Abstract
Background and Objectives:
Measuring a visible core length during macroscopic on-site evaluation (MOSE) can be useful for accurate diagnoses during an EUS-guided fine needle biopsy (EUS-FNB). We aimed to estimate visible core cutoff lengths predictive of a correct diagnosis when using 22-gauge Franseen needles for biopsies from pancreatic masses.
Materials and Methods:
We assessed 77 consecutive patients who underwent EUS-FNB using 22-gauge Franseen needles for pancreatic masses between March 2018 and October 2018. At least two needle passes were performed in all patients, irrespective of the findings on MOSE. The endoscopists measured the visible cores using a ruler during MOSE. The first two passes were analyzed on a per pass basis, and the correlation between visible core lengths and diagnostic accuracy was evaluated.
Results:
We evaluated 150 needle passes of 75 patients. The accuracy per pass was 92% (138/150). The median length of the visible cores was 15 (range: 0–60) mm and they were significantly longer in the correct diagnosis group than in the incorrect diagnosis group. The accuracy correlated positively with the visible core length. Receiver-operating characteristic curve analysis of the visible core length for accuracy demonstrated an optimal cutoff value of 10 mm. On multivariate logistic regression, visible core lengths >10 mm independently affected the correct diagnosis (odds ratio: 5.1, P = 0.02).
Conclusions:
Visible cores exceeding 10 mm may be useful for correct diagnosis while using a 22-gauge Franseen needle for EUS-FNB from pancreatic masses.
Keywords: EUS, EUS-FNA, fine-needle biopsy, Franseen needle, macroscopic on-site evaluation
INTRODUCTION
Owing to its high diagnostic accuracy, EUS-FNA is the standard method for the pathological assessment of pancreatic tissue samples.[1,2] However, since one needle passes using a standard needle provide inadequate sample tissues, three to four passes are required for correct diagnoses.[3,4,5,6,7] These may cause complications such as tumor seeding, pancreatitis, and bleeding, which necessitates the reduction of the number of needle passes.[8,9,10,11,12,13,14,15]
Rapid-on site cytopathological evaluation (ROSE) may reduce the number of FNA needle passes; however, in Asia and Europe, it is not commonly performed due to shortages of pathological staff and additional costs.[16,17] Direct observation of specimens obtained by FNA, known as macroscopic on-site evaluation (MOSE) or gross visual inspection, can be an alternative to ROSE because of its feasibility and readily available resources.[18,19] A visible core of the EUS-FNA sample using standard needles indicates sample adequacy and is predictive of correct pathological diagnoses.[18,19] Nonetheless, the clinical significance of MOSE has not been fully elucidated.
Tissues volumes procured with the Franseen needle for EUS-guided fine needle biopsy (EUS-FNB) are larger than those procured with the standard needles; furthermore, fewer passes are required to obtain adequate diagnostic samples with the Franseen needle.[20,21,22,23] Hence, the Franseen needle is preferred over the standard needles. However, since one needle pass is inadequate for a correct FNB diagnosis,[2,24,25,26] evaluation of FNB specimens, which predict a correct diagnosis, by MOSE would avoid unnecessary needle passes.
Nonetheless, false negatives due to macroscopic rather than pathological evaluation may be a drawback of MOSE. Measuring the length of the visible core of FNB specimens may aid in correct diagnoses and reduce false negatives. Studies reporting the use of MOSE for FNB specimens are scarce. Here, we assess the efficacy of MOSE to confirm the adequate core sizes for correct FNB diagnoses of pancreatic masses using a 22-gauge (G) Franseen needle.
MATERIALS AND METHODS
Patients and ethical considerations
This retrospective study was conducted at a tertiary referral cancer center for pancreatobiliary diseases, where more than 700 pancreatobiliary EUS procedures are performed annually. Consecutive patients who underwent EUS-FNB of pancreatic masses using a 22-G Franseen needle between April 2018 and October 2018 were included. Patients with inadequate MOSE data in the endoscopic reports were excluded. Written informed consent for EUS-FNB was obtained from all the patients. The present study was approved by the Ethics Committee of our institution, and the study protocols conformed to the ethical guidelines outlined in the Declaration of Helsinki.
