Abstract
The “gold” standard radiological method for the diagnosis of the lung findings in COVID-19 patients is known to be the chest high-resolution computed tomography. However, in a mass casualty scenario, as in times of COVID-19 epidemics, in which emergency departments, intensive care units, and whole hospitals are massive overcrowded and continue to change their original configuration, a more rapid, flexible, and performant diagnostic approach is required. Moreover, the high contagiousness of these patients and the risk of transporting critical patients make chest computed tomography (CT) a limited option for them. Lung ultrasonography, a rapid, reliable, bedside, nonradiating and repeatable examination, with its sensitivity closed to chest CT and much higher than the chest X-ray for COVID patients, has proved to be in COVID-19 pandemic as crucial diagnostic and monitoring tool of patients with acute respiratory failure. It could be performed in the prehospital setting, in the emergency department (as part of the diagnostic approach), up to the normal wards and the intensive care unit. The aim of this article is to describe the central role of LUS in the management of COVID-19 critically ill patients with acute respiratory distress syndrome, as valid diagnostic and monitoring point-of-care technique.
Keywords: Acute respiratory distress syndrome, COVID-19, lung ultrasound, pandemic, pneumonia
INTRODUCTION
The “gold” standard radiological method for the diagnosis of the lung findings in COVID-19 patients is known to be the chest high-resolution computed tomography (HRCT).
However, in a mass casualty scenario, as in time of COVID-19 epidemics, in which emergency departments, intensive care units, and whole hospitals are massive overcrowded and continue to change their original configuration, a more rapid, flexible, and performant diagnostic approach is required.
Moreover, the high contagiousness of these patients, and the risk of transporting critical patients, make chest computed tomography (CT) a limited option for them.
Lung ultrasonography, a rapid, reliable, bedside, non-radiating, and repeatable examination, with its sensitivity closed to chest CT and much higher than the chest X-ray for COVID patients, has been widely used in COVID-19 pandemic as the crucial diagnostic and monitoring tool of patients with acute respiratory failure. It could be performed in the prehospital setting, in the emergency department (as part of the diagnostic approach), up to the normal wards and the Intensive Care Unit.
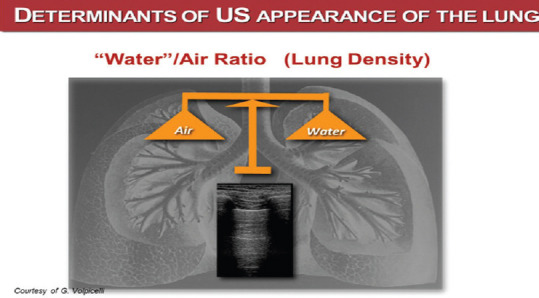
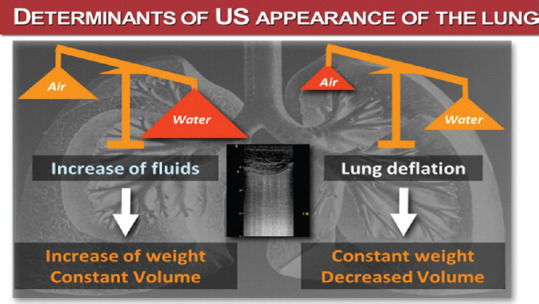
Bedside LUS allows to “read” reliably and dynamically the “water/air” ratio in the lung [Figures 1 and 2]. The interpretation of the LUS not only helps understanding the physiology and the pathophysiology of the lung, but also guides the correct clinical decision, once the diagnosis is done, and most of all, the choice of the appropriate ward for the patient, taking also into account the amount of oxygen supply or the ventilation support required.
Figure 1.
A pattern on LUS longitudinal thoracic scan. It reveals a normal lung Courtesy of G. Volpicelli. Water/air ratio (Lung density)
Figure 2.
B pattern on LUS longitudinal thoracic scan. A change in the fluids-air ratio (air decrease or fluid increase) generates this pathological pattern Courtesy of G. Volpicelli
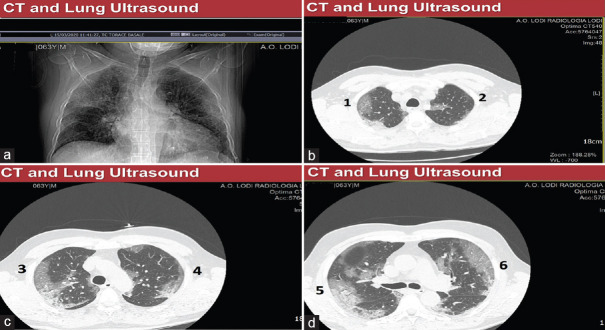
The respiratory failure of COVID-19 patients is due to pneumonia, with interstitial infiltrates, sometimes confluent and bilateral and basal/dorsal consolidations on chest HRCT, that could be associated, in more severe cases, to an acute respiratory distress syndrome (ARDS) an acute (onset 1 week or less), diffuse inflammatory lung injury, characterized by evidence of bilateral lung infiltrates and hypoxemia, in the absence of cardiogenic pulmonary edema [Figure 3].
