Abstract
Objective
Interstitial lung disease (ILD) is a major cause of morbidity and mortality in connective tissue diseases (CTDs). We aimed to assess the effect of rituximab ± mycophenolate mofetil (MMF) compared with MMF on pulmonary function and prednisone dosage in patients with CTD‐related ILD (CTD‐ILD).
Methods
This retrospective study included 83 patients from Stanford and Centre Hospitalier de l’Universite de Montreal. Fifteen patients received rituximab ± MMF (rituximab group), and 68 patients received MMF only (control group).
Results
Median ILD duration at the start of treatment was longer in the rituximab group at 47 months (range: 4‐170) versus 6.5 months (range: 0‐164) in controls. Forced vital capacity (FVC) decreased by 3.0% (range: 11%‐21%) after treatment in the rituximab group, whereas it increased by 2.0% (range: 14%‐25%) in the control group (p = 0.025). Diffusing capacity of carbon monoxide (DLCO) decreased by 3.0% (range: 10%‐12%) after treatment in the rituximab group, whereas it increased by 4.5% (range: 30%‐36%) in the control group (p = 0.046). Mixed model analysis controlling for ILD duration, baseline DLCO, systemic sclerosis, pulmonary hypertension, and prednisone use showed no significant difference in FVC or DLCO between groups at 6 months or 1 year. The average daily prednisone dose score decreased after treatment in the rituximab group, whereas it remained unchanged in the control group (p = 0.017).
Conclusion
Rituximab ± MMF did not significantly change pulmonary function compared with MMF alone, but it did result in a relative decrease in average daily prednisone dose in a population with recalcitrant CTD‐ILD.
Introduction
Interstitial lung disease (ILD) is a feature of many connective tissue diseases (CTDs), including systemic sclerosis (SSc), dermatomyositis (DM), polymyositis (PM), mixed connective tissue disease, rheumatoid arthritis (RA), systemic lupus erythematosus, and Sjogren syndrome. CTD‐ILD has typically been treated with nontargeted immunosuppressants, namely cyclophosphamide and mycophenolate mofetil (MMF) based on multicenter clinical trials in scleroderma‐related ILD (SSc‐ILD) (1, 2). However, a quarter of patients continue to progress despite these therapies (3). Evidence on the benefit of corticosteroids is conflicting, though the adverse effects, such as increased infection risk, hypertension, hyperglycemia, neuropsychiatric effects and osteoporosis, are well‐established (4).
Rituximab, a monoclonal antibody against the B‐cell surface antigen CD20, causes sustained depletion of B cells from the peripheral circulation for 6 to 9 months. Studies from animal and human models suggest that B cells play a critical role in the fibrotic process (5), and that B‐cell depletion with rituximab is increasingly being used for the treatment of CTD‐ILD with promising results (6, 7, 8).
The first randomized controlled trial (RCT) evaluating rituximab in SSc‐ILD found that patients treated with rituximab (half of whom were also on MMF) experienced improvement in pulmonary function but no significant change in high‐resolution computed tomography (HRCT) score compared with standard therapy, which varied but generally consisted of prednisone ± MMF or cyclophosphamide (9). The second RCT demonstrated a significant improvement in FVC with rituximab compared with a significant decline with cyclophosphamide in immunosuppression‐naive patients with recent‐onset diffuse cutaneous SSc‐ILD (10).
There are no studies that specifically compare the effects of rituximab with MMF in patients with CTD‐ILD. We chose to compare rituximab ± MMF with MMF alone, as MMF is typically the first‐line treatment in this population.
PATIENTS AND METHODS
Study design and ethics
This was a retrospective study performed at two sites: Stanford University and Centre Hospitalier de l’Universite de Montreal (CHUM). The protocol was approved by the respective Institutional Review Board at each site.
Patient selection
Inclusion criteria were age 18 years or older, diagnosis of a specific CTD based on validated classification criteria, and clinically significant ILD defined as FVC less than 80% predicted, fibrotic and/or inflammatory changes on chest CT not attributable to infection, and no evidence of obstructive lung disease. All patients had at least one set of pulmonary function tests (PFTs) 0 to 6 months prior to treatment initiation (pretreatment) and at least one set of PFTs 6 to 12 months after treatment initiation (posttreatment). Inclusion in the control group and the rituximab + MMF subgroup required documentation of at least 6 months of MMF use between March 1, 2006 and July 1, 2017. Inclusion in the rituximab group required documentation of at least one cycle of rituximab (1000 mg IV on day 1 and day 15) administered between March 1, 2006 and July 1, 2017. Patients were excluded if they received cyclophosphamide or if they were on chronic (>3 months) prednisone at doses greater than 20 mg per day concurrently with MMF and/or rituximab. Average daily prednisone dose during the pretreatment and posttreatment periods were categorized using a scale from 0 to 3 (0 = no prednisone use, 1 = prednisone ≤10 mg/d, 2 = prednisone 10‐20 mg/d, 3 = prednisone 21‐60 mg/d).