EUS-guided fine needle biopsy procedure
The EUS-FNB was performed using a curved linear-array echoendoscope (GF-UCT260; Olympus Medical Systems Corp., Tokyo, Japan) with patients under conscious sedation. All procedures were performed or supervised by an expert endoscopist who had performed more than 1000 EUS-FNA/B procedures. The procedures were also performed by three nonexpert endoscopists with experience of 50-200 EUS-FNA/B procedures. The pancreatic masses were punctured using a 22-G Franseen needle (Acquire™, Boston Scientific Corporation, Natick, MA, USA). The stylet was removed after advancing the needle in the target; suction was then applied using a 10 mL syringe, and the needle was moved to-and-fro in the lesion ten times. After EUS-FNB, the patients were monitored for 2 h in the recovery room and discharged if they were asymptomatic. After leaving the hospital, if the patients experienced symptoms, such as abdominal pain or fever, they made a telephone call to the hospital and received medical advice on the best way to manage their symptoms.
Macroscopic on-site evaluation
We introduced a standard MOSE protocol at our institution in March 2018. As per the protocol, after removing the FNB needle from the echoendoscope, the specimen was expelled onto a petri dish using the stylet. MOSE was performed by two endoscopists and the presence and length of a visible core was evaluated by inspecting the specimen. In cases where their interpretations differed, a decision was made after discussion. A visible core defined as white pieces of tissue was extracted from the specimen and measured using a ruler [Figure 1]. In cases where the visible cores were fragmented, the fragments were gathered and aligned using a 23-G injection needle followed by measuring the entire length. In cases where visible cores were not obtained, the length was recorded as 0 mm. At least two punctures or needle passes were performed for each target. Additional punctures were performed if a visible core was not obtained in the first two consecutive passes. ROSE was not performed at our center because of a shortage of pathological staff.
Figure 1.
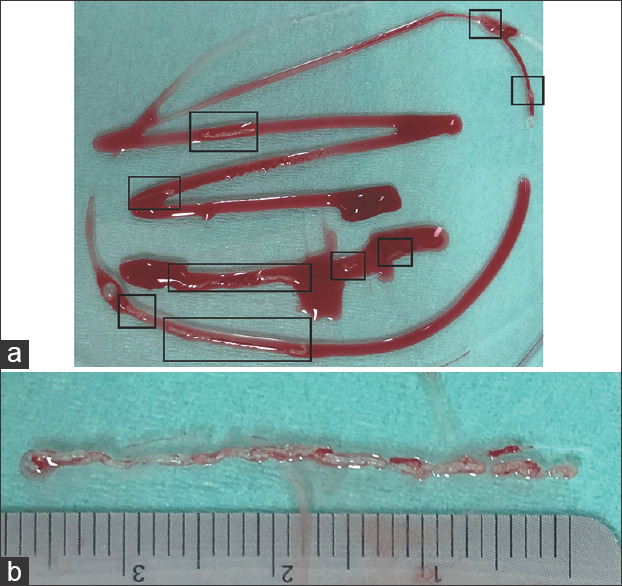
Measurement of the visible core during macroscopic on-site evaluation. (a) A whole specimen with scattered visible cores (in boxes). (b) Total length of multiple aligned visible cores measured using a linear rule
Sample preparation and pathologic assessment
The specimens of the first and second passes were placed in saline and formalin bottles, respectively, in the endoscopy room. These bottles were subsequently sent to the pathology department, where cytotechnologists processed the specimens and prepared the slides. A portion of the first pass specimen was smeared onto two glass slides, which were stained with hematoxylin and eosin (HE) and Papanicolaou for cytological analysis. The remaining portion of the specimen was fixed in formalin and embedded in paraffin; sections from it were then stained with HE for histological evaluation. The entire second pass specimen was embedded in paraffin, and a portion from it was used for histological evaluation after HE staining. Immunohistochemical staining was performed, if necessary. The cell block method[27] was not used at our center. Specimens that were considered suspicious or positive for malignancy and neuroendocrine neoplasms were categorized as positive for malignancy; conversely, those that were considered negative or atypical were categorized as negative for malignancy. Pathological evaluations were performed for each needle pass. The pathological diagnosis for the first pass specimen was provided after cytological and histological evaluation. The assessment was performed by an experienced pathologist.
Final diagnosis
In cases found to be positive for malignancy on pathological evaluation of specimens obtained by EUS-FNB, the final diagnosis was confirmed based on pathological findings during surgery or the clinical course (true-positive). In cases found to be negative for malignancy, the benign nature was confirmed through pathological findings during surgery or a clinical follow-up followed by imaging after 6 months or more. The masses were considered benign if stable or resolved (true-negative). The accuracy of EUS-FNB was defined as the sum of the true-positive and true-negative results divided by the total number of analyzed passes.
