracic neurogenic tumors. AJR Am J Roentgenol. 2016;207(3):552–561. doi: 10.2214/AJR.16.16018. [DOI] [PubMed] [Google Scholar]
- 21.Park JY, Park H, Park NJ, Park JS, Sung HJ, Lee SS. Use of calretinin, CD56, and CD34 for differential diagnosis of schwannoma and neurofibroma. Korean J Pathol. 2011;45:30–35. doi: 10.4132/KoreanJPathol.2011.45.1.30. [DOI] [Google Scholar]
- 22.Woodruff JM, Selig AM, Crowley K, Allen PW. Schwannoma (neurilemoma) with malignant transformation. A rare, distinctive peripheral nerve tumor. Am J Surg Pathol. 1994;18(9):882–895. doi: 10.1097/00000478-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Angelopoulos E, Eleftheriou K, Kyriakopoulos G, Athanassiadi K, Rontogianni D, Routsi C. A giant intrathoracic malignant schwannoma causing respiratory failure in a patient without von Recklinghausen’s disease. Case Rep Med. 2016;2016:2541290. doi: 10.1155/2016/2541290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39(5):311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123(3):295–319. doi: 10.1007/s00401-012-0954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JY, Lee KS, Han J, Yoon HK, Kim TS, Han BK, Kim J, Shim YM. Spectrum of neurogenic tumors in the thorax: CT and pathologic findings. J Comput Assist Tomogr. 1999;23(3):399–406. doi: 10.1097/00004728-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Pirvu A, Angelescu D, Savu C. Localized fibrous tumor of the pleura an unusual cause of severe hypoglycaemia. Case report. Rev Med Chir Soc Med Nat Iasi. 2016;120(3):628–630. [PubMed] [Google Scholar]
- 28.Savu C, Melinte A, Balescu I, Bacalbasa N. Azygos vein aneurysm mimicking a mediastinal mass. In Vivo. 2020;34(4):2135–2140. doi: 10.21873/invivo.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar S, Rafiq MU, Ahamed I, Ansari J, Cowen ME. Asymptomatic giant thoracic schwannoma. Ann Thorac Surg. 2006;82(3):e26. doi: 10.1016/j.athoracsur.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 30.Savu C, Melinte A, Posea R, Galie N, Balescu I, Diaconu C, Cretoiu D, Dima S, Filipescu A, Balalau C, Bacalbasa N. Pleural solitary fibrous tumors-a retrospective study on 45 patients. Medicina (Kaunas) 2020;56(4) doi: 10.3390/medicina56040185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosking MP, Mongan PD, Peterson RE. Removal of a large intrathoracic tumor in a child: neurogenic motor-evoked potential monitoring of spinal cord integrity and anesthetic management. Anesth Analg. 1992;74(3):460–463. doi: 10.1213/00000539-199203000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Wang B, Lv G, Li L. Thoracoscopy-assisted mini-open approach for resection of neurogenic tumors arising at the thoracic apex. Int J Clin Exp Med. 2017;10(3):5175–5181. [Google Scholar]
- 33.Liu HP, Yim AP, Wan J, Chen H, Wu YC, Liu YH, Lin PJ, Chang CH. Thoracoscopic removal of intrathoracic neurogenic tumors: a combined Chinese experience. Ann Surg. 2000;232(2):187–190. doi: 10.1097/00000658-200008000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han PP, Dickman CA. Thoracoscopic resection of thoracic neurogenic tumors. J Neurosurg. 2002;96(3 Suppl):304–308. doi: 10.3171/spi.2002.96.3.0304. [DOI] [PubMed] [Google Scholar]
- 35.Yang C, Zhao D, Zhou X, Ding J, Jiang G. A comparative study of video-assisted thoracoscopic resection versus thoracotomy for neurogenic tumours arising at the thoracic apex. Interact Cardiovasc Thorac Surg. 2015;20(1):35–39. doi: 10.1093/icvts/ivu328. [DOI] [PubMed] [Google Scholar]
- 36.Yang C, Zhao D, Zhang P, Fei K, Jiang G. Intrathoracic neurogenic tumor with malignant transition-20 years operation experience in a medical center of China. Neurosci Lett. 2017;637:195–200. doi: 10.1016/j.neulet.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Fraga JC, Rothenberg S, Kiely E, Pierro A. Video-assisted thoracic surgery resection for pediatric mediastinal neurogenic tumors. J Pediatr Surg. 2012;47(7):1349–1353. doi: 10.1016/j.jpedsurg.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 38.Horvat T, Savu C, Motas C, Tetu M. Pneumopericardium--complication of an unknown tuberculosis in a HIV positive patient. Eur J Cardiothorac Surg. 2004;26(5):1043. doi: 10.1016/j.ejcts.2004.08.014. [DOI] [PubMed] [Google Scholar]