Abstract
Background: Survival after allogeneic hematopoietic stem cell transplantation (allo-HSCT) has increased but so have long-term sequelae. New-onset post-transplant diabetes mellitus (PTDM) occurs frequently following allo-HSCT. Patients and Methods: Study endpoints were incidence and risk factors of PDTM. We studied 599 adult patients suffering from either acute myeloid leukemia n=220), acute lymphoblastic leukemia (n=79), chronic myeloid leukemia (n=22), myelodysplastic syndrome/myeloproliferative neoplasm (n=105), chronic lymphocytic leukemia (n=37), lymphoma/myeloma (n=116, or non-malignant disorders (e.g. bone marrow failure, hemoglobinopathies) (n=20) who underwent myeloablative (466; 77.8%) or non-myeloablative (131; 21.9%) allo-HSCT between 2006 and 2016. Results: Altogether, 39 patients (6.5%) developed PTDM. In a competing-risk analysis, time to PTDM was associated with acute grade 2-4 graft-versus-host-disease (p=0.017). Further cardiovascular risk factors were hypertension (n=145; 24.2%), coronary artery disease (n=36, 6%), dyslipidemia (n=139; 23.3%), and stroke (n=12; 2%). Conclusion: After allo-HSCT, a significant number of patients developed PTDM and patients with acute graft-versus-host-disease were found to have a higher risk for PTDM. Long-term and continuous follow-up for diabetes and cardiovascular risk factors after HSCT is important in order to be able to provide timely and appropriate treatment.
Keywords: Allogeneic hematopoietic stem cell transplantation, diabetes, insulin therapy, cardiovascular risk factors, long-term survivors
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a well-established therapy for hematological malignancies (1). HSCT is associated with many non-disease-related late effects (2). Furthermore, endocrinopathies such as ovarian insufficiency, spermatogenesis damage and hypothyroidism are among the most common late effects after allo-HSCT (3,4). Advances in transplantation strategies and supportive care have contributed to the improvement in outcome and to a growing number of long-term survivors. However, due to this development, new health issues are arising. One major problem is that of metabolic syndrome, a cluster characterized by diabetes mellitus (DM), abdominal obesity, atherogenic dyslipidemia, elevated blood pressure, insulin resistance and proinflammatory and prothrombotic state (5-7). Post transplantation diabetes mellitus (PTDM) occurs in 10-30% of patients undergoing allo-HCST (8,9). Despite its burden, its physiology remains poorly understood (9). A timely identification of cardiovascular risk factors can lead to early initiation of risk modification therapy, which in turn reduces the risk of late cardiovascular morbidity and mortality (2,6,10). We herein conducted a retrospective observational study to ascertain incidence and causes of PTDM and other features of metabolic syndrome in patients after allo-HCST.
Patients and Methods
Patients. This retrospective single-center cohort study at the Department of Hematology of the University Hospital Basel was performed according to the regulations of the local Ethics Committee (EKNZ 2019-01810). In total, 599 patients who underwent allo-HSCT between 2006 and 2016 were included. Data were analyzed until 11/2018.
Definitions. DM was diagnosed according to American Diabetes Association criteria and the recommendations of an international consensus meeting on PTDM: Fasting plasma glucose ≥126 mg/dl (7.0 mmol/l), random plasma glucose ≥200 mg/dl (11.1 mmol/l), 2-h plasma glucose after an oral glucose tolerance test ≥200 mg/dl (11.1 mmol/l), or hemoglobin A1c (HbA1c) ≥6.5% (11,12). Although these initial criteria were established for DM after solid organ transplantation, these criteria also seem to be useful in DM after allo-HSCT [as reviewed in (13)]. Early intervention during the asymptomatic stage of hyperglycemia improves the long-term outcome in the general population. Therefore, screening for hyperglycemia and PTDM is indicated in high-risk patients at the early-stage post HSCT and during the long-term surveillance (13). However, given the high incidence of new-onset PTDM during the first 100 days, it may be of benefit to screen all allo-HSCT recipients routinely early after transplant (13). This is the reason why we also included patients with DM in the early phase after allo-HSCT in contrary to solid organ transplantations where patients should be stable on their likely maintenance immunosuppression, with stable kidney allograft function and in the absence of acute infections (12). Caution was exercised in interpreting HbA1c results as our patients with hematological disorders often received transfusion support before and after allo-HSCT that might confound the interpretation of the HbA1c results (13). For this reason, a patient was classified as having diabetes when the diagnosis was documented in the patient’s medical record or they received insulin or oral hypoglycemic medication. Further cardiovascular risk factors such as hypertension, dyslipidemia, body mass index (BMI) with overweight and obesity were recorded parameters as already published elsewhere (2,10,14).
