Abstract
Objective
The present study was designed in order to elucidate the roles of serum interleukin 6 (IL‐6) in the pathogenesis in neuropsychiatric systemic lupus erythematosus (NPSLE).
Methods
Paired serum and cerebrospinal fluid (CSF) samples were obtained from 101 patients with SLE when they presented active neuropsychiatric manifestations (69 patients with diffuse psychiatric/neuropsychological syndromes [diffuse NPSLE] and 32 patients with neurologic syndromes or peripheral nervous system involvement [focal NPSLE]) and from 22 control patients without SLE with noninflammatory neurological diseases. The levels of albumin and IL‐6 in CSF and sera were measured by enzyme‐linked immunosorbent assay.
Results
Serum IL‐6 and CSF IL‐6 were elevated in NPSLE compared with non‐SLE controls. Among NPSLE, serum IL‐6 and CSF IL‐6 were significantly elevated in acute confusional state (ACS) compared with non‐ACS diffuse NPSLE (anxiety disorder, cognitive dysfunction, mood disorder, and psychosis) or focal NPSLE. Q albumin (CSF/serum albumin quotient) was also significantly higher in ACS than in the other two groups of NPSLE. Of note, serum IL‐6 (r = 0.2801, p = 0.0207), but not CSF IL‐6 (r = 0.1602, p = 0.1918), was significantly correlated with Q albumin in patients with diffuse NPSLE, including ACS and non‐ACS.
Conclusion
These results indicate that serum IL‐6 as well as CSF IL‐6 is involved in the pathogenesis of NPSLE. Moreover, it is suggested that serum IL‐6 might play a most important role in blood‐brain barrier breakdown in NPSLE.
INTRODUCTION
Neuropsychiatric manifestation in systemic lupus erythematosus (NPSLE) is one of the most recalcitrant complications of the disease, leading to substantial impairment of quality of life as well as disability (1). Among a variety of manifestations of NPSLE, acute confusional state (ACS) in diffuse psychiatric/neuropsychological syndromes (diffuse NPSLE) is the most serious one. Treatment of ACS often requires a combination of glucocorticoids with immunosuppressive agents (1, 2).
A number of studies have demonstrated that cerebrospinal fluid (CSF) interleukin 6 (IL‐6) was elevated in patients with NPSLE, including diffuse NPSLE and focal NPSLE (3, 4, 5). Moreover, several recent studies have disclosed that CSF IL‐6 levels were higher in those with ACS compared with those with diffuse NPSLE other than ACS (anxiety disorder, cognitive dysfunction, mood disorder and psychosis) or in focal NPSLE, indicating that CSF IL‐6 is a surrogate marker for the severity of NPSLE (6, 7).
On the other hand, little is known of the role serum IL‐6 has in NPSLE. Thus, serum IL‐6 was not found to be elevated in patients with NPSLE in a previous study using a bioassay with MH60.BSF2 cells (3). Because such a bioassay is influenced by serum components other than IL‐6, immunoassay, such as ELISA, is mandatory for appropriate evaluation of cytokines in sera. Several studies could not find any significant elevation of serum IL‐6 in NPSLE (8). However, it is possible that the small sample size as well as the lack of evaluation of severity of NPSLE might lead to negative results.
Recent studies have demonstrated that blood‐brain barrier (BBB) damages play a crucial role in the pathogenesis of diffuse NPSLE, especially ACS (7, 9). However, the mechanism of the BBB breakdown in NPSLE remained unclear. Of note, recent studies have revealed the roles of IL‐6 in the damages of a fully human in vitro model of BBB (10). It is therefore possible that IL‐6 might also play a role in the BBB damages in patients with NPSLE. The current study was therefore designed in order to study the association of serum IL‐6 with different NPSLE manifestations, with special attention to its role in BBB damages.
PATIENTS AND METHODS
Patients and samples
One hundred one patients with active SLE that required admission were included in the present study. All patients fulfilled the American College of Rheumatology (ACR) 1982 revised criteria for the classification of SLE (11). Of the 101 patients with SLE, 69 showed diffuse psychiatric/neuropsychological syndromes (diffuse NPSLE) according to the 1999 ACR definition of NPSLE (1), whereas 32 patients showed neuropsychiatric manifestations other than diffuse NPSLE, including neurologic syndromes and peripheral nervous system involvement (focal NPSLE) (Table 1). Eighteen of the sixty‐nine patients with diffuse NPSLE had cases complicated with seizures. The diagnosis of each manifestation of NPSLE was carefully made by neurologists and rheumatologists according to the definition, diagnostic criteria, and methods for ascertainment proposed by ACR in 1999 as appendix A (1) with the aid of psychiatrists. In addition, the diagnosis of all syndromes of NPSLE was further confirmed by the elevation of CSF immunoglobulin G (IgG) index (12) and/or CSF IL‐6 levels (13) with careful exclusion of other conditions that lead to similar neuropsychiatric manifestations. All the patients with NPSLE were hospitalized in Teikyo University Hospital, Kitasato University Hospital, or other correlated hospitals between 1993 and 2015.
Table 1.
