Abstract
Background: Although both chemotherapy and radiotherapy (RT) can sufficiently maintain tumor suppression of colorectal cancer (CRC), these treatments may trigger the expression of nuclear factor kappa B (NF-ĸB) and compromise patients’ survival. Regorafenib suppresses NF-ĸB activity in various tumor types. However, whether regorafenib may act as a suitable radiosensitizer to enhance therapeutic efficacy of RT remains unknown. Materials and Methods: Here, we established a CRC-bearing animal model to investigate the therapeutic efficacy of regorafenib in combination with RT, through measurement of tumor growth, body weight, whole-body computed tomography (CT) scan and immunohisto-chemistry staining. Results: Smallest tumor size and weight were found in the combination treatment group. In addition, RT-induced up-regulation of NF-ĸB and downstream proteins were diminished by regorafenib. Moreover, the body weight and liver pathology in the treated group were similar to those of the non-treated control group. Conclusion: Regorafenib may enhance the anti-CRC efficacy of RT.
Keywords: Radiotherapy, radiosensitizing effect, apoptosis, NF-ĸB
Recent innovative radiotherapy (RT) technologies, including intensity-modulated RT, image-guided RT, volumetric-modulated arc therapy, intraoperative RT, and stereotactic body RT (SBRT), are widely used to treat many types of cancer (1-3). RT as neoadjuvant strategy offers therapeutic benefits for patients with colorectal cancer (CRC). The combination of RT and surgical resection or chemotherapy has been shown to reduce the local recurrence rate and improve the survival of patients with CRC (4,5). For increasing anti-CRC efficacy of RT, novel radiosensitizers that sensitize cancer cells to radiation have been developed (6).
Regorafenib, a Food and Drug Administration-approved oral multi-kinase inhibitor, as well as an analog of sorafenib, has been demonstrated to prolong the overall survival of patients with CRC. Regorafenib induces tumor regression through inactivation of angiogenic and oncogenic kinases such as vascular endothelial growth factor receptors 1-3, fibroblast growth factor receptor 1, epidermal growth factor receptor, RAF, tyrosine-protein kinase, and rearranged during transfection (7-10). The combination of regorafenib and SBRT was demonstrated to increase survival benefits in patient with metastatic CRC (11). However, the anti-CRC effect and mechanism of regorafenib when used along with RT is not fully understood. To harness the beneficial properties of regorafenib on CRC radiotherapy, in vivo investigative experiments were carried out to better understand its feasibility in radiosensitization and the underlying mechanism involved.
Materials and Methods
Cells, antibodies and reagents. Human CRC CT26 cells were purchased from the Bioresource Collection and Research Center (BCRC, Hsinchu, Taiwan, ROC). Cells were cultured in RPMI-1640 medium with additional 10% fetal bovine serum, 10 U/ml of penicillin and 100 mg/ml streptomycin sulfate at 37˚C under a humidified atmosphere with 5% CO2. Primary antibodies purchased from different companies as follow, Ki-67 (Elabscience, Houston, TX, USA), cyclin D1 (Elabscience), survivin, NF-ĸB p65 (Ser276) (Cell Signaling Technology, Danvers, MA, USA), cleaved caspase-3 (Elabscience), cleaved caspase-8 (Elabscience), cleaved caspase-9 (Proteintech, Rosemont, IL, USA), Fas cell surface death receptor (FAS) (Elabscience), Fas ligand (FAS-L) (Elabscience), myeloid cell leukemia-1 (MCL1) (Elabscience), X-linked inhibitor of apoptosis protein (XIAP) (Elabscience), and cellular FADD-like interleukin-1β-converting enzyme-inhibitory protein (C-FLIP) (Elabscience). Regorafenib and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO, USA).