Figure 3.
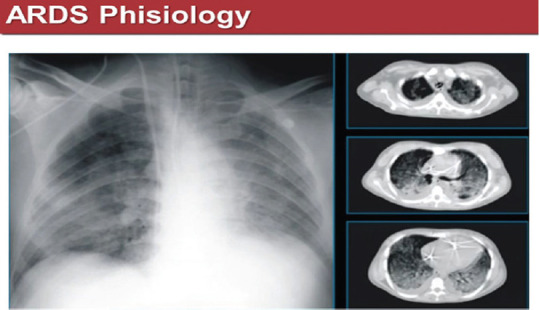
Positive chest X-ray (left) and coronal sections of chest high-resolution computed tomography (right) in an acute respiratory distress syndrome COVID-19 positive patient. Ground-glass zones are evident on chest X-ray and high-resolution computed tomography and dorsal consolidations only on high-resolution computed tomography sections
PATHOPHYSIOLOGY OF ACUTE RESPIRATORY DISTRESS SYNDROME
In ARDS, the water/air ratio is biased toward the water component. Figure 4 shows the CT number or Hounsfield scale, a value that expresses radio density (air is defined as −1000 Hounsfield Units, distilled water is defined as 0 HU and bone is defined as +1000 HU). According to CT number, four lung compartments can be defined: hyperinflated (−1000 to −900 HU), normally aerated (−900 to − 500 HU), poorly aerated (−500 to −100 HU), and nonaerated (−100 to +100 HU).
Figure 4.
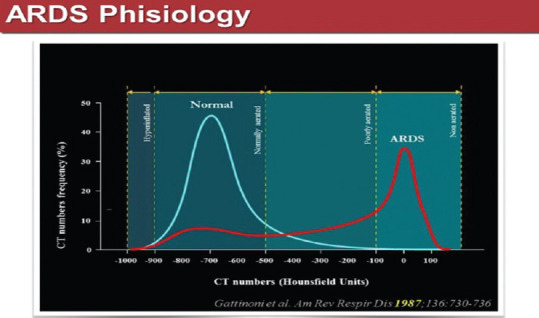
Distribution of computed tomography numbers in healthy versus acute respiratory distress syndrome patients[1]
Indeed, the hyperinflated compartment refers to the overfilling of the acini with gas. The “poorly aerated” compartment (which is approximately the quantitative equivalent of the ground-glass pattern) refers to a gas/tissue ratio that is present also in normal subjects, at least at end expiration, but is more extensive in ARDS. The nonaerated compartment, in most cases, includes the voxels between −100 HU and 0 HU, that is, with a gas/tissue ratio from 1/10 down to zero. Figure 4 shows the CT number distribution in the lung parenchyma of a healthy patient and of an ARDS affected patient: most of the lung parenchyma in the healthy patient is normally ventilated, whereas in ARDS poorly or non-aerated areas are predominant. Therefore, the gas exchange characteristics of the lung in COVID-19-positive patients with ARDS, are massively compromised, whereas healthy people lung has a very high capacity to buffer a functional deficit.[1]
The ARDS lung is not only more consolidated, but also its ratio (gas exchange capacity/volume of air exchanged) is impaired [Figure 5]. Moreover, there is also a ventral-dorsal gradient, gravity correlated, with the gravity zones more compromised than the antigravity ones. This asymmetry and the consequent different compliance between two important compartments (ventral and dorsal) determines a ventilation challenge in these patients.
Figure 5.