Profiles of the patients studied
| Diagnosis | No. of patients | Gender (male/ female) |
Age (mean ± SD) |
Disease duration of SLE, mo (mean ± SD) |
|---|---|---|---|---|
| Total NPSLE | 101 | 12/89 | 39.4 ± 14.6 | 54.8 ± 94.2 |
| Diffuse NPSLE | 69 | 8/61 | 38.1 ± 14.4 | 46.5 ± 89.6 |
| ACS | 34 a | 5/29 | 37.8 ± 15.5 | 34.0 ± 73.9 |
| Non‐ACS | 35 | 3/32 | 38.5 ± 13.5 | 58.5 ± 102.2 |
| Anxiety disorder | 4 | |||
| Cognitive dysfunction | 7 | |||
| Mood disorder | 12 | |||
| Cognitive dysfunction/mood disorder | 2 | |||
| Psychosis | 10 | |||
| Focal NPSLE | 32 | 4/28 | 42.2 ± 14.9 | 74.8 ± 97.1 |
| Cerebrovascular disease (CVD) | 10 | |||
| Demyelinating syndrome | 1 | |||
| Headache | 3 | |||
| Movement disorder | 2 | |||
| Myelitis | 1 | |||
| Seizure disorder | 12 | |||
| Aseptic meningitis | 1 | |||
| Polyneuropathy | 2 | |||
| Non‐SLE control | 22 | 21/1 | 49.4 ± 10.5 | … |
Abbreviations: ACS, acute confusional state; NPSLE, neuropsychiatric SLE; SLE, systemic lupus erythematosus.
Three patients also presented with myelitis.
In addition, 22 patients with non‐SLE noninflammatory neurologic diseases (eight with cervical spondylosis, seven with cerebrovascular diseases, three with neurodegenerative diseases, two with hyperventilation syndrome, one with headache, and one with diabetic neuropathy) were studied as non‐SLE controls. No patients had complications with severe infection or tumors that would result in the elevation of serum IL‐6. All 123 patients gave informed consent in accordance with the Helsinki Declaration, and the study was approved by the institutional ethical committee of Teikyo University School of Medicine.
CSF specimens were obtained from the patients by a lumbar puncture on the same day serum samples were obtained, when the diagnosis of NPSLE was made by neurologists and rheumatologists. These samples were kept frozen at −30°C until they were assayed. All assays were performed without knowledge of the diagnosis or clinical presentations. Furthermore, upon entering the present study, the diagnosis of the 101 patients with NPSLE and its classification was reconfirmed by hospital case records.
Measurement of IL‐6 and albumin
IL‐6 in CSF and sera was measured by ELISA (R&D Systems, Minneapolis, Minnesota). In some patients, CSF IL‐6 was determined by bioassay using IL‐6 dependent cell line MH60.BSF2 (3). The IL‐6 values in CSF determined by MH60.BSF2 cell bioassay have been confirmed to be closely correlated with those determined by ELISA. Albumin in CSF and sera was measured by ELISA using Human Albumin ELISA Quantitation Set (Bethyl Laboratories, Montgomery, Texas) (9, 14). BBB function was evaluated by Q albumin (CSF albumin × 1000 / serum albumin) as previously described (9, 12, 14).
Statistical analysis
Differences in various parameters among various groups of NPSLE were analyzed by the Kruskal‐Wallis test with the Dunn multiple comparison test. Correlations between various variables were determined by Spearman rank correlation test. All tests were carried out using GraphPad Prism 7.03 (GraphPad Software, Inc., San Diego, California).
RESULTS
CSF IL‐6 levels in various subsets of SLE
Initial experiments examined CSF levels of IL‐6 in various diseases. The baseline variables in various groups of NPSLE are shown in Table 2. There were no significant differences in the parameters in the four groups of NPSLE, except for CH50, C3, and C4, which were significantly lower in ACS or diffuse NPSLE compared with focal NPSLE and for anti‐Sm antibodies, which were significantly higher in ACS compared with non‐ACS diffuse NPSLE or focal NPSLE. As shown in Figure 1, CSF IL‐6 levels were significantly elevated in patients with NPSLE (336.7 ± 149.7 pg/ml [mean ± SEM]) compared with patients with non‐SLE control (2.6±1.0 pg/ml). Of note, CSF IL‐6 levels were significantly higher in ACS (863.9 ± 431.5 pg/ml) than they were in non‐ACS diffuse NPSLE (anxiety disorder, cognitive dysfunction, mood disorder, and psychosis) (41.5 ± 15.4 pg/ml) or in focal NPSLE (94.3 ± 53.7 pg/ml). The results thus confirm that CSF IL‐6 levels are elevated in patients with NPSLE. Moreover, the data have underscored that CSF IL‐6 levels are associated with the severity of NPSLE, being highest in ACS in diffuse NPSLE.
Table 2.
Baseline variables in various groups of neuropsychiatric systemic lupus erythematosus
| Diagnosis | n | Anti‐DNA antibodies (IU/ml) | Anti‐Sm antibodies (index) | CH50 (U/ml) | C3 (mg/l) | C4 (mg/l) |
|---|---|---|---|---|---|---|
| Diffuse NPSLE | 69 |
94.2 ± 16.7 (n = 66) |
60.5 ± 9.3 (n = 69) |
24.2 ± 22 b (n = 61) |
56.2 ± 3.9 b (n = 62) |
11.8 ± 1.3 b (n = 62) |
| ACS | 34 |
103.5 ± 23.6 (n = 34) |
81.7 ± 16.0 a (n = 34) |
21.3 ± 2.4 b (n = 33) |
52.2 ± 4.5 b (n = 34) |
11.1 ± 1.7 b (n = 34) |
| Non‐ACS diffuse NPSLE | 35 |
84.3 ± 23.9 (n = 32) |
40.0 ± 8.8 (n = 35) |
27.6 ± 3.9 (n = 28) |
61.1 ± 6.5 (n = 28) |
12.6 ± 2.0 (n = 28) |
| Focal NPSLE | 32 |
73.0 ± 34.8 (n = 32) |
23.6 ± 6.3 (n = 32) |
34.2 ± 3.1 (n = 31) |
78.7 ± 5.7 (n = 30) |
16.3 ± 1.8 (n = 30) |
Data are reported as means ± SEM. Numbers in parenthesis indicate the number of patients with available data.