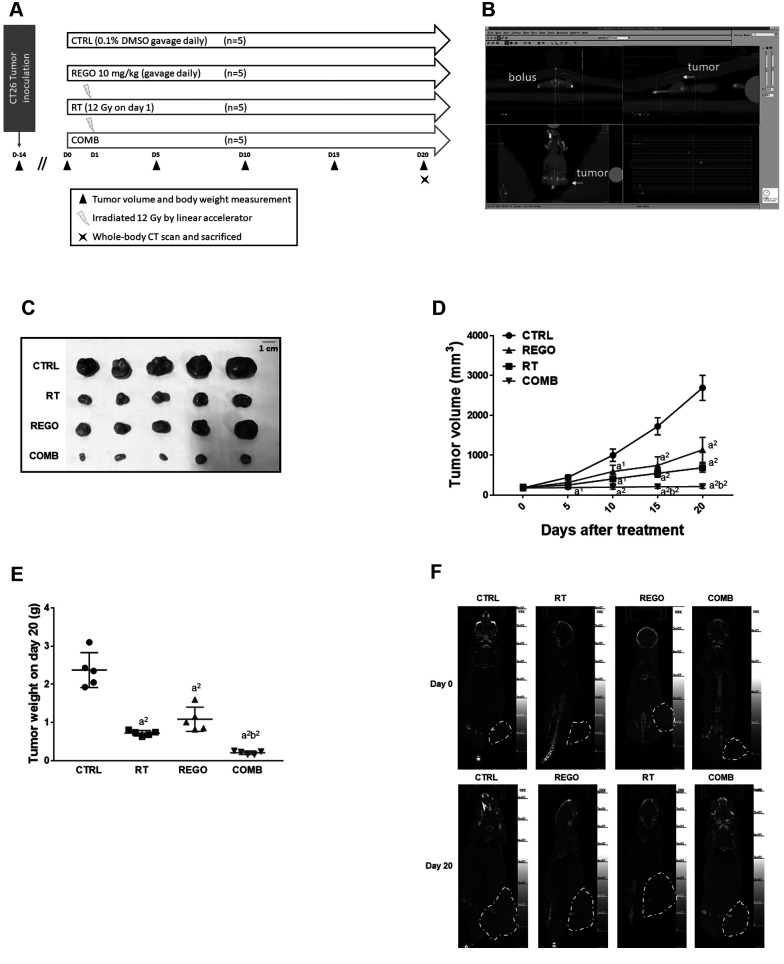
Animal experiment. Male BALB/c and CAnN.Cg-Foxn1nu/CrlNarl mice, approximately 22-28 g in weight and 4-6 weeks of age, were obtained from the National Laboratory Animal Center (Taipei, Taiwan, ROC). CT26 cells (5×106 cells/in 0.1-ml phosphate-buffered saline/mouse) were subcutaneously injected into the right flank of mice and developed into CRC in 2 weeks. A total of 20 mice were randomly divided into four groups (n=5 each) when tumor reached 100-120 mm3 and subsequently received different treatments: Control mice were treated with 0.1% DMSO in 100 μl double distilled water; regorafenib-treated mice were gavage-treated with 10 mg/kg/day regorafenib in 100 μl double distilled water; RT-treated mice were treated with 12-Gy radiation once on day 1 by linear accelerator; and the mice of the combination treatment group were treated with both regorafenib and radiation as described above (Figure 1A). Ethical approval was provided by the Institutional Animal Care and Use Committee of China Medical University (CMUIACUC-2019-288). Tumor volume was recorded every 5 days, evaluated by caliper and calculated by the equation, tumor volume=length × width2 ×0.523. The mice were sacrificed with CO2 for further studies on day 20 post-treatment.
Figure 1. Regorafenib (REGO) enhanced radiotherapy (RT) inhibition of tumor growth in colorectal cancer-bearing mice. A: Experimental flow chart of animal experiment. B: Irradiation dosage and area definition are analyzed by XiO® treatment planning software. C: Photo of extracted tumors from each group of mice on day 20. D: Tumor size. E: Tumor weight. F: Whole-body computed tomographic (CT) scan images. CTRL: Control, 0.1% dimethyl sulfoxide; COMB: combination therapy. Significantly different at: a1: p<0.05, and a2: p<0.01 vs. control group; b2: p<0.01 vs. single treatment group.
