Abstract
Background/Aim: High-dose chemotherapy (HDCT) and stem cell transplantation (SCT) have been established as the standard of care in patients with relapsed germ cell tumours (GCTs). We evaluated the safety, efficacy and tolerability of HDCT/ SCT in patients with relapsed GCTs. Patients and Methods: Twenty-eight patients with relapsed GCTs, treated with HDCT, were included in this study. The conditioning regime was carboplatin, etoposide, cyclophosphamide and paclitaxel. Clinical, radiological imaging and tumour markers determined treatment outcomes. Results: Median age was 35 years (range=21-57 years) with 26 males and 2 females. Median time to first relapse was 6 months. Median time to progression after 2nd line chemotherapy was 17.3 months. Fourteen patients hadMedian survival was 62 months and 16 patients (57%) are in clinical follow-up with surveillance. Conclusion: In relapsed GCT patients, median survival may exceed 5 years post-HDCT and SCT.
Keywords: Relapsed germ cell cancer, high dose chemotherapy, stem cell transplant, survival
Germ cell tumours (GCTs) are responsible for 1% of cancer diagnoses, affecting around 5-7/100,000 men and 0.4/100,000 women annually, typically occurring during the reproductive years. Of these, the majority will be cured with surgery with or without the addition of platinum-based chemotherapy. Cure rates are approximately 90-95% for patients with localised disease and around 80% for patients with metastatic disease (1). Bleomycin, etoposide and cisplatin (BEP) chemotherapy is the most widely used first-line regimen for higher risk or metastatic patients (2-4).
For the patients that do relapse or are refractory to primary treatment (20-30% of patients in the context of metastatic disease), approximately 50% will gain durable remission with salvage treatment with combination therapy using cisplatin, ifosfamide and a third agent - typically etoposide, paclitaxel or gemcitabine (5,6).
For such patients, options include treatment with conventional dose chemotherapy (CDC) or high dose chemotherapy (HDCT) and autologous stem cell transplant. Given the rarity of this cancer type as well as the low rates of relapse/refractory disease, outcomes for further lines of treatments are limited due to the small number of patients, while further stratification by risk makes the patient numbers even smaller. Therefore, comparison between strategies is challenging, more so because data are retrospective and therefore extrapolation is further limited.
Question remains over the benefit of high-dose chemotherapy against standard-dose chemotherapy with transplant support. Since the 1980s, HDCT and SCT has been investigated as a means of allowing treatment intensification and maintenance of dose density (7,8).
Einhorn et al. demonstrated efficacy of HDCT in relapsed GCT, using high-dose carboplatin and etoposide with autologous stem-cell support. In their retrospective cohort of 184 patients, 63% had complete remission at a median follow-up of 48 months. Survival was seen to be lower in second or later relapse (6).
Pico et al. demonstrated no significant difference in 3-year event-free survival or overall survival (OS) between patients who received 4 cycles of cisplatin, ifosfamide and etoposide (alternatively, vinblastine) or three cycles of this regimen and then high-dose carboplatin, etoposide and cyclophosphamide (CarboPEC) with stem cell transplantation. The authors concluded that “uncontrolled studies should not be used to justify routine use of a toxic and expensive treatment without confirmation in a randomised trial” (9).
Retrospective analysis of 1,594 patients treated with high-dose chemotherapy vs. standard dose in the salvage setting, however, demonstrated a progression-free survival (PFS) hazard ratio of 0.44 (95%CI=0.39-0.51) and OS of 0.65 (95%CI=0.56-0.75) indicating benefit of high-dose chemotherapy. 5-year OS was 40.8% for conventional dose chemotherapy and 53.2% for HDCT with SCT. Failure-free survival was also higher in the HDCT with SCT arm (10).
Overall, HDCT and SCT can offer cure rates of up to 60% in the relapsed GCT setting. Differing chemotherapy regimens have been used in varying settings from phase I and II studies and retrospective analyses, with a clear demonstration of curative potential, however, the optimal regimen remains undefined (11,12).
To provide clarity on how best to treat refractory or relapsed GCT patients, the TIGER trial is currently underway. It is a phase III trial, which is prospectively comparing conventional-dose chemotherapy for first relapse using TIP (paclitaxel, ifosfamide and cisplatin) against high-dose chemotherapy, with autologous stem cell transplantation, utilising paclitaxel and ifosfamide and then high dose carboplatin and etoposide (TI-CE) (13).
We aimed to add to the published data sets our experience of HDCT treated patients, their toxicities and survival outcomes.