Abbreviations: ACS, acute confusional state; NCSLE, neuropsychiatric SLE; SLE, systemic lupus erythematosus.
Compared with non‐ACS diffuse NPSLE or with focal NPSLE, p < 0.05.
Compared with focal NPSLE, p < 0.05.
Figure 1.
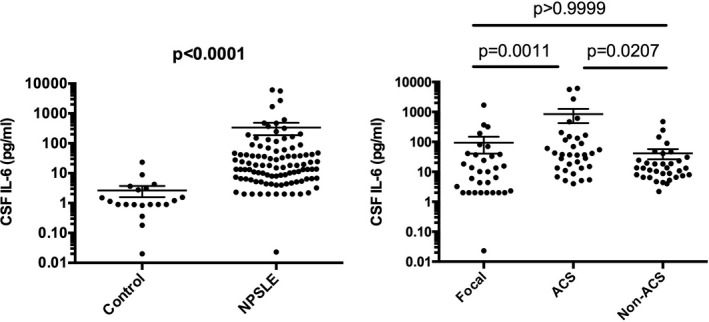
Cerebrospinal fluid (CSF) interleukin 6 (IL‐6) in neuropsychiatric systemic lupus erythematosus (NPSLE). A, Comparison between NPSLE and non‐SLE control. B, Comparison among various subtypes of NPSLE. Non‐ACS is representative of diffuse NPSLE other than ACS, including anxiety disorder, cognitive dysfunction, mood disorder, and psychosis. Focal represents focal NPSLE. Statistical analysis was performed by, A, Mann‐Whitney U test and, B, by Kruskal‐Wallis test with Dunn multiple comparison. ACS, acute confusional state.
Serum IL‐6 levels in various subsets of SLE
The next experiments explored serum levels of IL‐6 in various diseases. As shown in Figure 2, serum IL‐6 levels were significantly elevated in patients with NPSLE (89.8 ± 35.05 pg/ml [mean ± SEM]) compared with patients with non‐SLE control (2.0 ± 0.9 pg/ml). More interestingly, serum IL‐6 levels were also significantly higher in ACS (204.8 ± 94.9 pg/ml) compared with non‐ACS diffuse NPSLE (anxiety disorder, cognitive dysfunction, mood disorder, and psychosis) (16.0 ± 5.4 pg/ml) or in focal NPSLE (52.1 ± 44.3 pg/ml). The results indicate that serum IL‐6 levels are elevated in patients with NPSLE. Moreover, the data have disclosed that serum IL‐6 levels are also associated with the severity of NPSLE, being highest in ACS in diffuse NPSLE.
Figure 2.
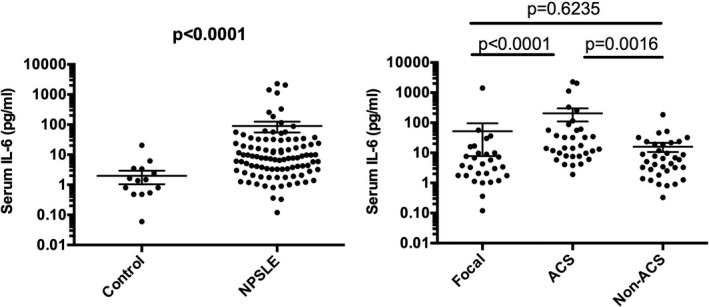
Serum interleukin 6 (IL‐6) in NPSLE. A, Comparison between neuropsychiatric systemic lupus erythematosus (NPSLE) and non‐SLE control. B, Comparison among various subtypes of NPSLE. Non‐ACS is representative of diffuse NPSLE other than ACS, including anxiety disorder, cognitive dysfunction, mood disorder, and psychosis. Focal represents focal NPSLE. Statistical analysis was performed by, A, Mann‐Whitney U test and, B, by Kruskal‐Wallis test with Dunn multiple comparison. ACS, acute confusional state.
Relationship between serum IL‐6 and CSF IL‐6 in NPSLE
We next compared CSF IL‐6 with serum IL‐6 in patients with NPSLE. As shown in Figure 3, there is a significant correlation between CSF IL‐6 and serum IL‐6 in diffuse NPSLE or total NPSLE. IL‐6 in serum and CSF in various groups of NPSLE is summarized in Table 3. We have compared serum IL‐6 among subgroups of diffuse NPSLE, including ACS, mood disorder, and psychosis. Although serum IL‐6 in ACS appeared to be higher than that in mood disorder or in psychosis, it did not reach statistical significance. There was no significant difference in serum IL‐6 between mood disorder and psychosis. Again, although CSF IL‐6 appeared to be higher in ACS than that in mood disorder or in psychosis, it did not reach statistical significance. There was no significant difference in CSF IL‐6 between mood disorder and psychosis. As for focal NPSLE, profiles of CSF IL‐6 and serum IL‐6 appear to differ between cerebrovascular disease (CVD) and seizures, both of which may involve antiphospholipid antibodies, although there was no statistical difference.
Figure 3.