Tumor, spleen, kidney and liver tissue collection. Tumor, spleen, kidney and liver samples were isolated from all mice after sacrificed and prepared for further processing of formalin fixing and paraffin embedding. Tumor tissues were then weighed individually.
Radiation exposure. Mice were anesthetized by 1-3% isoflurane during the irradiation procedure. For dose build up, the tumor was covered with 1.0 cm bolus and scanned with computed tomography (CT). After CT simulation, XiO® treatment planning software was used to evaluate the dose distribution in the mouse. The prescribed dose given by a linear accelerator (6 MV photons; Elekta Synergy linear accelerator, Crawley, UK) to the gross tumor volume was 12 Gy (12).
Whole-body CT scan. One of the mice from each group was anesthetized by 1-3% isoflurane during whole-body CT scan on day 0 and 20. Whole-body CT scan was acquired by Mediso CT (Mediso Ltd., Budapest, Hungary). The scanning resolution was 145×145×145 μm, the tube potential peak was 55 kVp and the tube current was 145 μA (13).
Immunohistochemistry (IHC) staining. The protein expression level in tumor tissue including, Ki-67, cyclin D1, survivin, NF-ĸB p65 (Ser536), cleaved caspase-3, cleaved caspase-8, cleaved caspase-9, FAS, FAS-L, MCL1, XIAP, and C-FLIP were investigated in tumor tissue sections by IHC staining as described in previous studies (9,14). Stained slides were imaged under a Nikon microscope at ×100 (Nikon ECLIPSE Ti-U; Nikon, Minato City, Japan). Protein expression levels were measured by ImageJ software version 1.50 (National Institutes of Health, Bethesda, MD, USA).
Hematoxylin and eosin (H&E) staining. Tissue sections underwent H&E staining by by Bio-Check Laboratories Ltd (New Taipei City, Taiwan, ROC) as described elsewhere (9). H&E-stained sections were then imaged under microscopy and quantified by ImageJ software version 1.50.
Statistical analysis. Data are presented as the mean±standard deviation (n=5). All data were analyzed using GraphPad Prism version 7. Differences between the control and experimental groups were investigated by independent Student’s t-test analysis when p<0.05 was considered as a statistically significant difference.
Results
Regorafenib effectively induced anti-CRC ability of RT. To investigate whether regorafenib may trigger toxicity of RT, we established CT26 tumor-bearing animal model. As shown in Figure 1A, mice were divided into four different groups: Control treated with 0.1% DMSO; RT alone (12 Gy/once); regorafenib alone (10 mg/kg/day); and combination treatment. RT treatment plan was analyzed XiO® treatment planning software and irradiation was delivered by linear accelerator (Figure 1B). A 1-cm bolus for dose build up was placed on mouse tumors during irradiated procedure. Isolated tumors from each group at the endpoint (day 20) were photographed and displayed in Figure 1C. Smallest tumor size was found in the combination treatment group. Disparity in tumor volume between the combination treatment and control groups began at day 5 after treatment. After 15 days of treatment, the treatment efficacy of combination treatment group was better than that of the single treatment group (Figure 1D). Tumors extracted from mice on day 20 were weighed and the tumor weight of the combination treatment group was less than that of single treatment groups (Figure 1E). Whole-body CT scan indicated marked tumor growth inhibition in the combination treatment group as compared to single treatment groups (Figure 1F). In summary, as found from the tumor growth pattern, regorafenib effectively sensitized CRC to RT.