Statistical analysis. Statistical analyses, including distribution analysis and descriptive statistics, were performed with IBM SPSS 25.0 (IBM, Armonk, NY, USA). Comparison between groups was performed using chi-squared test for categorical variables and Mann-Whitney or Student’s t-test for continuous variables. Study endpoints were incidence and risk factors of PDTM. Overall survival was defined as the time from day of stem cell infusion until death from any cause or last follow-up. Kaplan-Meier curves were calculated for survival estimates. In the multivariate analyses, the following risk factors for PTDM were considered: Age at transplantation, gender, human leukocyte antigen -mismatch, graft-versus-host disease (GvHD), and total body irradiation (TBI) in the conditioning regimen. p-Values less than 0.05 were considered as significant. Two-sided tests were used throughout.
Results
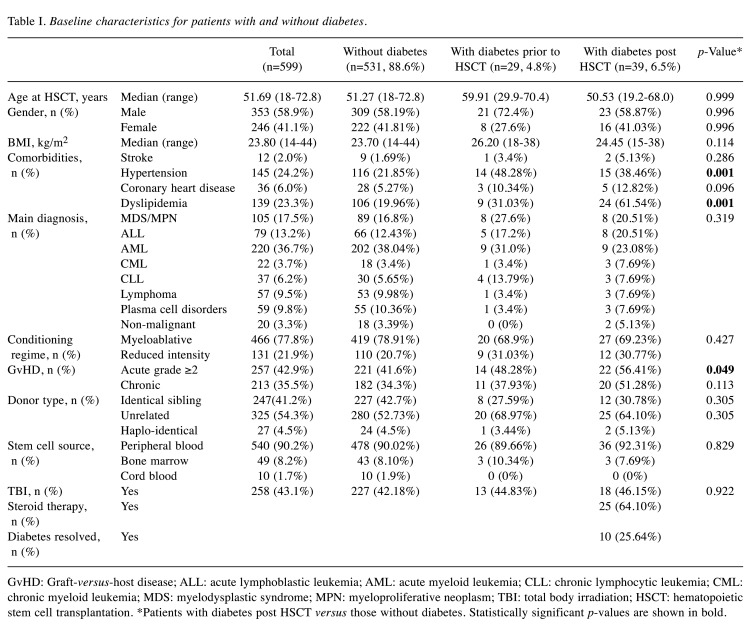
Patient characteristics. The 599 patients with allo-HSCT had a median age of 51.69 (range=18-72.8) years. A total of 353 were male (58.9%), and included patients receiving transplants from identical siblings (n=247; 41.2%), unrelated (n=325; 54.3%), and haplo-identical (n=27; 4.5%) donors. Stem cell source was peripheral blood (90.2%), bone marrow (8.2%), or cord blood (1.8%). Conditioning regimen, GvHD prophylaxis and therapy as performed at our center were published previously (15). The patients’ baseline characteristics are listed in Table I. Conditioning regimens were largely myeloablative (n=466; 77.80%), as opposed to reduced intensity (n=131; 21.9%). TBI was used in 258 patients (43.10%). Clinically relevant grade 2 or more acute GvHD were reported in 257 patients (42.9%), and any grade chronic GvHD in 213 (35.5.%). Overall survival at 3 years was 54% and at 5 years was 48%, with a median follow-up period of 18 (range=0-142) months (last follow-up date 11/2018).
Table I. Baseline characteristics for patients with and without diabetes.