Data analyses
The first two passes of each EUS-FNB procedure, including MOSE, were analyzed on a per pass basis. Continuous variables pertaining to patient characteristics and MOSE results were presented as medians with ranges and compared using the Mann–Whitney U-test. Categorical variables were analyzed using the Fisher's exact test. The correlation between the total length of the visible cores and the accuracy of EUS-FNB was analyzed using the Cochran-Armitage test for trends. Receiver-operating characteristic (ROC) curves of the visible core lengths for ensuring a correct FNB diagnosis were analyzed to assess the accuracy of the area under the curve of the ROC (AUC); they were also analyzed to determine the optimal cutoff length of the visible core for obtaining a correct FNB diagnosis. Univariate and multivariate logistic regression analyses for factors affecting the correct diagnosis were performed based on the following predictor variables: tumor size (<20 vs. ≥20 mm), lesion location (head vs. body or tail), pass number ( first pass vs. second pass), operator expertise (expert vs. nonexpert), and visible core length (> cutoff value vs. ≤ cutoff value). The P < 0.05 was considered statistically significant for all tests. All statistical analyses were performed using the EZR (version 3.4.1) software package” to “EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics. Additionally, adverse events (AEs), such as bleeding and pancreatitis, were recorded according to the American Society for Gastrointestinal Endoscopy workshop report.[28]
RESULTS
Overall, 77 patients with pancreatic masses who underwent EUS-FNB using a 22-G Franseen needle were included; two patients were excluded because of insufficient details in their endoscopic reports. Finally, 150 passes in 75 patients were analyzed. The patients' characteristics and EUS-related data are presented in Table 1. The final diagnoses were based on the evaluation of the surgical specimen and clinical course in 21 and 54 patients, respectively. The diagnostic accuracy of the first pass was 95% (71/75), and that of the second pass was 89% (67/75). The total accuracy of EUS-FNB per pass was 92% (138/150). The accuracy values of EUS-FNB per pass in malignancy and benign at the final diagnosis were 91% (120/132) and 100% (18/18), respectively. No AEs were reported during and after EUS-FNB.
Table 1.
Patient characteristics
| Characteristics | Value |
|---|---|
| Median age, year (range) | 71 (45-87) |
| Sex, male, n (%) | 42 (56) |
| Median tumor size, mm (range) | 26 (7-57) |
| Lesion location, head: body or tail, n | 45:30 |
| Operator expertise, expert, n (%) | |
| 1st pass | 17 (23) |
| 2nd pass | 20 (27) |
| Final diagnosis, n (%) | |
| Malignant | 66 (88) |
| Adenocarcinoma | 60 (80) |
| Neuroendocrine tumor | 4 (5) |
| Metastatic pancreatic cancer | 2 (3) |
| Benign | 9 (12) |
| Autoimmune-related pancreatitis | 3 (4) |
| Chronic pancreatitis | 6 (8) |
According to MOSE, a visible core was observed in 95% of all passes (142/150), with a median length of 15 mm (range; 0–60 mm). The visible cores in the correct diagnosis group were longer than in those with an incorrect diagnosis (median: 15 mm vs. 7 mm, P = 0.007) [Figure 2].
Figure 2.
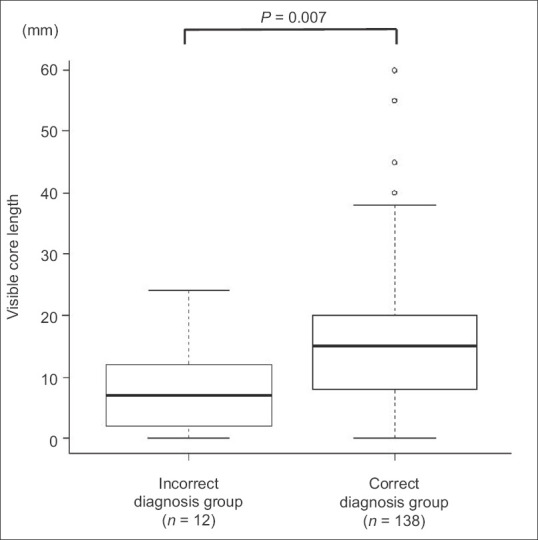
The median visible core length and box plot for incorrect and correct diagnosis groups showing a significantly longer visible core length in the correct diagnosis group than incorrect diagnosis group (median: 15 mm vs. 7 mm, P = 0.007)
The accuracy of EUS-FNB showed a positive correlation with the visible core length (P = 0.016) [Figure 3]. ROC curve analysis of the visible core length for accuracy identified a cutoff value of 10 mm with an AUC of 0.74 (95% confidence interval: 0.60–0.87).