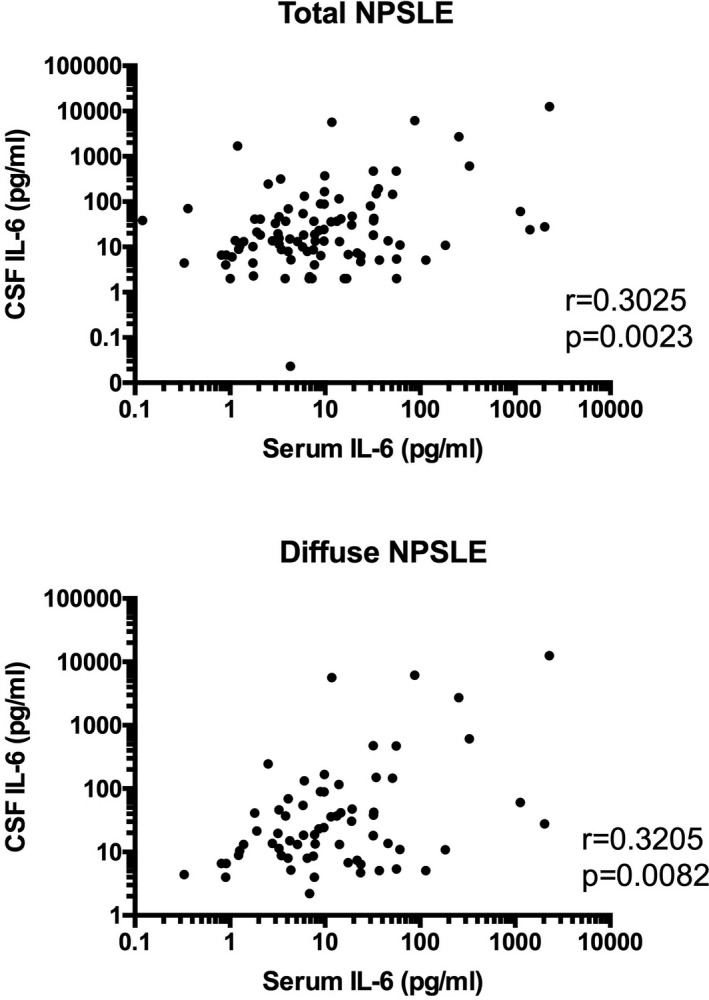
Significant correlation between serum interleukin 6 (IL‐6) and cerebrospinal fluid (CSF) IL‐6 in patients with diffuse neuropsychiatric systemic lupus erythematosus (NPSLE) or with total NPSLE. Statistical significance was analyzed by Spearman rank correlation test.
Table 3.
Interleukin 6 in serum and cerebrospinal fluid in various groups of neuropsychiatric systemic lupus erythematosus
| Diagnosis | No. of patients | Serum IL‐6 (pg/ml) a | CSF IL‐6 (pg/ml) a |
|---|---|---|---|
| Total NPSLE | 101 | 89.8 ± 35.1 | 336.7 ± 149.7 |
| Diffuse NPSLE | 69 | 107.6 ± 47.2 | 452.7 ± 220.1 |
| ACS | 34 | 204.8 ± 94.9 | 863.9 ± 431.5 |
| Non‐ACS | 35 | 16.0 ± 5.4 | 41.5 ± 15.4 |
| Anxiety disorder | 4 | ||
| Cognitive dysfunction | 7 | 8.8 ± 3.3 | 16.9 ± 5.4 |
| Mood disorder | 12 | 27.2 ± 14.9 | 43.0 ± 21.6 |
| Cognitive dysfunction/mood disorder | 2 | 3.4 ± 2.6 | 12.5 ± 5.9 |
| Psychosis | 10 | 14.1 ± 5.2 | 70.9 ± 51.2 |
| Focal NPSLE | 32 | 52.1 ± 44.3 | 94.3 ± 53.7 |
| Cerebrovascular disease (CVD) | 10 | 145.9 ± 141.9 | 18.3 ± 6.7 |
| Demyelinating syndrome | 1 | ||
| Headache | 3 | ||
| Movement disorder | 2 | ||
| Myelitis | 1 | ||
| Seizure disorder | 12 | 5.5 ± 1.7 | 203.7 ± 140.3 |
| Aseptic meningitis | 1 | ||
| Polyneuropathy | 2 | ||
| Non‐SLE control | 22 | 2.0 ± 0.9 | 2.6 ± 1.1 |
Abbreviations: ACS, acute confusional state; CSF, cerebrospinal fluid; IL‐6, interleukin 6; NCSLE, neuropsychiatric SLE; SLE, systemic lupus erythematosus.
Data are presented as means ± SEM.
Relationship between BBB integrity and serum IL‐6 in NPSLE
Previous studies have revealed that BBB damages play a crucial role in the pathogenesis of diffuse NPSLE, especially ACS (7, 9). Consistently, Q albumin values were significantly elevated in ACS (11.32 ± 1.95 [mean ± SEM]) compared with those in non‐ACS diffuse NPSLE (4.39 ± 0.70) or in focal NPSLE (3.65 ± 0.52) in our series of patients (Figure 4). Of note, as shown in Figures 5 and 6, serum IL‐6 (r = 0.2801, p = 0.0207), but not CSF IL‐6 (r = 0.1602, p = 0.1918), was significantly correlated with Q albumin in patients with diffuse NPSLE, including ACS and non‐ACS. The results suggested that serum IL‐6 might play a most important role in BBB breakdown in NPSLE.
Figure 4.