Patients and Methods
Patients treated with HDCT and ASCT between December 1999 to December 2019 were identified retrospectively. Patients were treated at Mount Vernon Hospital, Northwood and received their ASCT at the transplantation unit of the Hammersmith Hospital, London. Relapse was identified through elevation of tumour markers or positive histology. Patients with first and second relapse were included.
The HDCT conditioning regime used was carboplatin, etoposide, cyclophosphamide and paclitaxel (CarboPEC-Taxol). Paclitaxel 75 mg/m2, etoposide 450 mg/m2 and carboplatin AUC 10 were given on days -7, -5 and -3 from SCT, with cyclophosphamide 60 mg/kg on days -5 and -3.
Baseline patient and tumour characteristics, chemotherapy regimens, time to relapse, toxicity and PFS and OS outcomes were obtained from consultation outcomes and documentation and determined treatment outcomes. Through clinical review and retrospective evaluation of notes, PFS and OS as well as toxicity including acute myeloid leukaemia (AML) rates in the cohort were assessed. Toxicities were classified according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
Statistical analysis. Patients who had at least one autologous stem cell infusion were included in the analysis. PFS was derived from date of first chemotherapy for relapse until date of verifiable disease progression.
Similarly, OS was evaluated from date of first chemotherapy for relapse until date of death from any cause. PFS and OS were derived from Kaplan–Meier curves. Statistical analysis was achieved through the use of Microsoft Excel.
Results
Twenty-eight patients were treated with HDCT-ASCT and their baseline characteristics are shown in Table I. Median age was 35 years (range=21-57 years). Twenty-six (92%) patients had non-seminomatous disease and 2 (8%) had seminomatous disease. The majority of patients had IGCCG stage 4 disease. Twenty-five patients were treated for first relapse and three treated in the third line setting for relapse/refractory disease.
Table I. Baseline characteristics.
IGCCCG: International Germ Cell Cancer Collaborative Group; POMBACE: cisplatin, vincristine, methothrexate, bleomycin- actinomycin-d, cyclophosphamide, etoposide; BEP: bleomycin, etoposide, cisplatin
Median time to progression after first line treatment was 6 months (range=1-106 months). POMB-ACE (cisplatin, vincristine, methotrexate, bleomycin, actinomycin, cyclophosphamide, and etoposide) was the most frequently used primary chemotherapy regimen (15/28) with the remaining patients receiving BEP-based chemotherapy.
Table II outlines second line management. Three patients had second line chemotherapy prior to priming. GEM-TIP was the most commonly utilised priming chemotherapy combination (26/28 patients) and Carbo-PEC-taxol was the most frequently used HDCT regime (27/28 patients). Twelve of 28 patients (43%) had tandem transplants, defined as two sequential stem cell infusions. Long-term toxicities of grade 2 or worse were most commonly peripheral neuropathy, occurring in 14 patients, ototoxicity in 5 patients and renal failure in 4 patients. There was 1 case of acute myeloid leukaemia.
Table II. Second line management and survival outcomes of second line treatment.
GEM-TIP: Gemcitabine-paclitaxel, ifosfamide, cisplatin; HDCT: high dose chemotherapy, SCT: stem cell transplant; PEC: paclitaxel, etoposide, cyclophosphamide
Median follow-up was 38 months (range=2-145 months) (Table III). Of the total number of patients, 47% progressed after HDCT-SCT. Table IV shows the other treatment modalities used in the third line setting. The majority of the patients requiring further treatment had retroperitoneal lymph node dissection (RPLND) and 5 patients received external beam radiotherapy to the para-aortic nodes following HDCT and SCT.
Table III. Outcomes.
HDCT: High dose chemotherapy; AML: acute myeloid leukaemia
Table IV. Additional treatment modalities used in the second line setting after HDCT and SCT.
RPLND: Retroperitoneal lymph node dissection
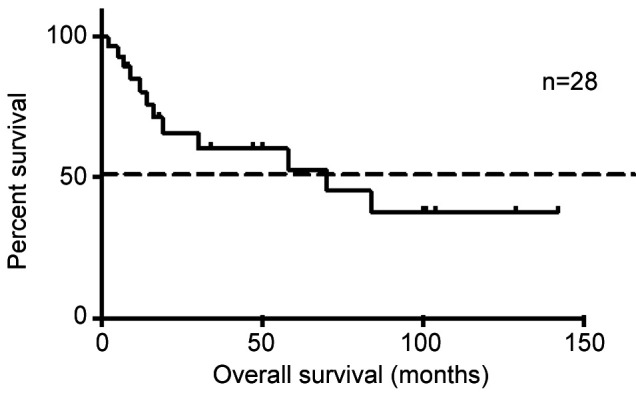
Median TTP post-HDCT was 4.5 months (mean 7.6 months, range=2-24 months) with a median survival of 62 months (range=2-145 months) (Figure 1). Sixteen patients (57%) are in clinical follow-up with surveillance.