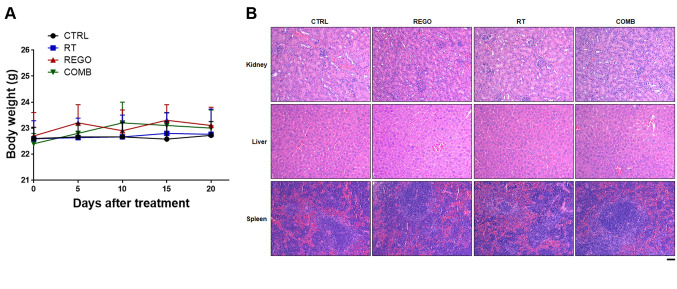
Regorafenib combined with RT in CRC-bearing mice appeared not to induce general toxicity. To investigate whether regorafenib combined with RT influenced body weight alteration, we measured mouse body weight once every 5 days. As shown in Figure 2A, no significant difference change of body weight was found between untreated and treated groups. In addition, we also extracted liver, spleen, and kidney tissues from each group for pathology evaluation (Figure 2B). No noticeable pathological change was found in H&E staining between untreated and treated groups. Taken together, regorafenib combined with RT appeared not to induce any general toxicity effect.
Figure 2. No general toxicity is found in mice treated with regorafenib (REGO) combined with radiotherapy (RT). A: Mouse body weight is measured every 5 days. B: Kidney, liver and spleen tissues from each group of mice on day 20 are displayed. CTRL: Control, 0.1% dimethyl sulfoxide; COMB: combination therapy. Scale bar: 100 μm.
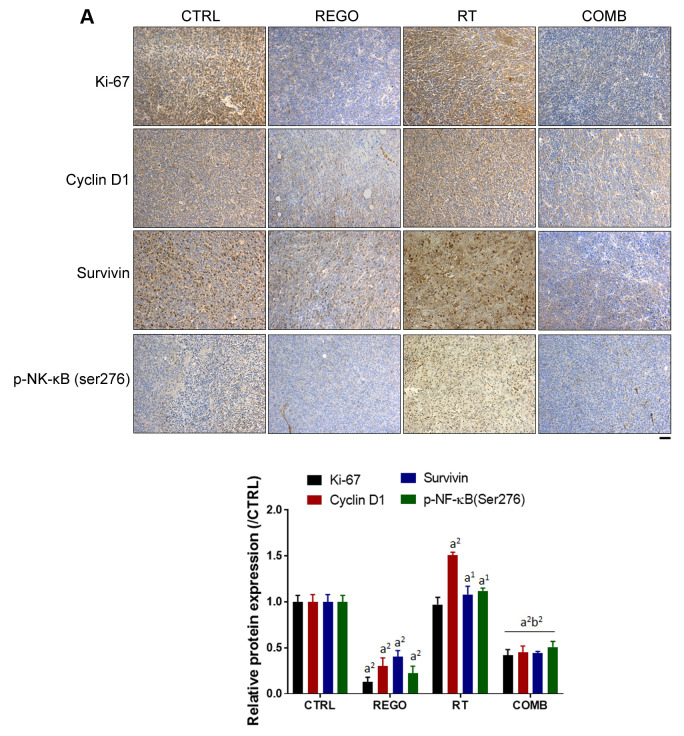
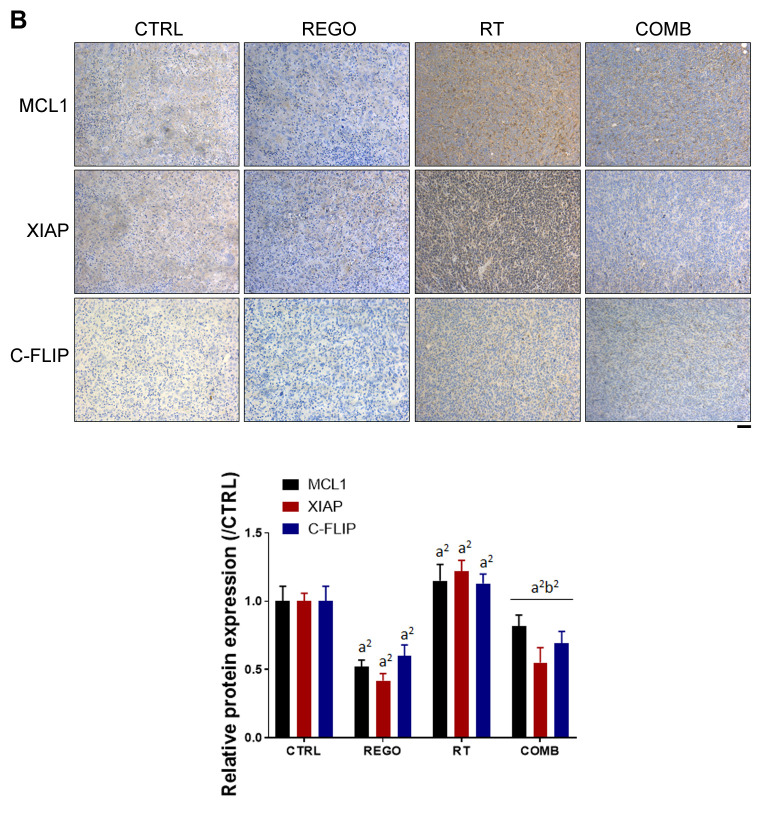