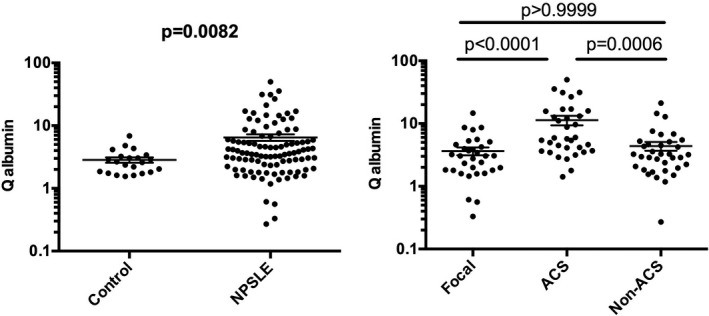
Blood‐brain barrier functions (Q albumin) in neuropsychiatric systemic lupus erythematosus (NPSLE). A, Comparison between NPSLE and non‐SLE control. B, Comparison among various subtypes of NPSLE. Non‐ACS is representative of diffuse NPSLE other than ACS, including anxiety disorder, cognitive dysfunction, mood disorder, and psychosis. Focal represents focal NPSLE. Statistical analysis was performed by, A, Mann‐Whitney U test and, B, by Kruskal‐Wallis test with Dunn multiple comparison. ACS, acute confusional state.
Figure 5.
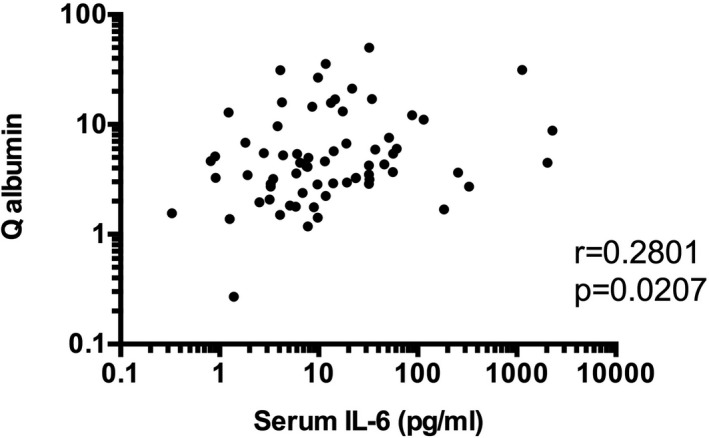
Significant correlation between serum interleukin 6 (IL‐6) and Q albumin in patients with diffuse neuropsychiatric systemic lupus erythematosus (NPSLE). Statistical significance was analyzed by Spearman rank correlation test.
Figure 6.
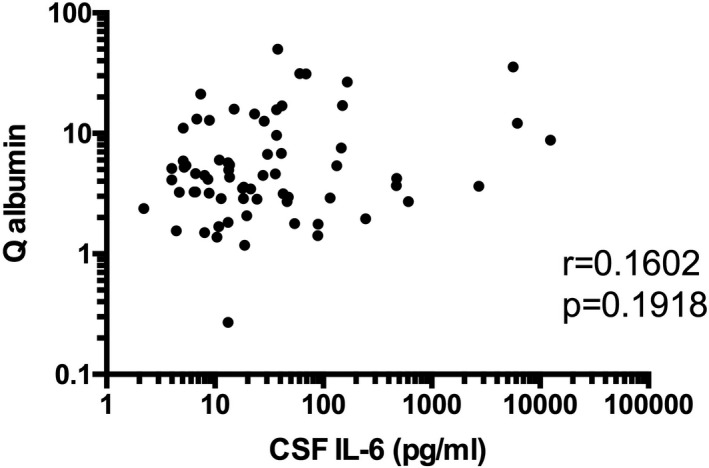
Lack of correlation between cerebrospinal fluid (CSF) interleukin 6 (IL‐6) and Q albumin in patients with diffuse NPSLE. Statistical significance was analyzed by Spearman rank correlation test.
Previous studies have reported on the association between anti‐Sm antibodies and ACS (9). In addition, it has been suggested that anti‐Sm antibodies might play a role in the BBB damages (15). Therefore, we next examined the association between anti‐Sm antibodies and serum IL‐6 levels. As shown in Figure 7, serum anti‐Sm antibodies were significantly correlated with serum IL‐6. It is therefore suggested that serum IL‐6 might be involved in BBB damages mediated by anti‐Sm antibodies.
Figure 7.
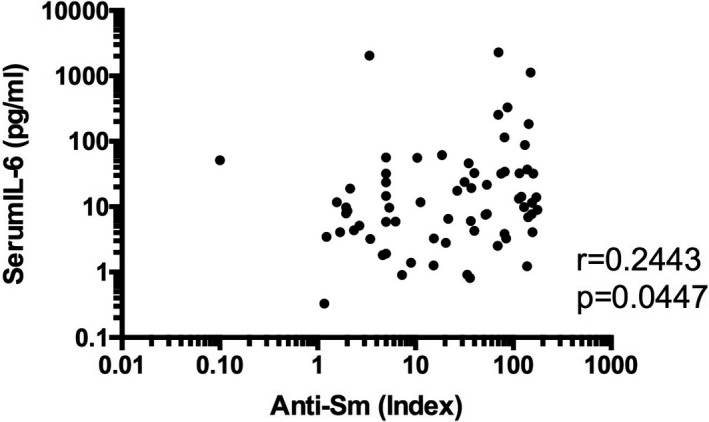
Significant correlation between serum anti‐Sm antibodies and serum interleukin 6 (IL‐6) in patients with diffuse neuropsychiatric systemic lupus erythematosus (NPSLE). Statistical significance was analyzed by Spearman rank correlation test.