Figure 1. Kaplan-Meier curve demonstrating survival post-high dose chemotherapy and stem cell transplant.
Discussion
Our results show that in a median follow-up of 38 months, 57% of patients were alive post-HDCT. This is in line with the case series by Lewin et al. demonstrating a 2-year OS of 71% (14). Comparatively, both their sample size of 17 and the sample size presented here [28] are small, but also exemplify the challenge in this rare tumour type, with rare event rates and high first line cure rates. Similarly, our series also report a mixed risk and gender cohort. Furthermore, the frequency of further multidisciplinary treatment is evident in this group of patients, with the majority of patients having either surgery or radiotherapy after HDCT and SCT.
The single case of AML reflects the anticipated toxicity and published rates of AML occurrence in these patients. There was one unexplained death that was most likely attributable to non-treatment related cardiac events. It is notable that there was no transplant-related mortality among the patients included who underwent SCT.
Patients with relapsed or refractory GCTs reflect a small proportion of patients with GCTs, as a majority of patients respond well to first line treatment. There are some available data that reflect our results and highlight a benefit in both PFS and OS from HDCT as initial salvage chemotherapy in relapsed or refractory GCTs; however, a degree of uncertainty has remained. The IT-94 trial showed a superior objective response rate in patients treated with HDCT, but due to several notable limitations, failed to show a significant clinical benefit in the use of HDCT. The trial failed to recruit the projected number of patients and mortality was higher in the experimental arm of the trial using HDCT, which potentially obscured any benefit from HDCT. Furthermore, the trial was particularly limited by using a single HDCT cycle in the experimental arm, rather than sequential treatment, and over a quarter of the patients in this arm did not receive this cycle of HDCT (15).
As such, the TIGER trial, an international, multi-centre phase III trial prospectively comparing conventional-dose chemotherapy using TIP against high-dose chemotherapy using TI-CE followed by autologous stem cell transplantation, is currently underway to determine the optimal salvage treatment for patients with refractory or relapsed GCTs, thus providing sufficient evidence for the use of HDCT in this cohort of patients (14,15).
Conclusion
This retrospective work adds to the already published data to support HDCT with SCT treatment in relapsed or refractory GCT. Our single-centre experience demonstrates that in unselected patients of variable risk, median survival exceeded 5 years post-HDCT and SCT, thereby offering an effective salvage option.
Conflicts of Interest
The Authors have no conflicts of interest to declare regarding this study.
Authors’ Contributions
AS, DSB, PVJ, NV and MH contributed to the concept and design of the paper. AS, PVJ, AG, MJ, AY and EK contributed to the patient care and case presentation. AG contributed to the radiological examination. All Authors signed and approved the final manuscript.