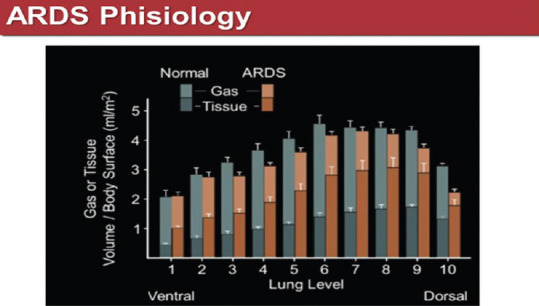
Gas-tissue volume ratio from ventral to dorsal lung zones of acute respiratory distress syndrome patients. From Pelosi et al. Am J Respir Critical Care Med 1994, Jan: 149 (1):8-13
Figure 6 summarizes the three aeration zones of the lung in ARDS patients and their gravity distribution. This is important not only from a pathophysiological point of view, but also for a better comprehension of the suggested therapy. The sternal (white) zone represents a normal lung zone, the intermediate one (a shaded area)-a heavy, edematous lung which tends to collapse under its own weight and the dorsal zone– of consolidation. Collapse and consolidation are not synonyms, as often thought, and we will explain below why the two entities are different [Figure 6].
Figure 6.
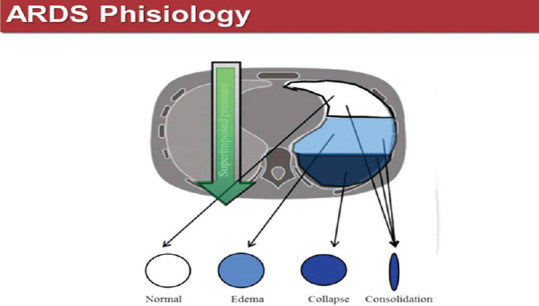
Aeration zones in the acute respiratory distress syndrome lung
Among the nonaerated lung zones, the collapsed zones represent recruitable lung volume, which can be reaerated, applying an appropriate level of pressure to the lung, while consolidations remain unrecruitable, no matter the applied pressure. Trying to apply high pressures to open the consolidated zones of the lung of ARDS patients, the only effect is an over distension of the ventral zones, with normal compliance, running the risk of generating a pneumothorax [Figure 7].
Figure 7.
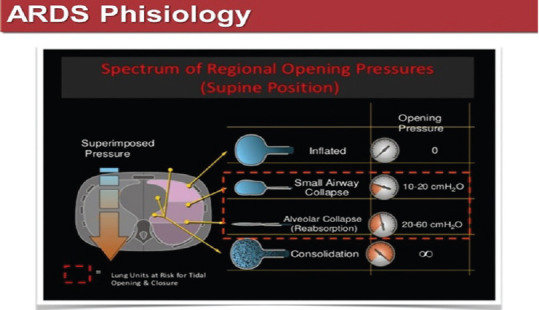
Regional opening pressures in different areas of an acute respiratory distress syndrome lung. From Tobin MJ: principles and practice of mechanical ventilation, 3rd ed.., Mc Graw Hills
LUS PATTERNS AND LUNG AERATION SCORE
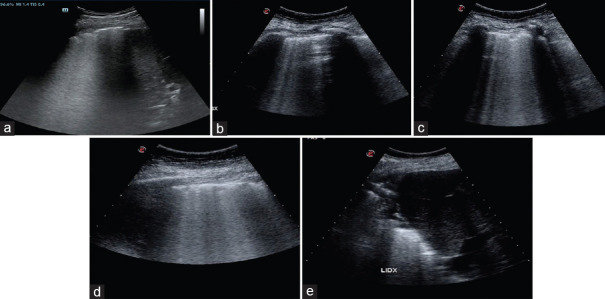
In time of epidemics, with a known gap between the resources and number of patients to be treated, LUS is proving to be a valid and simple tool to value the entity of lung aeration loss, technique that can be learned “on the field,” no matter the health professionals involved in the diagnostic process, in the ED, ICU or in a normal ward, as well. After a relatively short-learning curve, physicians could “read,” by means of LUS, the transition from an aerated lung to a completely collapsed one, as shown in Figure 8.
Figure 8.
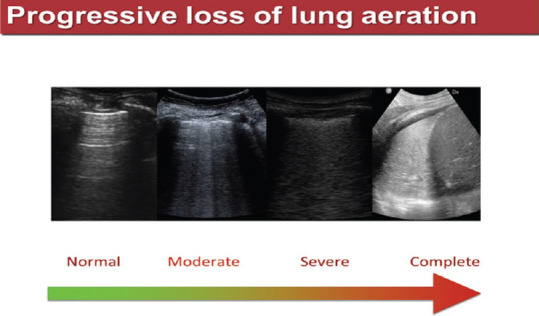
Progressive loss of lung aeration on different longitudinal thoracic scans
In ICU, we use to perform the 12 regions LUS technique with the convex transducer (and sometimes, to examine better the pleural line, also with a high-frequency linear transducer), acquiring 4 anterior, 4 lateral, and 4 posterior scans, by dividing each hemithorax in six zones, resulting from the intersection of parasternal right and left lines, anterior and posterior axillary right and left lines, paravertebral right and left lines with the inter-mammillary line.[2] An oblique basal scan is often performed to value the pleural effusion.