Imaging
Chest CTs were reviewed and scored in consensus by two blinded, centralized thoracic radiologists (HG and ZG) using the system described by Goldin et al (11). Each lung was divided into three zones (upper, middle, and lower), and the extent of four different types of pulmonary abnormalities (pure ground‐glass opacities, pure fibrosis, honeycomb cysts, and emphysema) in each of the six zones was scored using a scale from 0 to 4, with 4 indicating the most severe disease. Posttreatment CTs were ordered at the discretion of the treating clinician. The clinicians assessed change in PFTs and symptoms to determine whether or not to repeat CT imaging. For each patient, the total score of the posttreatment CT (performed 6‐18 months after treatment initiation) was compared with that of the pretreatment CT (performed 0‐6 months prior to treatment initiation) and categorized as better/same or worse.
Statistical analysis
The difference in FVC, DLCO, and CT score posttreatment versus pretreatment was compared between the rituximab and control groups by Wilcoxon rank‐sum test for continuous variables and Fisher exact test for categorical variables. Variables with p < 0.15 were retained for covariates in multivariate linear mixed models accounting for repeated measures to assess changes in PFTs over time. As a sensitivity analysis, up to three control patients were matched to each rituximab patient by propensity score matching on baseline DLCO to account for confounding by indication, and identical linear mixed models were applied (12). Additionally, a subgroup analysis assessing patients treated with rituximab + MMF versus MMF alone was performed. All tests were two‐sided and p < 0.05 was considered significant. Statistical software SAS version 9.4 (SAS Institute Inc) was used.
RESULTS
Baseline characteristics
This study included 83 patients (46 from Stanford and 37 from CHUM), 15 of whom received rituximab ± MMF (rituximab group) and 68 of whom received MMF only (control group). Ten of the 15 patients in the rituximab group received both rituximab and MMF. There was no significant difference in the proportion of patients with SSc versus non‐SSc CTD between groups (p = 0.13), but there were nonsignificantly higher proportions of patients with RA and DM/PM in the rituximab group. ILD duration prior to treatment initiation was significantly longer in the rituximab group versus in the control group, and baseline DLCO was numerically lower in the rituximab group. The control group had longer median follow‐up time than the rituximab group. There were no significant differences in the other baseline characteristics (Table 1).
Table 1.
Baseline characteristics
| Characteristic |
Rituximab (n = 15) |
Control (n = 68) |
p value |
|---|---|---|---|
| Age at treatment start in years, median (range) | 57 (43‐82) | 61 (25‐78) | 0.45 |
| Female (%) | 7 (46.7) | 48 (70.6) | 0.13 |
| Race/ethnicity (%) | 0.43 | ||
| White | 8 (53.3) | 38 (55.9) | |
| African American | 2 (13.3) | 5 (7.3) | |
| Hispanic | 0 (0) | 8 (11.8) | |
| Asian | 2 (13.3) | 10 (14.7) | |
| Other/multiple races | 3 (20.0) | 6 (8.8) | |
| Unknown | 0 (0) | 1 (1.5) | |
| Baseline FVC, % predicted, median (range) | 60 (32‐79) | 63 (31‐87) | 0.55 |
| Baseline DLCO, % predicted, median (range) | 52 (28‐76) | 62 (31‐87) | 0.058 |
| ILD duration at treatment start, months (range) | 47 (4‐170) | 7 (0‐164) | 0.0003* |
| Pulmonary hypertension (%) | 4 (26.7) | 8 (11.8) | 0.22 |
| CTD (%) | 0.02* | ||
| SSc | 2 (13.3) | 24 (35.3) | |
| Rheumatoid arthritis | 6 (40.0) | 9 (13.2) | |
| MCTD | 2 (13.3) | 6 (8.8) | |
| Dermatomyositis/polymyositis | 5 (33.3) | 11 (16.2) | |
| Sjogren syndrome | 0 (0) | 12 (17.6) | |
| UCTD | 0 (0) | 6 (8.8) | |
| CTD (SSc vs other) | 0.13 | ||
| Concurrent immunomodulator use (%) | |||
| Prednisone | 14 (93.3) | 49 (72.1) | 0.1 |
| Hydroxychloroquine | 5 (33.3) | 20 (29.4) | 0.76 |
| Azathioprine | 2 (13.3) | 3 (4.4) | 0.22 |
| Leflunomide | 0 (0) | 2 (2.9) | 1 |
| TNF‐alpha inhibitor | 1 (6.7) | 1 (1.5) | 0.33 |
| Abatacept | 0 (0) | 2 (2.9) | 1 |
| IVIg | 2 (13.3) | 2 (2.9) | 0.15 |
| Follow‐up times in months, median (range) | 34.3 (12.7‐56.8) | 41.6 (7.4‐118.7) | 0.017* |
Abbreviations: CTD, connective tissue disease; DLCO, diffusing capacity of carbon monoxide; FVC, forced vital capacity; IVIg, intravenous immunoglobulin; MCTD, mixed CTD; SSc, systemic sclerosis; TNF, tumor necrosis factor; UCTD, undifferentiated CTD.