References
- 1.Popovic L, Matovina-Brko G, Popovic M, Petrovic D, Cvetanovic A, Vukojevic J, Jovanovic D. High dose chemotherapy with stem cell support in the treatment of testicular cancer. World J Stem Cells. 2015;7(11):1222–1232. doi: 10.4252/wjsc.v7.i11.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma A, Alifrangis C, Milic M, Hall M, Vasdev N, Wilson P, Gogbashian A, Hrouda D, Berney D, Shamash J. Somatic transformation in metastatic testicular germ cell tumours – a different disease entity. Anticancer Res. 2019;39(9):4911–4916. doi: 10.21873/anticanres.13678. [DOI] [PubMed] [Google Scholar]
- 3.McCaffrey JA, Mazumdar M, Bajorin DF, Bosl GJ, Vlamis V, Motzer RJ. Ifosfamide- and cisplatin-containing chemotherapy as first-line salvage therapy in germ cell tumors: response and survival. J Clin Oncol. 1997;15(7):2559–2563. doi: 10.1200/JCO.1997.15.7.2559. [DOI] [PubMed] [Google Scholar]
- 4.Saxman SB, Finch D, Gonin R, Einhorn LH. Long-term follow-up of a phase III study of three versus four cycles of bleomycin, etoposide, and cisplatin in favourable prognosis germ-cell tumors: The Indian University experience. J Clin Oncol. 1998;16(2):702–706. doi: 10.1200/JCO/1998.16.2.702. [DOI] [PubMed] [Google Scholar]
- 5.Adra N, Abonour R. High-dose chemotherapy and autologous stem cell transplant. Urol Clin North Am. 2019;46(3):439–448. doi: 10.1016/j.ucl.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Einhorn LH, Williams SD, Chamness A, Barmes MJ, Perkins SM, Abonour R. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumours. N Eng J Med. 2007;357(4):340–348. doi: 10.1056/NEJMoa067749. [DOI] [PubMed] [Google Scholar]
- 7.Beyer J, Kramar A, Mandanas R, Linkesch W, Greinix A, Droz JP, Pico JL, Diehl A, Bokemeyer C, Schmoll HJ, Nichols CR, Einhorn LH, Siegert W. High-dose chemotherapy as salvage treatment in germ cell tumors: a multivariate analysis of prognostic variables. J Clin Oncol. 1996;14(10):2638–2645. doi: 10.1200/JCO.1996.14.10.2638. [DOI] [PubMed] [Google Scholar]
- 8.De Giorgi U, Rosti G, Salvioni R, Papiani G, Ballardini M, Pizzocaro G, Marangolo M. Long-term outcome of salvage high-dose chemotherapy in patients with germ cell tumor with poor prognostic features. Urol Oncol. 2011;29(3):284–290. doi: 10.1016/j.urolonc.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Pico J-L, Rosti G, Kramar A, Wandt H, Koza V, Salvioni R, Theodore C, Lelli G, Siegert W, Horwich A, Marangolo M, Linkesch W, Pizzocaro G, Schmoll H-J, Bouzy J, Droz J-P, Biron P, Genito-Urinary Group of the French Federation of Cancer Centres (GETUG-FNCLCC), European Group for Blood and Marrow Transplantation (EBMT) A randomised trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advance germ cell tumours. Ann Oncol. 2005;16(7):1152–1159. doi: 10.1093/annonc/mdi228. [DOI] [PubMed] [Google Scholar]
- 10.Lorch A, Kleinhans A, Kramar A, Kollmannsberger CK, Hartmann JT, Bokemeyer C, Rick O, Beyer J. Sequential versus single high-dose chemotherapy in patients with relapsed or refractory germ cell tumors: long-term results of a prospective randomised trial. J Clin Oncol. 2012;30(8):800–805. doi: 10.1200/JCO.2011.38.6391. [DOI] [PubMed] [Google Scholar]
- 11.Pagliaro LC. Role of high-dose chemotherapy with autologous stem-cell rescue in men with previously treated germ cell tumours. J Clin Oncol. 2017;35(10):1036–1040. doi: 10.1200/JCO.2016.70.6523. [DOI] [PubMed] [Google Scholar]
- 12.Schmoll HJ, Kollmannsberger C, Metzner B, Hartmann JT, Schleuner N, Schoffski P, Schleicher J, Rick O, Beyer J, Hossfeld D, Kanz L, Berdel WE, Andreesen R, Bokemeyer C, German Testicular Cancer Study Group Long-term results of first-line sequential high-dose etoposide, ifosfamide, and cisplatin chemotherapy plus autologous stem cell support for patients with advanced metastatic germ cell cancer: an extended phase I/II study of the German Testicular Cancer Study Group. J Clin Oncol. 2003;21(22):4083–4091. doi: 10.1200/JCO.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 13.Feldman DR, Huddart R, Hall E, Beyer J, Powles T. Is high dose therapy superior to conventional dose therapy as initial treatment for relapsed germ cell tumours? The TIGER trial. J Cancer. 2011;13(2):374–377. doi: 10.7150/jcs.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewin J, Dickinson M, Voskoboynik M, Collins M, Ritchie D, Toner G. High-dose chemotherapy with autologous stem cell transplantation in relapsed or refractory germ cell tumours: outcomes and prognostic variables in a case series of 17 patients. Intern Med J. 2014;44(8):771–778. doi: 10.1111/imj.12486. [DOI] [PubMed] [Google Scholar]
- 15.McHugh DJ, Feldman DR. Conventional-dose versus highdose chemotherapy for relapsed germ cell tumours. Adv Urol. 2018 doi: 10.1155/2018/7272541. [DOI] [PMC free article] [PubMed] [Google Scholar]