The lung aeration could also be quantified, using a LUS score, as illustrated in Figure 9, where the 0 score is assigned to an A pattern, that is a normal aerated lung, while 1 and 2 describe a moderate (Score 1) or severe (Score 2) loss of lung aeration, corresponding to a B pattern with multiple, well-defined B lines, respectively, with coalescent B lines. The Score 3 defines a lung consolidation.[2]
Figure 9.
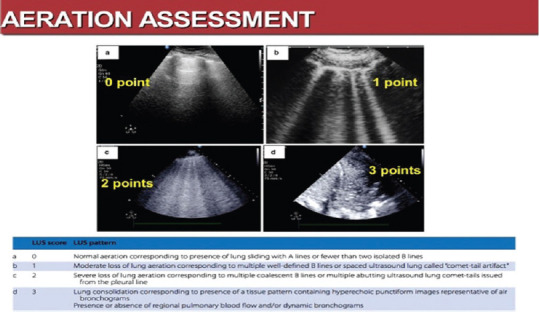
LUS aeration score and LUS patterns[2]
CHEST HIGH-RESOLUTION COMPUTED TOMOGRAPHY VERSUS LUS FINDINGS IN ACUTE RESPIRATORY DISTRESS SYNDROME
A central question is whether, in time of epidemics, performing LUS in our hospital instead of chest HRCT has been a well-motivated choice, that had prerequisites, eventually a winning choice, or not. There are already works issued from the beginning of this pandemics that confirm the great concordance between LUS and the gold standard-chest HRCT-in terms of findings that characterize the COVID-19 ARDS, as seen in the Figure 10.[3]
Figure 10.
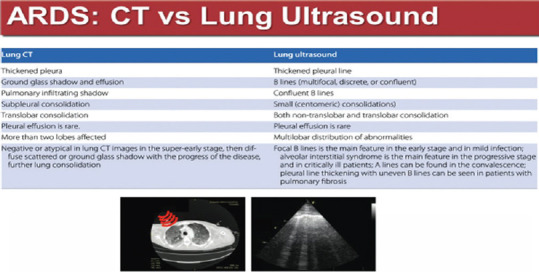
Chart high-resolution computed tomography versus LUS findings in acute respiratory distress syndrome[3]
From Figure 11a–d are visible some typical findings of a COVID-positive CT scan: ground-glass areas, well defined, almost a “crazy paving” pattern, reaching the pleural line and following an anterior-posterior gradient, more evident in the dorsal zones, sometimes absent at bases, in less severe cases.
Figure 11.
(a) Computed tomography scout view showing ground-glass areas in a COVID-19 patient. (b-d) Coronal section of chest high-resolution computed tomography showing ground-glass areas in a COVID-19 patient
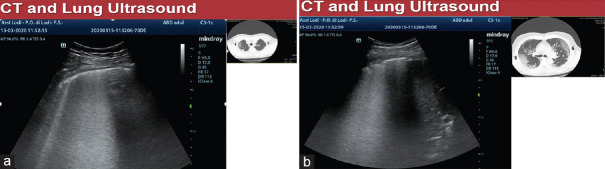
LUS can explore almost all the lung surface and the great majority of the lung findings in ARDS reach also the pleura, that's why we also found between the two techniques (LUS and chest HRCT) an almost total concordance for the lesions that reach the pleural line, clinically highly relevant and more than sufficient, that concerned us to modify our clinical decisions only on the LUS. Figure 12 shows, in the same COVID-19 patient, an interrupted pleural line and a white lung pattern on LUS, while on CT scan is seen a ground-glass pattern.
Figure 12.
(a and b) B pattern with interrupted pleural line on LUS corresponds to ground-glass pattern on chest high-resolution computed tomography
The B pattern with an irregular pleural line (and also lung pulse and reduced lung sliding on dynamic images) associated with adjacent spared areas (A profile, i.e., normal lung areas) on LUS [Figure 13a and b], as well as the corresponding ground-glass zones alternated with normal lung areas on chest HRCT, are pathognomonic findings of an ARDS. The reduced mobility of the pleural line and the evidence of lung pulse on LUS attest to the fact that the explored lung zone is a rigid and less ventilated one. Subpleural small as well as basal or dorsal consolidations represent also characteristic LUS findings in ARDS patients [Figure 13c–e].