p < 0.05.
Change in PFTs
The rituximab group demonstrated a decrease in FVC and DLCO posttreatment, whereas the control group demonstrated an increase in both. These differences were statistically significant (Table 2). However, mixed model analysis controlling for potential confounders showed no significant difference in FVC or DLCO between groups over time (Figure 1).
Table 2.
Changes in pulmonary function, imaging, and prednisone use in rituximab versus control groups
| Median change (range) | Rituximab | Control | p value |
|---|---|---|---|
| Absolute change in FVC (% predicted, posttreatment – baseline) | −3.0 (−11, 21) | 2.0 (−14, 25) | 0.03* |
| Absolute change in DLCO (% predicted, posttreatment – baseline) | −3.0 (−10, 12) | 4.5 (−30, 36) | 0.046* |
| Change in CT score (posttreatment – baseline) | 0 (−9, 1) | 0 (−5, 7) | 0.5 |
| Change in average daily prednisone dose (posttreatment – baseline) a | −0.5 (−2.0, 1.0) | 0 (−2.0, 1.0) | 0.02* |
Abbreviations: CT, computed tomography; DLCO, diffusing capacity of carbon monoxide; FVC, forced vital capacity.
Units based on aforementioned scale from 0 to 3 (0 = no prednisone use, 1 = prednisone ≤10 mg/d, 2 = prednisone 10‐20 mg/d, 3 = prednisone 20‐60 mg/d).
p < 0.05.
Figure 1.
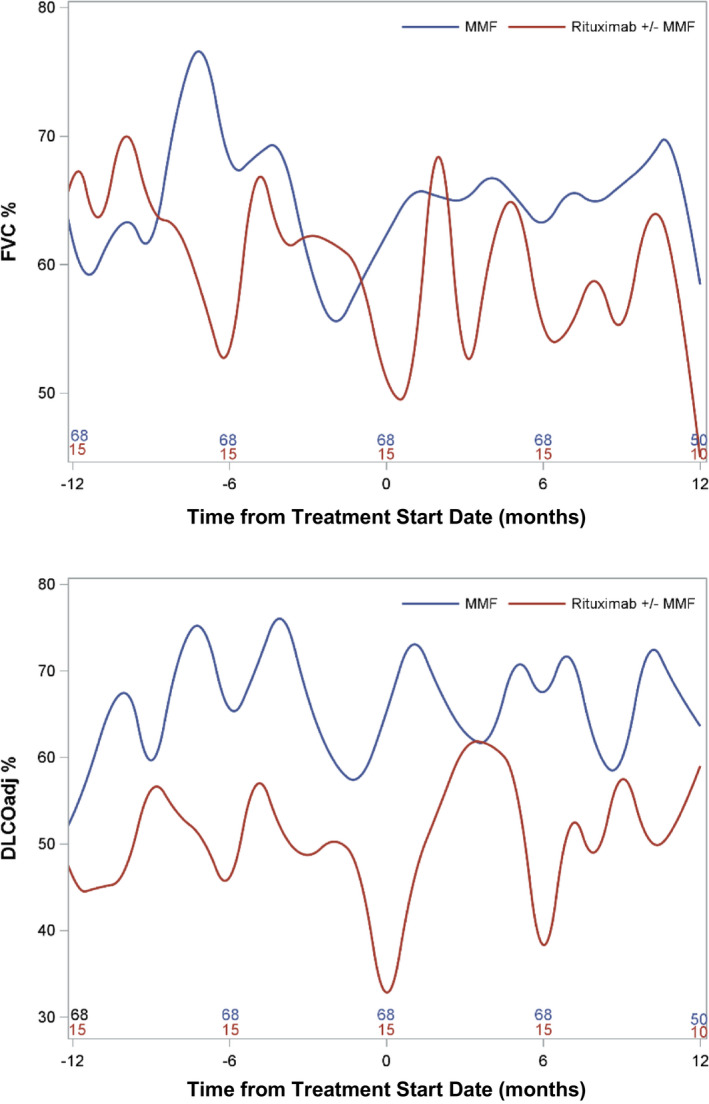
Mixed Model Analysis for Pulmonary Function Parameters Over Time.