Regorafenib diminished radiation-induced NF-ĸB and NF-ĸB-mediated protein expression on CRC-bearing mice. NF-ĸB phosphorylation and the expression of NF-ĸB-related proteins were detected by IHC staining of tumor sections. In Figure 3A, radiation-induced phosphorylation of NF-ĸB was reduced by combination with regorafenib. Tumor proliferation marker, Ki-67, was markedly reduced by regorafenib combined with RT. NF-ĸB-related proteins that regulate tumor proliferation, such as cyclin D1 and survivin were all reduced by combination therapy. Furthermore, NF-ĸB-related proteins that regulate apoptosis (12,15), such as MCL1, XIAP and C-FLIP were also effectively reduced by regorafenib combined with RT (Figure 3B). Regorafenib successfully diminished RT-induced expression of NF-ĸB and its associated proteins.
Figure 3. Regorafenib (REGO) reduced radiation (RT)-triggered nuclear factor kappa B (NF-ĸB) activation and expression of anti-apoptosis proteins. Tumor immunostaining for expression of A: Ki-67, cyclin D1, survivin, phospho (p)-NF-ĸB(ser276) and B: myeloid cell leukemia-1 (MCL1), X-linked inhibitor of apoptosis protein (XIAP), and cellular FADD-like interleukin-1β-converting enzyme-inhibitory protein (C-FLIP) from each group of mice are displayed (left) and quantified (right). CTRL: Control, 0.1% dimethyl sulfoxide; COMB: combination therapy. Significantly different at: a1: p<0.05, and a2: p<0.01 vs. control group; b2: p<0.01 vs. single treatment group. Scale bar: 100 μm.