Figure 3.
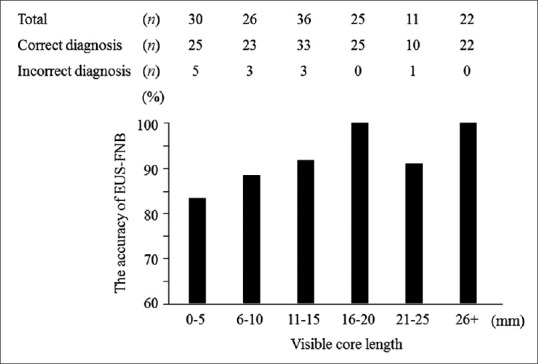
Results of the Cochran-Armitage test for trends showing the positive correlation between the accuracy of EUS-FNB and total length of visible cores (P = 0.016). EUS-FNB: EUS-guided fine needle biopsy
Univariate and multivariate analyses were also performed for factors affecting the correct diagnosis [Table 2]. On univariate analysis, the accuracy of EUS-FNB tended to be higher, with visible core lengths >10 mm (odds ratio: 3.8, 95% confidence interval: 1.1–13.1; P = 0.038). Multivariate logistic regression analysis found that only visible core lengths >10 mm independently affected the correct diagnosis (odds ratio: 5.1, 95% confidence interval: 1.3–20.0; P = 0.020).
Table 2.
Univariate and multivariate analyses of factors affecting the accuracy of EUS-FNB
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Tumor size (mm) | ||||
| >20 | 1.0 (0.3-3.5) | 1.00 | 0.6 (0.1-2.4) | 0.46 |
| ≤20 | 1 | 1 | ||
| Lesion location | ||||
| Body or tail | 2.0 (0.5-7.5) | 0.32 | 2.1 (0.5-8.8) | 0.30 |
| Head | 1 | 1 | ||
| Pass number | ||||
| 1st pass | 2.1 (0.6-7.4) | 0.24 | 1.9 (0.5-6.7) | 0.34 |
| 2nd pass | 1 | 1 | ||
| Operator expertise | ||||
| Expert | 0.63 (0.2-2.2) | 0.47 | 0.5 (0.1-2.0) | 0.31 |
| Nonexpert | 1 | 1 | ||
| Visible core length (mm) | ||||
| >10 | 3.8 (1.1-13.1) | 0.04* | 5.1 (1.3-20.0) | 0.02* |
| ≤10 | 1 | 1 | ||
*P<0.05. OR: Odds ratio; CI: Confidence interval.
DISCUSSION
Our study demonstrated that the visible core length from MOSE may predict the correct pathological diagnosis in cases of EUS-FNB of pancreatic masses using a 22-G Franseen needle. Visible cores longer than 10 mm can probably provide more accurate diagnoses and can indicate the need for terminating the EUS-FNB procedure.
The utility of MOSE has been reported in studies that employed standard 22-G and 19-G FNA needles.[18,19] For example, a prospective study using 19-G needles targeting the lymph nodes, submucosal tumors, and local tissues, as well as the pancreas reported that visible cores ≥4 mm on MOSE may indicate specimen adequacy for pathological interpretation.[18] Our findings demonstrated improvements in the accuracy of EUS-FNB with increasing visible core lengths; visible cores of at least 10 mm were strongly associated with the likelihood of obtaining a correct diagnosis. This length is considerably longer than that of the previous study. However, this may not be solely attributed to the difference in needle sizes; rather, it may be related to a significant difference in the indicators. The previous study was intended to assess the amount of tissue while the present study aimed to evaluate the likelihood of a correct diagnosis, which is of greater clinical significance. Therefore, we selected the indicator as a main outcome measure.