DISCUSSION
Some recent studies have suggested that CSF IL‐6 levels might be a surrogate marker for the severity of NPSLE. Thus, it has been shown that CSF IL‐6 levels were higher in ACS compared with those in diffuse NPSLE other than ACS (anxiety disorder, cognitive dysfunction, mood disorder, and psychosis) or in focal NPSLE (6, 7). The results in the current study have confirmed that CSF IL‐6 levels were the highest in ACS, the severest form in NPSLE. It is therefore concluded that CSF IL‐6 is a surrogate marker of disease activity of NPSLE.
As to serum IL‐6 and the severity of NPSLE, little has been known. Some reports have demonstrated that serum IL‐6 levels were elevated in NPSLE (98.5 ± 133.5 pg/ml (8) and 21.68 ± 14.34 pg/ml (16)), which were comparable with the serum IL‐6 levels in the present study. Notably, it has been reported that serum IL‐6 predicted viral meningitis at 66 pg/ml with maximal sensitivity and specificity (17). In addition, we had one patient with infectious meningitis who showed the elevation of serum IL‐6 to 50.1 pg/ml. Thus, the levels of elevation of serum IL‐6 in NPSLE in the present study are considered to be clinically meaningful. However, the differences among the subsets of NPSLE have not been examined, probably because of the paucity of samples. Notably, the data in the present study have disclosed that serum IL‐6 levels were higher in ACS compared with those in non‐ACS diffuse NPSLE or in focal NPSLE. Thus, it is also concluded that serum IL‐6 is also a surrogate marker of the severity of NPSLE. In fact, previous studies disclosed that serum IL‐6 significantly reduced the survival of patients with ACS (18).
We previously reported that CSF IL‐6 concentrations were useful in the diagnosis of lupus psychosis or diffuse NPSLE (13). There is a significant correlation between CSF IL‐6 and serum IL‐6 in diffuse NPSLE or total NPSLE in the present study. Therefore, it is possible that the diagnosis of diffuse NPSLE or ACS could be made by serum IL‐6 concentrations. Thus, the study to compare serum IL‐6 levels in ACS or diffuse NPSLE with those in ACS‐like symptoms or other diffuse NPSLE‐like symptoms due to causes other than SLE would deserve consideration.
The profiles of CSF IL‐6 and serum IL‐6 appear to differ between CVD and seizures, which both might involve antiphospholipid antibodies, although it did not reach statistical difference. Also, in some patients, both conditions might be caused by vasculitis (1, 19), which may result in the elevation of serum IL‐6 and CSF IL‐6. Therefore, it would be interesting to use a study with a greater number of patients to delineate the differences in the pathogenesis among various manifestations of focal NPSLE.
Recent studies have emphasized the importance of the breakdown of BBB in the pathogenesis of ACS in NPSLE (7, 9, 14). Thus, the bleached BBB might allow a variety of autoantibodies directed to neuronal cells to enter the central nervous system. Consistently, the present study has confirmed that Q albumin, a parameter of BBB integrity, was higher in ACS than in non‐ACS diffuse NPSLE or in focal NPSLE. More importantly, the data in the present study have revealed that Q albumin was significantly correlated with serum IL‐6 but not with CSF IL‐6 in patients with diffuse NPSLE. It is therefore suggested that serum IL‐6, but not CSF IL‐6, might be involved in the damages of the BBB.
In the present study, CSF IL‐6 was correlated with serum IL‐6. A recent study suggests that elevation of CSF IL‐6 might be due to transudation of IL‐6 from systemic circulation (7). Thus, the primary event is the elevation of serum IL‐6, which might result in BBB damages, whereas the elevation of CSF IL‐6 is a secondary event to BBB damages. Therefore, the relationship between serum IL‐6 and BBB damages might be more direct than that between CSF IL‐6 and BBB damages. The difference in the causal relationship might reflect the difference in the strength of correlation with Q albumin between serum IL‐6 and CSF IL‐6.
The expression of a variety of autoantibodies is a hallmark of SLE. In particular, several autoantibodies have been implicated in the pathogenesis of NPSLE (14, 15, 20). Among these, anti‐Sm has been found to be associated with ACS (9). More importantly, recent studies have disclosed that serum anti‐Sm was significantly correlated with Q albumin in patients with NPSLE (15). On the other hand, recent in vitro studies have demonstrated that anti‐Sm and anti‐RNP antibodies synergistically upregulate the expression of IL‐6 and tumor necrosis factor‐α at protein and messenger RNA levels in human monocytes (21). Moreover, the data in the present study have demonstrated that serum anti‐Sm antibodies were significantly correlated with serum IL‐6. Therefore, it is likely that IL‐6 might be involved, at least in part, in the damages of BBB mediated by anti‐Sm.
It should be noted that antiribosomal P protein antibody (anti‐P) and anti–N‐methyl D‐aspartate receptor antibody (anti‐NR2) have been detected in sera from patients with NPSLE (22, 23, 24). Moreover, these antibodies have been found to promote the production of proinflammatory cytokines, including IL‐6, when they bind to target cells (25, 26). It is therefore possible that these autoantibodies might damage BBB function through upregulation of IL‐6 production. Because anti‐Sm, anti‐NR2, and anti‐P bind endothelial cells (21, 26, 27), it is also possible that all these autoantibodies might affect BBB function through their direct action on endothelial cells.