Change in imaging
Eight patients in the rituximab group and 19 patients in the control group had pre‐ and posttreatment chest CTs. The proportion of these patients with a usual interstitial pneumonia pattern on imaging was 2/8 (25%) in the rituximab group and 4/19 (21%) in the control group. Patients who did not have posttreatment CTs had a 2% improvement in FVC after treatment, whereas patients who did have a posttreatment CT did not have any change; this difference was statistically significant (p = 0.019). There were no statistically significant differences in age at treatment start, gender, baseline FVCWe or DLCO, or change in DLCO after treatment between groups. As might be expected, the treating clinician was less likely to order a repeat CT if the patient’s FVC was improving after treatment.
The mean baseline chest CT score was higher in the rituximab group (17.5 ± 4.6) compared with that in the control group (12.6 ± 4.4). Pre‐ versus posttreatment CT scores were not different within groups (p = 1 for both groups) or between groups (p = 0.52). Similarly, there was no significant difference in the proportion of posttreatment CTs categorized as better/same versus worse between groups (p = 0.40), though the rituximab group had a numerically lower proportion of patients who worsened (25% vs 47%).
Prednisone use
Average daily prednisone dose pre‐ and posttreatment could be calculated in 14 patients in the rituximab group and 65 in the control group. The prednisone dose score decreased posttreatment in the rituximab group, whereas it remained unchanged in the control group (p = 0.017) (Table 2). Half (7/14) of patients in the rituximab group versus 20% (13/65) of patients in the control group had a decreased prednisone dose score posttreatment.
Sensitivity analyses
Repeating these analyses with propensity score matching on baseline DLCO confirmed the aforementioned results with worsening of FVC and DLCO in the rituximab compared with control group posttreatment, but with lower average daily prednisone dose in the rituximab group. A subgroup analysis assessing patients treated with rituximab + MMF versus MMF alone showed no differences in PFTs but again showed lower average daily prednisone dose in the rituximab group.
Adverse events
Four patients (27%) in the rituximab group had at least one documented infection occurring within 1 year of treatment. One patient required hospitalization for ILD exacerbation with possible superimposed pneumonia; the other three had infections that did not require hospitalization. Two patients had hypogammaglobulinemia documented within 1 year of treatment; however, the hypogammaglobulinemia was present prior to rituximab in both cases. One patient had a documented infusion reaction. No patients developed progressive multifocal leukoencephalopathy or malignancy during or after rituximab treatment. Mortality any time after treatment was 3/15 (20.0%) in the rituximab group versus 7/68 (10.3%) in the control group.
DISCUSSION
This retrospective study assessed the effect of rituximab ± MMF versus MMF alone on PFTs and prednisone dosage in a cohort of patients with CTD‐ILD. Patients in the rituximab group demonstrated a decline in FVC and DLCO posttreatment compared with pretreatment, whereas patients in the control group demonstrated an increase in FVC and DLCO. However, mixed model analysis did not reveal a significant difference in either parameter over time. Notably, despite the fact that patients in the rituximab group had longer disease duration and lower DLCO at baseline, they were able to reduce their average daily prednisone dose posttreatment to a greater degree compared with those treated with MMF alone.
The rituximab group had a numerically lower proportion of patients who had worsening findings on posttreatment chest CT, though this was not statistically significant. There were no significant baseline differences between patients who did versus did not have a posttreatment CT done, but the treating clinician was less likely to order a repeat CT if the patient’s FVC was improving posttreatment.