Figure 13.
(a) B pattern with irregular B lines (b) B pattern with irregular B lines and adjacent spared zones on LUS (c and d) B pattern with small subpleural consolidations (e) B pattern with basal consolidations on LUS
As the patients with COVID-19 ARDS are often not dyspneic at the beginning, despite low oxygenation values, a trial of noninvasive ventilation could be performed in the emergency department. The ultrasound assessment of the respiratory variation of the inferior vena cava (IVC) diameter in these patients, could help the physician, together with LUS, arterial blood gas analysis and LUS, decide the right timing of an upgrade of therapy to intubation. A rigid, plethoric IVC with loss of respiratory variation is a sign of worsening clinical picture in these patients [Figure 14].
Figure 14.
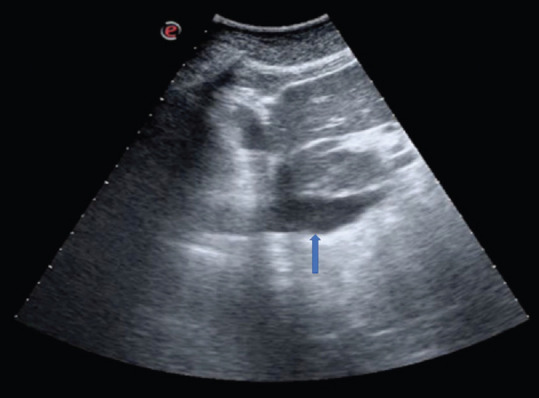
A plethoric inferior vena cava (blue arrow) on a subcostal longitudinal scan of a COVID-19 continuous positive airway pressure-supported patient
The great value of the LUS in these critical patients with ARDS is that, unlike the CT scan, LUS is repeatable, noninvasive and enables the physician to revalue the patient daily as well as in case of gas exchange worsening, before modifying the ventilation parameters, before performing recruitment maneuver.
LUS AND RECRUITMENT MANEUVER
Performed to open up collapsed lung under progressively higher airway pressures, it aims to improve oxygenation in ARDS hypoxic intubated patients. An appropriated PEEP will maintain open the recruited lung.
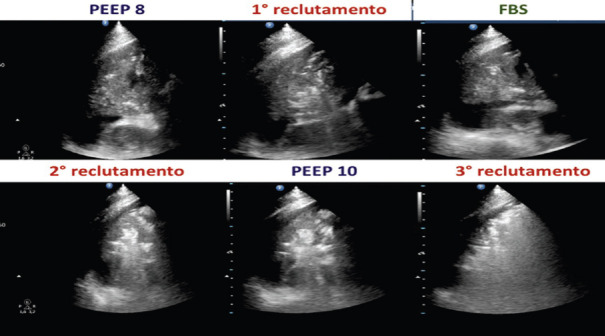
LUS could be used to assess the lung recruitment, maneuver performable only in intensive care patients, under a high-level monitoring. During effective recruitment maneuvers, the ultrasound could show areas changing from a tissue-like pattern, sometimes without any bronchogram, to patterns gradually more (and more) aerated, up to an A pattern. Figure 15 illustrates different LUS patterns corresponding to increasing pressure values to open a deflated lung area (on left side) and the return to the initial pattern in case of de-recruitment (on the right side).
Figure 15.
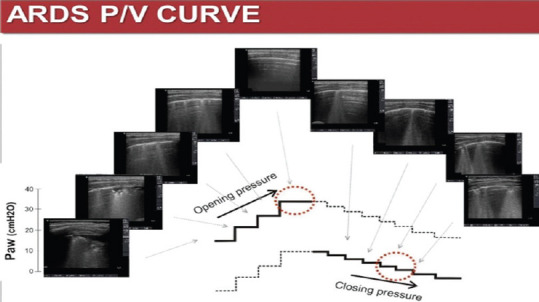
Different LUS pattern corresponding to increasing pressure values to open a deflated lung area (on left side) and the return to the initial pattern in case of de-recruitment (on the right side)
LUS performed before the recruitment maneuver helps identifying potentially recruitable lung zones (only the collapsed zones and non the consolidations) [Figure 16].
Figure 16.