Neuromyelitis optica spectrum disorder (NMOSD) is an inflammatory disease in which anti‐AQP4 antibodies play a crucial role (24). Anti‐AQP4 antibodies are produced mainly in the periphery, whereas they target astrocytes behind the BBB (28). Therefore, disruption of BBB has been considered to be crucial for the pathogenesis of NMOSD. Notably, recent studies have shown that the serum IgG from patients NMOSD (NMO‐IgG) plays a role in the BBB dysfunction in NMOSD. Thus, it has been disclosed in in vitro studies that NMO‐IgG induces IL‐6 production by AQP4‐positive astrocytes and that IL‐6 signaling to endothelial cells decreases barrier function (29). In fact, it has been recently demonstrated that monoclonal antibodies to human IL‐6 receptor, such as satralizumab and tocilizumab, reduced the risk of relapse in patients with NMOSD in randomized, multicenter, open‐labeled trials (30, 31), confirming the critical role of IL‐6 signaling in the pathogenesis. The elevation of serum IL‐6 and its positive correlation with BBB damages in ACS in NPSLE suggest that such therapies targeting IL‐6 signaling by tocilizumab or satralizumab might have beneficial effects in the treatment of ACS. Thus, further studies to explore the efficacy of these monoclonal antibodies deserve consideration.
The limitation of our study is that it is cross sectional and the observations are associations and not fully causal, though the involvement of IL‐6 in BBB dysfunction caused by NMO‐IgG suggests plausibility (29). Another limitation is the possibility that cytokines other than IL‐6 might have properties that damage endothelial cells, leading to BBB dysfunction. In fact, the correlation of serum IL‐6 and Q albumin was relatively low, although it was statistically significant. Therefore, it would be ideal to examine a variety of cytokines in sera, such as tumor necrosis factor‐α and vascular endothelial growth factor (32).
AUTHOR CONTRIBUTIONS
Hirohata had full access to all of the data in the study and takes responsibility for the decision to submit for publication. Hirohata participated in experimental procedures, drafted the manuscript, and critically revised the work. Kikuchi critically revised. All the authors read and approved the final version of the manuscript to be published.
Study conception and design
Hirohata.
Acquisition of data
Hirohata, Kikuchi.
Analysis and interpretation of data
Hirohata, Kikuchi.
Acknowledgments
The authors wish to thank Dr. Tamiko Yanagida and Ms. Terumi Mizuno for their technical assistance.
This work was supported by a grant‐in‐aid (c) from the Ministry of Education, Culture, Science, and Sports of Japan (grant 23591447)
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999;42:599–608. [DOI] [PubMed] [Google Scholar]
- 2. Bertsias GK, Ioannidis JP, Aringer M, Bollen E, Bombardieri S, Bruce IN, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis 2010;69:2074–82. [DOI] [PubMed] [Google Scholar]
- 3. Hirohata S, Miyamoto T. Elevated levels of interleukin‐6 in cerebrospinal fluid from patients with systemic lupus erythematosus and central nervous system involvement. Arthritis Rheum 1990;33:644–9. [DOI] [PubMed] [Google Scholar]
- 4. Trysberg E, Carlsten H, Tarkowski A. Intrathecal cytokines in systemic lupus erythematosus with central nervous system involvement. Lupus 2000;9:498–503. [DOI] [PubMed] [Google Scholar]
- 5. Fragoso‐Loyo H, Richaud‐Patin Y, Orozco‐Narváez A, Dávila‐Maldonado L, Atisha‐Fregoso Y, Llorente L, et al. Interleukin‐6 and chemokines in the neuropsychiatric manifestations of systemic lupus erythematosus. Arthritis Rheum 2007;56:1242–50. [DOI] [PubMed] [Google Scholar]
- 6. Katsumata Y, Harigai M, Kawaguchi Y, Fukasawa C, Soejima M, Takagi K, et al. Diagnostic reliability of cerebral spinal fluid tests for acute confusional state (delirium) in patients with systemic lupus erythematosus: interleukin 6 (IL‐6), IL‐8, interferon‐α, IgG index, and Q‐albumin. J Rheumatol 2007;34:2010–7. [PubMed] [Google Scholar]
- 7. Asano T, Ito H, Kariya Y, Hoshi K, Yoshihara A, Ugawa Y, et al. Evaluation of blood‐brain barrier function by quotient α2 macroglobulin and its relationship with interleukin‐6 and complement component 3 levels in neuropsychiatric systemic lupus erythematosus. PLoS One 2017;12:e0186414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoshio T, Okamoto H, Kurasawa K, Dei Y, Hirohata S, Minota S. IL‐6, IL‐8, IP‐10, MCP‐1 and G‐CSF are significantly increased in cerebrospinal fluid but not in sera of patients with central neuropsychiatric lupus erythematosus. Lupus 2016;25:997–1003. [DOI] [PubMed] [Google Scholar]
- 9. Hirohata S, Sakuma Y, Yanagida T, Yoshio T. Association of cerebrospinal fluid anti‐Sm antibodies with acute confusional state in systemic lupus erythematosus. Arthritis Res Ther 2014;16:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mantle JL, Lee KH. A differentiating neural stem cell‐derived astrocytic population mitigates the inflammatory effects of TNF‐α and IL‐6 in an iPSC‐based blood‐brain barrier model. Neurobiol Dis 2018;119:113–20. [DOI] [PubMed] [Google Scholar]
- 11. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 12. Winfield JB, Shaw M, Silverman LM, Eisenberg RA, Wilson HA III, Koffler D. Intrathecal IgG synthesis and blood‐brain barrier impairment in patients with systemic lupus erythematosus and central nervous system dysfunction. Am J Med 1983;74:837–44. [DOI] [PubMed] [Google Scholar]