Compared to other retrospective studies on this topic, our study is unique in that it includes a control group of patients treated with MMF, which is currently considered first‐line treatment for CTD‐ILD. This study has several limitations, including its small sample size and its retrospective nonrandomized design, such that the two groups were not balanced. Baseline DLCO was numerically lower in the rituximab group, so we used propensity score matching to address confounding by indication. We included a heterogeneous patient population with multiple types of CTD. Additionally, many patients had longstanding disease by the time of treatment initiation and may not have been as responsive to immunosuppressive therapy as patients with early disease. However, our data add to the existing body of evidence and support the need for RCTs to investigate the use of rituximab in CTD‐ILD.
Author Contributions
All authors critically revised the manuscript for intellectual content and provided final approval of the version submitted for publication.
Study conception and design
Zhu, Guo, Li, Morisset, Mooney, Raj, Chung.
Acquisition of data
Zhu, Chung, Gagne, Guo, Guenther, Jacobs, Mooney.
Analysis and interpretation of data
Zhu, Li, Morisset, Mooney, Raj, Chung.
Dr. Chung receives funding from the Training Program in Adult and Pediatric Rheumatology (grant 5T32AR050942‐12). Dr. Chung receives funding from the Scleroderma Research Foundation.
Dr. Mooney has received consulting fees from Genentech/Roche and Boehringer Ingelheim (less than $10,000 each). Dr. Chung has received consultant fees from Boerhinger Ingelheim (more than $10,000), Eicos Sciences (less than $10,000), Bristol Myers Squibb (less than $10,000) and Mitsubishi Tanabe (less than $10,000). She has served on the Data Safety Monitoring Board for Reata (less than $10,000). No other disclosures relevant to this article were reported.
References
- 1. Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006;354:2655–66. [DOI] [PubMed] [Google Scholar]
- 2. Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma‐related interstitial lung disease (SLS II): a randomised controlled, double‐blind, parallel group trial. Lancet Respir Med 2016;4:708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoffmann‐Vold AM, Allanore Y, Alves M, Graf N, Airò P, Ananyeva LP, et al. Progressive lung fibrosis with patients with systemic sclerosis‐associated interstitial lung disease in the EUSTAR database [abstract]. Arthritis Rheumatol 2018;70 Suppl 10 URL: https://acrabstracts.org/abstract/progressive‐lung‐fibrosis‐in‐patients‐with‐systemic‐sclerosis‐associated‐interstitial‐lung‐disease‐in‐the‐eustar‐database. [Google Scholar]
- 4. Iudici M, van der Goes MC, Valentini G, Bijlsma JW. Glucocorticoids in systemic sclerosis: weighing up the benefits and risks ‐ a systematic review. Clin Exp Rheumatol 2013;31 Suppl 76:S157–65. [PubMed] [Google Scholar]
- 5. Daoussis D, Liossis SN, Yiannopoulos G, Andonopoulos AP. B‐cell depletion therapy in systemic sclerosis: experimental rationale and update on clinical evidence. Int J Rheumatol 2011;2011:214013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keir GJ, Maher TM, Ming D, Abdullah R, de Lauretis A, Wickremasinghe M, et al. Rituximab in severe, treatment‐refractory interstitial lung disease. Respirology 2014;19:353–9. [DOI] [PubMed] [Google Scholar]
- 7. Jordan S, Distler JH, Maurer B, Huscher D, van Laar JM, Allanore Y, et al. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis 2015;74:1188–94. [DOI] [PubMed] [Google Scholar]
- 8. Sharp C, McCabe M, Dodds N, Edey A, Mayers L, Adamali H, et al. Rituximab in autoimmune connective tissue disease‐associated interstitial lung disease. Rheumatology (Oxford) 2016;55:1318–24. [DOI] [PubMed] [Google Scholar]
- 9. Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Kazantzi A, Sirinian C, et al. Experience with rituximab in scleroderma: results from a 1‐year, proof‐of‐principle study. Rheumatology (Oxford) 2010;49:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatology (Oxford) 2018;57:2106–13. [DOI] [PubMed] [Google Scholar]
- 11. Goldin JG, Lynch DA, Strollo DC, Suh RD, Schraufnagel DE, Clements PJ, et al. High‐resolution CT scan findings in patients with symptomatic scleroderma‐related interstitial lung disease. Chest 2008;134:358–67. [DOI] [PubMed] [Google Scholar]
- 12. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]