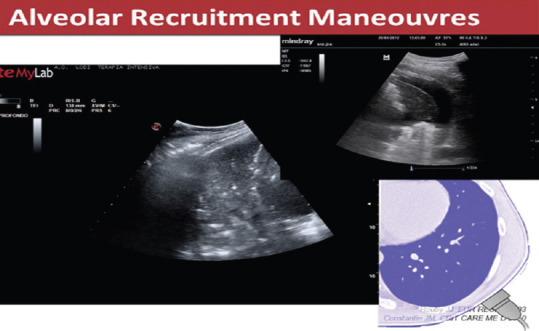
Lung consolidation with air bronchogram (seen as white spots on the left image) and a collapsed area surrounded by a pleural effusion (right image)
If static air or fluid bronchogram are visible on LUS, recruitment maneuver is suitable to be performed after a cleaning fibrobronchoscopy, to avoid a lung over distention (that would also mean running a high risk of pneumothorax) and if effective, would determine an improvement of the lung aeration, evident on LUS [Figure 17].
Figure 17.
Progressive increase of aeration in a consolidation on LUS after recruitment maneuver alone and repeated after a cleaning fibrobroncoscopy. Courtesy of Andrea Magnacavallo
PRONE POSITION
As in supine ARDS patients the dorsal lung zones are the most damaged and those with more impact on the resistive characteristics of the lung, prone positioning with consequent reversion of this gravitational gradient, generates a theoretical reopening of these dorsal (sloping) areas, and therefore, an improvement of the ventilation/perfusion ratio and of the respiratory gas exchange.[4]
PRONE POSITION IN EMERGENCY DEPARTMENT
In this time of pandemic, under the pressure of not having enough mechanical ventilators, oxygen supply machines, intensive care beds, and intensive care physicians to do this maneuver only in intensive care, our ED performed the prone positioning of COVID-19 ARDS patients, supported with helmet-continuous positive airway pressure. Although a nonconventional method, the prono-supination of the ARDS patients in the ED was very well tolerated (patients were awake, not sedated) and avoided intubation in a significant number of these patients [Figure 18].
Figure 18.
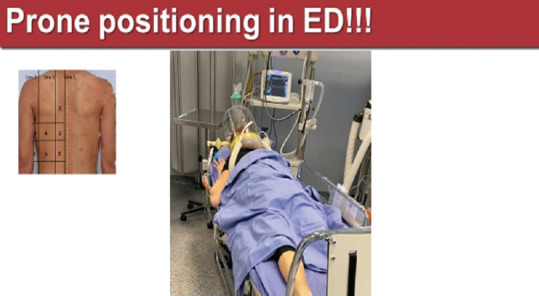
Prone positioning in a continuous positive airway pressure-supported COVID-19 patient in ED
CONCLUSIONS
The already consolidated value of the LUS in patients with respiratory failure was confirmed in times of COVID-19 pandemic, demonstrating its key role as point-of-care in triaging and monitoring critically ill patients. Although the gold standard radiological method is HRCT, only a small part the COVID-19 ARDS patients underwent a HRCT scan, because of their critical conditions and the massive hospital overcrowding.
In the management of COVID-19 ARDS patients, LUS helps physician diagnosing, detecting diffuse interstitial syndrome with a high sensitivity, close to the HRCT. It is also a valid monitoring tool assisting physician during recruitment maneuver, prone positioning, and respiratory weaning.
Moreover, it enables the clinician to identify and treat complications, to manage fluid therapy and to set ventilation parameters.
Financial support and sponsorship
Nil.
Conflicts of interest
Many thanks to Dr. G Volpicelli for having provided us the figures 1 and 2.
REFERENCES
- 1.Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M, et al. Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis. 1987;136:730–6. doi: 10.1164/ajrccm/136.3.730. [DOI] [PubMed] [Google Scholar]
- 2.Bouhemad B, Mongodi S, Via G, Rouquette I, et al. Ultrasound for “Lung monitoring” of ventilated patients. Anesthesiology. 2015;122:437–47. doi: 10.1097/ALN.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 3.Peng Q-J, Wang X-T. Zhang L-N and Chinese Critical Ultrasound Study Group (CCUSG) Intensive Care Med. 2020;46:849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X-T, Ding X, Zhang H, Chen H, Su L, Liu D and Chinese Critical Ultrasound Study Group (CCUSG) Lung ultrasound can be used to predict the potential of prone positioning and assess prognosis in patients with ARDS. Crit Care. 2016;20:385. doi: 10.1186/s13054-016-1558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]