GvHD: Graft-versus-host disease; ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; CLL: chronic lymphocytic leukemia; CML: chronic myeloid leukemia; MDS: myelodysplastic syndrome; MPN: myeloproliferative neoplasm; TBI: total body irradiation; HSCT: hematopoietic stem cell transplantation. *Patients with diabetes post HSCT versus those without diabetes. Statistically significant p-values are shown in bold
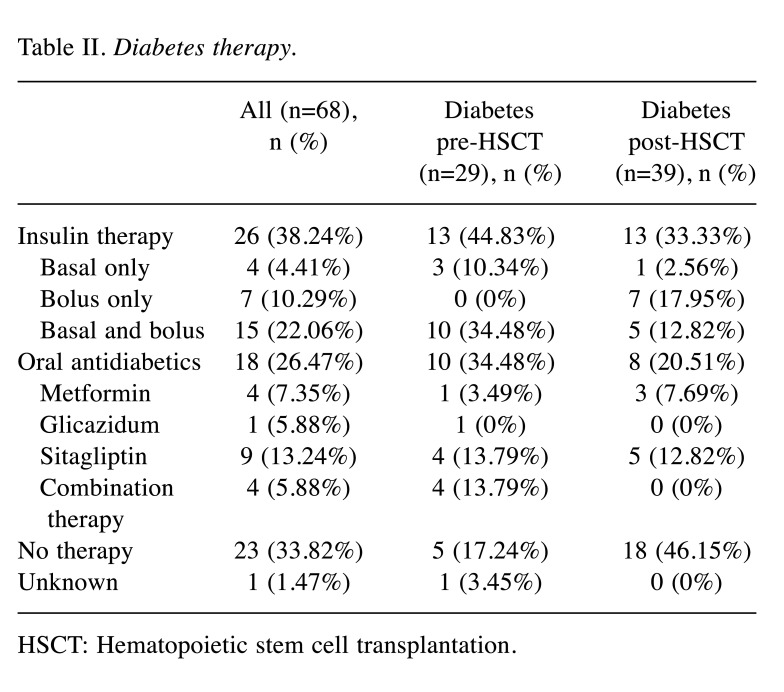
Diabetes and cardiovascular risk factors. Twenty-nine patients suffered from DM before allo-HSCT: 13 patients (44.83%) had insulin therapy, while 10 (34.48%) had therapy with oral antidiabetic drugs (Table II); five patients (17.24%) did not receive any drug therapy and had well-controlled DM with life-style modifications and in one patient therapy was unknown. During follow-up, 39 (6.5%) patients developed PTDM. In these patients, the median age at transplantation was 50.53 (range=19.2-68.0) years, and 23 patients (58.97%) were male. There was no difference in the type of diseases and treatment factors between groups (Table I).
Table II. Diabetes therapy.
HSCT: Hematopoietic stem cell transplantation
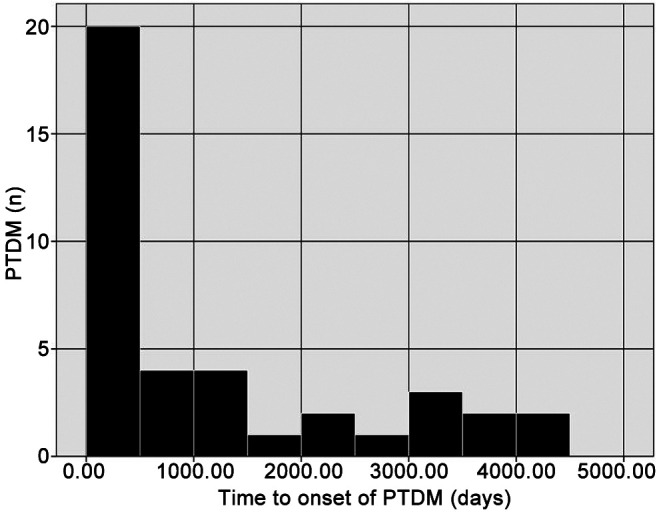
The median time from allo-HSCT to diagnosis of acute GvHD was 26 (range=4-417) days and median time of onset of PTDM was 477 (range=11-4,052) days (Figure 1). In patients with both aGvHD and DM (n=27), in all but two, aGvHD preceded DM by a median of 240 (range=5-3,622) days. Twenty-five (64.10%) of the patients developed DM during or shortly after initiation of steroid therapy (mainly but not only for acute GvHD, e.g. hemolytic anemia, immune thrombocytopenia). However, in 10/27 (37.04%) patients suffering from GvHD, the onset of PTDM was after stopping steroid therapy (median time interval=130, range=93-3,021 days). In 10 (25.64%) of these patients, diabetes resolved after discontinuation of the steroid therapy (median time interval from stopping steroid therapy until resolution of PTDM was 67 days (range=37-98 days). In the other patients (n=14; 35.9%), DM was not related to steroid therapy. The multivariate analysis showed that grade 2-4 acute GvHD was associated with the risk of developing PTDM (adjusted hazard ratio=2.237, 95% confidence intervaI=1.140-4.391; p=0.017). Conversely, no correlation was found with age, gender, underlying disease, conditioning regimen, stem cell source, donor, or chronic GvHD (Table I). Therapy for PTDM was insulin-based in 13 (33.33%), and oral antidiabetics in eight patients (20.51%), while 18 patients (46.15%) had only life-style modifications (Table II).