Evaluating the relationship between visible core lengths and correct diagnoses is more challenging than assessing the relationship between lengths and specimen adequacy. Obtaining large specimen quantities does not necessarily result in a correct diagnosis. For instance, in cases where the visible cores contain more tissue from nontarget lesions, the likelihood of an incorrect diagnosis (false negative) is higher. A prospective study on EUS-FNA using 19-G needles reported that pancreatic lesions were more prone to false negative diagnoses and the reliability of the visible cores while obtaining a correct diagnosis was reduced when targeting pancreatic lesions.[18] This may be because pancreatic lesions are frequently accompanied by fibrous tissue, such as desmoplastic changes and pancreatitis caused by pancreatic cancer. This would probably be recognized as a visible core on MOSE.[18,29,30] Since our study evaluated only pancreatic lesions, we presumed that the probability of false negatives, as observed in the previous studies, was higher; therefore, longer visible cores were needed to predict correct diagnoses. However, this may raise concerns regarding whether the method can be applied to small tumors. In the present study, 12 needle passes for six pancreatic masses measuring <10mm in diameter were included. Consequently, the accuracy of EUS-FNB was 83% (10/12), and the median length of the visible core was 12.5 mm (range: 0–26 mm). Thus, even for a small tumor, a visible core with a size larger than that of its tumor could be obtained, indicating that our result can be applied to small tumors.
It is argued that ROSE and MOSE are not necessary when using the Franseen needle because two needle passes are likely to provide a correct diagnosis in most cases.[20,22,23,24,25,26] Although a higher number of needle passes may be associated with a higher AE rate, another issue of particular concern is needle tract seeding. The reason is that needle tract seeding originating from EUS-FNA for pancreatic body or tail cancer was observed in 3.4% of the patients who underwent distal pancreatectomy, as reported by a recent multicenter retrospective cohort study.[12] This AE rate should not be overlooked. Therefore, if an infallible needle is developed, using which a correct diagnosis can be achieved with one needle pass, ROSE and MOSE will not be required. However, until then, an attempt to reduce the number of needle passes by MOSE may be beneficial in clinical practice, even in the era of the superior FNB needle.
A recent retrospective study reported the use of MOSE during EUS-FNB using 22-G Franseen needles.[31] In that cohort, a visible core was obtained in 93% (50/54) of patients; histological core fragments were confirmed in 94% (47/50) of those patients. The overall diagnostic accuracy was 94% (48/51), with a median of one pass. Since the accuracy rate per pass was not mentioned, the relationship between the visible core lengths and the accuracy of EUS-FNB was unclear. However, findings from that study indicated the utility of MOSE, and aligned with our findings. Unlike our case, the study also included several lesions, including pancreatic masses. As mentioned previously, the false negative rate of MOSE can increase while targeting pancreatic lesions.[18] Therefore, when targeting pancreatic lesions, evaluating the length of the visible cores may enhance the diagnostic yield.
The present study had several limitations. First, it was retrospective, which may have caused a selection bias. Second, only 22-G Franseen needles were evaluated in this study, and further studies with 19-G or 25-G needles may provide different outcomes. However, since 22-G needles are most commonly used for EUS-FNA/B, we speculate that our findings may be readily applied to clinical practice. Third, only pancreatic lesions were included in this analysis. Since the probability of a visible core providing a correct diagnosis varies between lesions, the results may not be applicable to other lesions, such as lymph nodes and submucosal masses. Further studies will be needed to evaluate the probability of an accurate diagnosis for these lesion types. Fourth, the proficiency of the endoscopists who performed the EUS-FNB procedures were not uniform. However, trainees who had experience with <50EUS-FNA/B procedures were not involved in this study. Nevertheless, multiple endoscopists are needed to ensure reproducibility of our findings. Fifth, the specimens of the first and second passes were assessed by different pathological methods in this study. Although the diagnostic accuracy of the first pass was higher than that of the second pass, there was no significant difference between these passes. Therefore, we analyzed the first and second passes together, and multivariate logistic regression analysis showed that the pass number did not independently affect the correct diagnosis. This study also had certain strengths; MOSE, a standardized method, was employed for evaluation.