- 13. Hirohata S, Kanai Y, Mitsuo A, Tokano Y, Hashimoto H. NPSLE Research Subcommittee. Accuracy of cerebrospinal fluid IL‐6 testing for diagnosis of lupus psychosis. A multicenter retrospective study. Clin Rheumatol 2009;28:1319–23. [DOI] [PubMed] [Google Scholar]
- 14. Hirohata S, Arinuma Y, Yanagida T, Yoshio T. Blood‐brain barrier damages and intrathecal synthesis of anti‐N‐methyl‐D‐aspartate receptor NR2 antibodies in diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematosus. Arthritis Res Ther 2014;16:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirohata S, Sakuma Y, Matsueda Y, Arinuma Y, Yanagida T. Role of serum autoantibodies in blood brain barrier damages in neuropsychiatric systemic lupus erythematosus. Clin Exp Rheumatol 2018;36:1003–7. [PubMed] [Google Scholar]
- 16. Wang JB, Li H, Wang LL, Liang HD, Zhao L, Dong J. Role of IL‐1β, IL‐6, IL‐8 and IFN‐γ in pathogenesis of central nervous system neuropsychiatric systemic lupus erythematous. Int J Clin Exp Med 2015;8:16658–63. [PMC free article] [PubMed] [Google Scholar]
- 17. Lee JY, Son M, Kang JH, Choi UY. Serum interleukin‐6 levels as an indicator of aseptic meningitis among children with enterovirus 71‐induced hand, foot and mouth disease. Postgrad Med 2018;130:258–63. [DOI] [PubMed] [Google Scholar]
- 18. Abe G, Kikuchi H, Arinuma Y, Hirohata S. Brain MRI in patients with acute confusional state of diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematosus. Mod Rheumatol 2017;27:278–83. [DOI] [PubMed] [Google Scholar]
- 19. Kimura M, Aramaki K, Wada T, Nishi K, Matsushita R, Iizuka N, et al. Reversible focal neurological deficits in systemic lupus erythematosus: report of 2 cases and review of the literature. J Neurol Sci 2008;272:71–6. [DOI] [PubMed] [Google Scholar]
- 20. Hanly JG, Kozora E, Beyea SD, Birnbaum J. Review: nervous system disease in systemic lupus erythematosus: current status and future directions. Arthritis Rheumatol 2019;71:33–42. [DOI] [PubMed] [Google Scholar]
- 21. Matsueda Y, Arinuma Y, Nagai T, Hirohata S. Synergistic enhancement of production of proinflammatory cytokines of human peripheral blood monocytes by anti‐Sm and anti‐RNP antibodies. PLoS One 2018;13:e0209282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Isshi K, Hirohata S. Association of anti‐ribosomal P protein antibodies with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum 1996;39:1483–90. [DOI] [PubMed] [Google Scholar]
- 23. DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti‐DNA antibodies cross‐reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med 2001;7:1189–93. [DOI] [PubMed] [Google Scholar]
- 24. Mader S, Jeganathan V, Arinuma Y, Fujieda Y, Dujmovic I, Drulovic J, et al. Understanding the antibody repertoire in neuropsychiatric systemic lupus erythematosus and neuromyelitis optica spectrum disorders: do they share common targets? Arthritis Rheumatol 2018;70:277–86. [DOI] [PubMed] [Google Scholar]
- 25. Nagai T, Arinuma Y, Yanagida T, Yamamoto K, Hirohata S. Anti‐ribosomal P protein antibody in human systemic lupus erythematosus up‐regulates the expression of proinflammatory cytokines by human peripheral blood monocytes. Arthritis Rheum 2005;52:847–55. [DOI] [PubMed] [Google Scholar]
- 26. Yoshio T, Okamoto H, Hirohata S, Minota S. IgG anti‐NR2 glutamate receptor autoantibodies from patients with systemic lupus erythematosus activate endothelial cells. Arthritis Rheum 2013;65:457–63. [DOI] [PubMed] [Google Scholar]
- 27. Frampton G, Moriya S, Pearson JD, Isenberg DA, Ward FJ, Smith TA, et al. Identification of candidate endothelial cell autoantigens in systemic lupus erythematosus using a molecular cloning strategy: a role for ribosomal P protein P0 as an endothelial cell autoantigen. Rheumatology (Oxford) 2000;39:1114–20. [DOI] [PubMed] [Google Scholar]
- 28. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic‐spinal multiple sclerosis binds to the aquaporin‐4 water channel. J Exp Med 2005;202:473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takeshita Y, Obermeier B, Cotleur AC, Spampinato SF, Shimizu F, Yamamoto E, et al. Effects of neuromyelitis optica–IgG at the blood–brain barrier in vitro. Neurol Neuroimmunol Neuroinflamm 2016;4:e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang C, Zhang M, Qiu W, Ma H, Zhang X, Zhu Z, et al. TANGO Study Investigators. Safety and efficacy of tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum disorder (TANGO): an open‐label, multicentre, randomised, phase 2 trial. Lancet Neurol 2020;19:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Traboulsee A, Greenberg BM, Bennett JL, Szczechowski L, Fox E, Shkrobot S, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double‐blind, multicentre, placebo‐controlled phase 3 trial. Lancet Neurol 2020;19:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Michalak S, Kalinowska‐Lyszczarz A, Rybacka‐Mossakowska J, Zaborowski M, Kozubski W. The associations between serum vascular endothelial growth factor, tumor necrosis factor and interleukin 4 with the markers of blood‐brain barrier breakdown in patients with paraneoplastic neurological syndromes. J Neural Transm (Vienna) 2019;126:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]