Figure 1. Histogram of onset of post-transplant diabetes mellitus (PTDM) after allogeneic hematopoietic stem cell transplantation.
The median age at transplantation was higher in patients with DM prior to HSCT compared to patients without DM (p=0.026) (Table I). However, there was no significant difference in age between patients with PTDM and patients without during follow-up [50.53 (range=19.2-68.0) versus 51.27 (range=18.0-72.8) years; p=0.999]. Overall, BMI levels were higher in patients with DM prior to HSCT as compared to patients without DM [26.2 (range=18-38) versus 23.70 (range=14-44) kg/m2; p=0.002]. However, BMI was similar in patients with PTDM compared to patients without [24.45 (range=15-38) versus 23.70 (range=14-44) kg/m2; p=0.114]. In patients with diagnosis of either pre- or post-HSCT DM, other cardiovascular risk factors were more common. Hypertension was diagnosed in 116 patients (21.85%) without DM and in 15 patients (38.46%) with post-HSCT DM (p=0.001). Similarly, patients with DM were more prone to suffering from dyslipidemia post-HSCT (n=24; 61.54%) as compared to patients without DM (n=106; 19.96%) (p=0.001). Patients with PTDM more often had dyslipidemia as compared to patients with a diagnosis of DM before HSCT (p=0.05), whereas the rate of hypertension was similar in both groups (p=0.62). Despite higher prevalence of cardiovascular risk factors, patients with pre- or post-HSCT DM did not have more events of ischemic stroke compared to patients with normal glucose values; p=0.146); the follow-up period may possibly be too short for proper evaluation of this. But patients with DM prior to or post HSCT had slightly but not significantly more coronary heart disease compared to patients without DM [n=5 (12.82%) and n=28 (5.27%) versus n=3 (10.34%); p=0.096)].
Discussion
Our study showed that PTDM is a relevant complication in long-term survivors after allo-HSCT and acute GvHD is associated in many but not all patients with PTDM. Furthermore, further cardiovascular risk factors such as dyslipidemia or hypertension were more frequent in patients with PTDM. Endocrine diseases are well-known common complications of HSCT (5,8,12). Risk factors for the development of PTDM such as age, non-White ethnicity, corticosteroids, parenteral nutrition and TBI have been evaluated in several studies. However, complete mechanisms causing PTDM are still not well understood (9,16). Several studies showed that exposure to high-dose corticosteroids increase the likelihood of developing PTDM and diabetogenic immunosuppressive drugs are traditionally believed to be responsible for the development of DM after HSCT (6,16,17).
In our study, we found a correlation between acute GvHD and PTDM. The association between acute GvHD and development of metabolic syndrome including DM is not completely clear. In a study including 86 adults after allo-HSCT, GvHD status was not associated with metabolic syndrome (7). Since patients suffering from acute GvHD mostly need high-dose corticosteroids, this might be a risk factor for the development of PTDM (13).