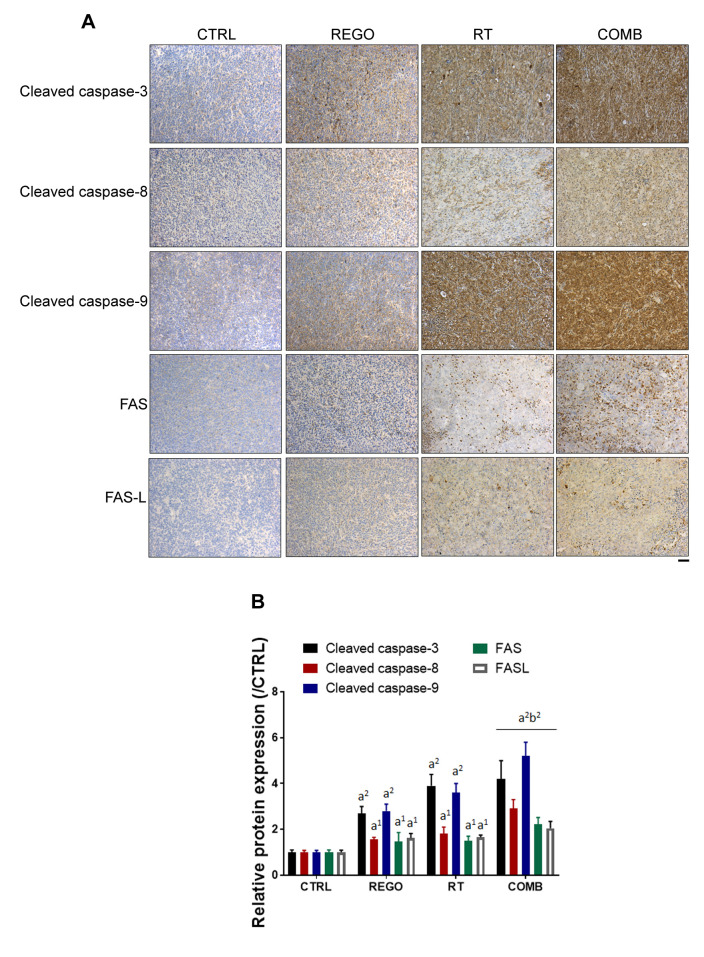
Regorafenib enhanced RT-induced expression of extrinsic and intrinsic apoptosis-associated proteins in CRC-bearing mice. The levels of extrinsic and intrinsic apoptosis-related proteins, cleaved caspase-3, -8 and -9, were detected by IHC staining of tumor sections. As illustrated in Figure 4A, overall apoptosis-related marker cleaved caspase-3 was significantly expressed in the combination treatment group as compared to single treatment groups. Expression of FAS, FAS-L, and cleaved caspase-8, which are extrinsic apoptosis-related proteins, were induced by regorafenib combined with RT (Figure 4B). Moreover, cleavage of caspase-9, an intrinsic apoptosis-related protein was also triggered in the combination treatment group as compared to single treatment group. In conclusion, RT-induced expression of extrinsic and intrinsic apoptotic-related proteins were successfully enhanced by regorafenib.
Figure 4. Regorafenib (REGO) increased expression of radiation (RT)-induced apoptotic proteins. A: Immunohistochemistry staining images and B: quantification of cleaved caspase-3, -8 and -9, Fas cell surface death receptor (FAS), and Fas ligand (FAS-L) expression in tumor from each group of mice are displayed. CTRL: Control, 0.1% dimethyl sulfoxide; COMB: combination therapy. Significantly different at: a1: p<0.05, and a2: p<0.01 vs. control group (0.1% dimethyl sulfoxide; CTRL); b2: p<0.01 vs. single treatment group. Scale bar: 100 μm.
Discussion
The combination of regorafenib and SBRT has been demonstrated to control disease progression and extend progression-free survival to approximately 3 years in patients with late-stage metastatic CRC (11). Our results indicate that regorafenib, RT, and their combination significantly diminished tumor growth of CT-26 tumor-bearing mice (Figure 1C and D). The combination group exhibited small tumor volume and tumor weight compared to mice from individual single treatment groups (Figure 1E). We found that regorafenib, as a radiosensitizer, effectively diminished CRC tumor growth with RT in vivo.
Cleaved caspase-8 and -9 are required for apoptosis induced by radiation through extrinsic (via death receptor) and intrinsic (mitochondrial) apoptotic pathways. Caspase-3 can be activated or cleaved by caspase-8 or caspase-9, leading to initiation of apoptotic DNA fragmentation (16,17). The increased protein expression of cleaved caspase-3 in tumor and tumor-associated stroma was associated with better prognosis in patients with CRC (18). Our results showed that expression of FAS, FAS-L, and cleaved caspase-3, -8, and -9 was significantly augmented with regorafenib, RT, and their combination (Figure 4). The combination treatment increased the expression of these proteins to a significantly higher level than that increased by regorafenib or radiation alone.