Furthermore, insulin resistance is a well-known feature of the metabolic syndrome and is highly prevalent in survivors of HSCT (9). It was shown that impaired glucose tolerance and hyperinsulinemia are increased in survivors of allo-HSCT independently of exposure to immunosuppressive medications or corticosteroids (18). Additionally, a study by Engelhart et al. revealed that insulin resistance prior to HCST is a determinant for PTDM (9). In line with these results, another study showed that patients with PTDM had higher levels of C-peptide before transplantation, suggesting that pre-transplantation features other than the use of immunosuppressive drugs might play a role in the development of PTDM (16). Our results are in line with this in that patients without GvHD and steroid therapy also developed PTDM.
Several studies show a high prevalence of metabolic syndrome among patients with allo-HCST, the most common features being dyslipidemia and hypertension (2,6,10). In our studyI a reliable number of patients had other features of metabolic syndrome other than DM. Overall, these cardiovascular risk factors were more common among (both pre- and post-HSCT) diabetic patients compared to non-diabetic patients.
The following limitations of our study must be taken into account: Firstly, investigating DM after HSCT was not a predefined endpoint of this transplantation cohort. Thus, diagnosis of pre/post HSCT DM was assigned retrospectively by chart review assessed by a Board-certified endocrinologist. Secondly, the number of patients with PTDM in our cohort was rather small. Furthermore, the diagnosis of DM is difficult because in many cases in the ambulatory setting in the follow-up period, no fasting glucose levels were feasible and there were difficulties in interpreting HbA1c results. There are some differences regarding the incidence/prevalence of cardiovascular risk factors after allo-HSCT in our study. Reasons for this might be differences in definitions used, patient populations analyzed, time periods, and retrospective versus prospective study.
In conclusion, our study showed that PTDM is an important complication in long-term survivors after allo-HSCT and is associated with acute GvHD. A timely identification and therapy thereof are recommended. Further evaluation is required to confirm the correlation between acute GvHD and PTDM and to assess whether acute GvHD is causally associated with PTDM.
Conflicts of Interest
The Authors have no conflicts of interest to declare.
Authors’ Contributions
NCN and MM designed the study. VDV, MM, and NCN collected or provided data that were analyzed by VDV, NCN, MM, JPH, and JP. JP performed statistical analysis. JPH, SG, CA, AT, and DH treated and observed the patients. The draft of the article written by VDV, MM and NCN, was reviewed and approved by all Authors.
Acknowledgements
The Authors thank the data team for always keeping the database up-to-date.
References
- 1.Passweg JR, Baldomero H, Chabannon C, Basak GW, Corbacioglu S, Duarte R, Dolstra H, Lankester AC, Mohty M, Montoto S, Peffault de Latour R, Snowden JA, Styczynski J, Yakoub-Agha I, Kröger N, European Society for Blood and Marrow Transplantation The EBMT activity survey on hematopoietic-cell transplantation and cellular therapy 2018: CAR-T’s come into focus. Bone Marrow Transplant. 2020;55(8):1604–1613. doi: 10.1038/s41409-020-0826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tichelli A, Bucher C, Rovó A, Stussi G, Stern M, Paulussen M, Halter J, Meyer-Monard S, Heim D, Tsakiris DA, Biedermann B, Passweg JR, Gratwohl A. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2008;110(9):3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 3.Medinger M, Zeiter D, Heim D, Halter J, Gerull S, Tichelli A, Passweg J, Nigro N. Hypothyroidism following allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia. Leuk Res. 2017;58:43–47. doi: 10.1016/j.leukres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Orio F, Muscogiuri G, Palomba S, Serio B, Sessa M, Giudice V, Ferrara I, Tauchmanovà L, Colao A, Selleri C. Endocrinopathies after allogeneic and autologous transplantation of hematopoietic stem cells. Scientific World J. 2014;2014:282147. doi: 10.1155/2014/282147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker KS, Ness KK, Steinberger J, Carter A, Francisco L, Burns LJ, Sklar C, Forman S, Weisdorf D, Gurney JG, Bhatia S. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: A report from the bone marrow transplantation survivor study. Blood. 2007;109(4):1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majhail NS, Challa TR, Mulrooney DA, Baker KS, Burns LJ. Hypertension and diabetes mellitus in adult and pediatric survivors of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(9):1100–1107. doi: 10.1016/j.bbmt.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Majhail NS, Flowers ME, Ness KK, Jagasia M, Carpenter PA, Arora M, Arai S, Johnston L, Martin PJ, Baker KS, Lee SJ, Burns LJ. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2009;43(1):49–54. doi: 10.1038/bmt.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelhardt BG, Jagasia SM, Crowe JE Jr, Griffith ML, Savani BN, Kassim AA, Lu P, Weitkamp JH, Moore DJ, Yoder SM, Rock MT, Jagasia M. Predicting posttransplantation diabetes mellitus by regulatory T-cell phenotype: implications for metabolic intervention to modulate alloreactivity. Blood. 2012;119(10):2417–2421. doi: 10.1182/blood-2011-10-384750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelhardt BG, Savani U, Jung DK, Powers AC, Jagasia M, Chen H, Winnick JJ, Tamboli RA, Crowe JE Jr, Abumrad NN. New-onset post-transplant diabetes mellitus after allogeneic hematopoietic cell transplant is initiated by insulin resistance, not immunosuppressive medications. Biol Blood Marrow Transplant. 2019;25(6):1225–1231. doi: 10.1016/j.bbmt.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Premstaller M, Perren M, Koçack K, Arranto C, Favre G, Lohri A, Gerull S, Passweg JR, Halter JP, Leuppi-Taegtmeyer AB. Dyslipidemia and lipid-lowering treatment in a hematopoietic stem cell transplant cohort: 25Years of follow-up data. J Clin Lipidol. 2018;12(2):464–480.e3. doi: 10.1016/j.jacl.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 12.Sharif A, Hecking M, de Vries AP, Porrini E, Hornum M, Rasoul-Rockenschaub S, Berlakovich G, Krebs M, Kautzky-Willer A, Schernthaner G, Marchetti P, Pacini G, Ojo A, Takahara S, Larsen JL, Budde K, Eller K, Pascual J, Jardine A, Bakker SJ, Valderhaug TG, Jenssen TG, Cohney S, Säemann MD. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: Recommendations and future directions. Am J Transplant. 2014;14(9):1992–2000. doi: 10.1111/ajt.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuji S, Rovó A, Ohashi K, Griffith M, Einsele H, Kapp M, Mohty M, Majhail NS, Engelhardt BG, Tichelli A, Savani BN. How do I manage hyperglycemia/post-transplant diabetes mellitus after allogeneic HSCT. Bone Marrow Transplant. 2016;51(8):1041–1049. doi: 10.1038/bmt.2016.81. [DOI] [PubMed] [Google Scholar]
- 14.Arranto CA, Burkard T, Leuppi-Taegtmeyer AB, Gerull S, Passweg JR, Pfister O, Halter JP. Prevalence of untreated and uncontrolled cardiovascular risk factors in survivors of allogeneic cell transplantation. Bone Marrow Transplant. 2020;Jul 14 doi: 10.1038/s41409-020-00997-x. [DOI] [PubMed] [Google Scholar]
- 15.Kraft S, Bollinger N, Bodenmann B, Heim D, Bucher C, Lengerke C, Kleber M, Tsakiris DA, Passweg J, Tzankov A, Medinger M. High mortality in hematopoietic stem cell transplant-associated thrombotic microangiopathy with and without concomitant acute graft-versus-host disease. Bone Marrow Transplant. 2019;54(4):540–548. doi: 10.1038/s41409-018-0293-3. [DOI] [PubMed] [Google Scholar]
- 16.Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356(9234):993–997. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian S, Trence DL. Immunosuppressive agents: effects on glucose and lipid metabolism. Endocrinol Metab Clin North Am. 2007;36(4):891–905. doi: 10.1016/j.ecl.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Jenssen T, Hartmann A. Emerging treatments for post-transplantation diabetes mellitus. Nat Rev Nephrol. 2015;11(8):465–477. doi: 10.1038/nrneph.2015.59. [DOI] [PubMed] [Google Scholar]