NF-ĸB, an oncogenic transcription factor composed of p50 and p65 dimers, can be activated with chemotherapeutic agents or radiation (19,20). Increased NF-ĸB activity attenuates the anticancer efficacy of RT through promoting the expression of proliferative and anti-apoptotic proteins, such as cyclin-D1, survivin, MCL1, XIAP, and C-FLIP (12,21). It has been found that worse 3- and 5-year overall survival of patients with CRC was correlated with overexpression of NF-ĸB activation (22). In addition, cell and animal models revealed suppression of NF-ĸB signaling enhanced radiosensitivity of CRC (12,15,21). Correspondingly, we found that radiation-triggered NF-ĸB activation, expression of proliferative, and anti-apoptotic proteins were significantly diminished by regorafenib concurrent treatment (Figure 3).
In conclusion, regorafenib was found to enhance the anti-CRC efficacy of RT. Induction of apoptosis and reduction of NF-ĸB signaling are involved in the radiosensitizing effect of regorafenib.
Conflicts of Interest
The Authors declare no competing financial interests regarding this study.
Authors’ Contributions
YCL, JHH, MCW and FTH performed the experiments. YHL, ITC and CSC prepared the initial version of the article. YCL, JGC and SSL designed the study, performed the literature review, and prepared the final versions of the article.
Acknowledgements
This study were supported by Chang Bing Show Chwan Memorial Hospital (ID: BRD-18010), Taipei Veterans General Hospital, Yuan-Shan/Su-Ao Branch, Yilan (ID: YSVH10904) and Central Taiwan University of Science and Technology (ID: CTU108-P-103), respectively.
References
- 1.Fiorino C, Guckemberger M, Schwarz M, van der Heide UA, Heijmen B. Technology-driven research for radiotherapy innovation. Mol Oncol. 2020;14(7):1500–1513. doi: 10.1002/1878-0261.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C-H, Chen C-Y, Yeh C-T, Lin K-H. Radiosensitization of hepatocellular carcinoma through targeting radio-associated microRNA. Int J Mol Sci. 2020;21(5):1859. doi: 10.3390/ijms21051859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tam SY, Wu VWC. A review on the special radiotherapy techniques of colorectal cancer. Front Oncol. 2019;9:208. doi: 10.3389/fonc.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Zhang L, Li G, Gao Z. Xanthohumol protects against azoxymethane-induced colorectal cancer in Sprague-Dawley rats. Environ Toxicol. 2020;35(2):136–144. doi: 10.1002/tox.22849. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH. Controversial issues in radiotherapy for rectal cancer: A systematic review. Radiat Oncol J. 2017;35(4):295–305. doi: 10.3857/roj.2017.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvaruso M, Pucci G, Musso R, Bravatà V, Cammarata FP, Russo G, Forte GI, Minafra L. Nutraceutical compounds as sensitizers for cancer treatment in radiation therapy. Int J Mol Sci. 2019;20(21):5267. doi: 10.3390/ijms20215267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercier J, Voutsadakis IA. A systematic review and meta-analysis of retrospective series of regorafenib for treatment of metastatic colorectal cancer. Anticancer Res. 2017;37(11):5925–5934. doi: 10.21873/anticanres.12039. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamoorthy SK, Relias V, Sebastian S, Jayaraman V, Saif MW. Management of regorafenib-related toxicities: A review. Therap Adv Gastroenterol. 2015;8(5):285–297. doi: 10.1177/1756283X15580743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YC, Tsai JJ, Weng YS, Hsu FT. Regorafenib suppresses epidermal growth factor receptor signaling-modulated progression of colorectal cancer. Biomed Pharmacother. 2020;128:110319. doi: 10.1016/j.biopha.2020.110319. [DOI] [PubMed] [Google Scholar]
- 10.Jensen LH, Olesen R, Petersen LN, Boysen AK, Andersen RF, Lindebjerg J, Nottelmann L, Thomsen CEB, Havelund BM, Jakobsen A, Hansen TF. NPY gene methylation as a universal, longitudinal plasma marker for evaluating the clinical benefit from last-line treatment with regorafenib in metastatic colorectal cancer. Cancers. 2019;11(11):1649. doi: 10.3390/cancers11111649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberto M, Falcone R, Mazzuca F, Archibugi L, Castaldi N, Botticelli A, Osti MF, Marchetti P. The role of stereotactic body radiation therapy in oligometastatic colorectal cancer: Clinical case report of a long-responder patient treated with regorafenib beyond progression. Medicine. 2017;96(48):e9023. doi: 10.1097/MD.0000000000009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YS, Sun R, Chen WL, Yau YC, Hsu FT, Chung JG, Tsai CJ, Hsieh CL, Chiu YM, Chen JH. The in vivo radiosensitizing effect of magnolol on tumor growth of hepatocellular carcinoma. In Vivo. 2020;34(4):1789–1796. doi: 10.21873/invivo.11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng MC, Li MH, Chung JG, Liu YC, Wu JY, Hsu FT, Wang HE. Apoptosis induction and AKT/NF-ĸB inactivation are associated with regroafenib-inhibited tumor progression in non-small cell lung cancer in vitro and in vivo. Biomed Pharmacother. 2019;116:109032. doi: 10.1016/j.biopha.2019.109032. [DOI] [PubMed] [Google Scholar]
- 14.Su CM, Weng YS, Kuan LY, Chen JH, Hsu FT. Suppression of PKCδ/NF-ĸB signaling and apoptosis induction through extrinsic/intrinsic pathways are associated magnolol-inhibited tumor progression in colorectal cancer in vitro and in vivo. Int J Mol Sci. 2020;21(10):3527. doi: 10.3390/ijms21103527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandur SK, Deorukhkar A, Pandey MK, Pabón AM, Shentu S, Guha S, Aggarwal BB, Krishnan S. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int J Radiat Oncol Biol Phys. 2009;75(2):534–542. doi: 10.1016/j.ijrobp.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahmanian N, Hosseinimehr SJ, Khalaj A. The paradox role of caspase cascade in ionizing radiation therapy. J Biomed Sci. 2016;23(1):88. doi: 10.1186/s12929-016-0306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manente L, Pecoraro S, Picillo E, Gargiulo U, Gargiulo P, De Luca A, Politano L. Molecular evidence of apoptotic pathway activation in semen samples with high DNA fragmentation. In Vivo. 2015;29(2):289–294. [PubMed] [Google Scholar]
- 18.Noble P, Vyas M, Al-Attar A, Durrant S, Scholefield J, Durrant L. High levels of cleaved caspase-3 in colorectal tumour stroma predict good survival. Br J Cancer. 2013;108(10):2097–2105. doi: 10.1038/bjc.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celec P. Nuclear factor kappa B–molecular biomedicine: The next generation. Biomed Pharmacother. 2004;58(6-7):365–371. doi: 10.1016/j.biopha.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Li F, Sethi G. Targeting transcription factor nuclear factor kappa B to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805(2):167–180. doi: 10.1016/j.bbcan.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, Krishnan S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor kappa B-regulated gene products. Clin Cancer Res. 2008;14(7):2128–2136. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 22.Wu D, Wu P, Zhao L, Huang L, Zhang Z, Zhao S, Huang J. NF-ĸB expression and outcomes in solid tumors: A systematic review and meta-analysis. Medicine. 2015;94(40):e1687. doi: 10.1097/MD.0000000000001687. [DOI] [PMC free article] [PubMed] [Google Scholar]