CONCLUSIONS
Our findings suggest that visible cores exceeding 10 mm may predict the likelihood of a correct diagnosis when using 22-G Franseen needles for EUS-FNB of pancreatic masses. In the absence of ROSE, obtaining a visible core exceeding the cutoff length may be a useful indicator for terminating the procedure. Further prospective studies including larger cohorts are needed to validate our findings
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Voss M, Hammel P, Molas G, et al. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244–9. doi: 10.1136/gut.46.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline – March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 4.Puli SR, Bechtold ML, Buxbaum JL, et al. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass. A meta-analysis and systematic review? Pancreas. 2013;42:20–6. doi: 10.1097/MPA.0b013e3182546e79. [DOI] [PubMed] [Google Scholar]
- 5.Matsubayashi H, Matsui T, Yabuuchi Y, et al. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: Clinical aspects to improve the diagnosis. World J Gastroenterol. 2016;22:628–40. doi: 10.3748/wjg.v22.i2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujie S, Ishiwatari H, Sasaki K, et al. Comparison of the diagnostic yield of the standard 22-gauge needle and the new 20-gauge forward-bevel core biopsy needle for endoscopic ultrasound-guided tissue acquisition from pancreatic lesions. Gut Liver. 2019;13:349–55. doi: 10.5009/gnl18189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato J, Ishiwatari H, Sasaki K, et al. Benefit of high negative pressure during endoscopic ultrasound-guided fine-needle aspiration with standard 22-gauge needles for pancreatic lesions: A retrospective comparative study. Scand J Gastroenterol. 2019;54:108–13. doi: 10.1080/00365521.2018.1564788. [DOI] [PubMed] [Google Scholar]
- 8.LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–81. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 9.Bang JY, Magee SH, Ramesh J, et al. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–50. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishiwatari H, Hayashi T, Kawakami H, et al. Randomized trial comparing a side-port needle and standard needle for EUS-guided histology of pancreatic lesions. Gastrointest Endosc. 2016;84:670–8. doi: 10.1016/j.gie.2016.03.1329. [DOI] [PubMed] [Google Scholar]
- 11.Minaga K, Takenaka M, Katanuma A, et al. Needle tract seeding: An overlooked rare complication of endoscopic ultrasound-guided fine-needle aspiration. Oncology. 2017;93(Suppl 1):107–12. doi: 10.1159/000481235. [DOI] [PubMed] [Google Scholar]
- 12.Yane K, Kuwatani M, Yoshida M, et al. Non-negligible rate of needle tract seeding after endoscopic ultrasound-guided fine-needle aspiration for patients undergoing distal pancreatectomy for pancreatic cancer. Dig Endosc. 2019 doi: 10.1111/den.13615. [doi: 101111/den13615] Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Mohamadnejad M, Mullady D, Early DS, et al. Increasing number of passes beyond 4 does not increase sensitivity of detection of pancreatic malignancy by endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. 2017;15:1071–800. doi: 10.1016/j.cgh.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Kawakubo K, Yane K, Eto K, et al. A prospective multicenter study evaluating bleeding risk after endoscopic ultrasound-guided fine needle aspiration in patients prescribed antithrombotic agents. Gut Liver. 2018;12:353–9. doi: 10.5009/gnl17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribeiro A, Goel A. The risk factors for acute pancreatitis after endoscopic ultrasound guided biopsy. Korean J Gastroenterol. 2018;72:135–40. doi: 10.4166/kjg.2018.72.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wani S, Mullady D, Early DS, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: A prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015;110:1429–39. doi: 10.1038/ajg.2015.262. [DOI] [PubMed] [Google Scholar]
- 17.van Riet PA, Cahen DL, Poley JW, et al. Mapping international practice patterns in EUS-guided tissue sampling: Outcome of a global survey. Endosc Int Open. 2016;4:E360–70. doi: 10.1055/s-0042-101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwashita T, Yasuda I, Mukai T, et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: A single-center prospective pilot study (MOSE study) Gastrointest Endosc. 2015;81:177–85. doi: 10.1016/j.gie.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 19.Ishiwatari H, Sato J, Fujie S, et al. Gross visual inspection by endosonographers during endoscopic ultrasound-guided fine needle aspiration. Pancreatology. 2019;19:191–5. doi: 10.1016/j.pan.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Bang JY, Hebert-Magee S, Navaneethan U, et al. EUS-guided fine needle biopsy of pancreatic masses can yield true histology. Gut. 2018;67:2081–4. doi: 10.1136/gutjnl-2017-315154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asokkumar R, Yung Ka C, Loh T, et al. Comparison of tissue and molecular yield between fine-needle biopsy (FNB) and fine-needle aspiration (FNA): A randomized study. Endosc Int Open. 2019;7:E955–63. doi: 10.1055/a-0903-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukai S, Itoi T, Yamaguchi H, et al. A retrospective histological comparison of EUS-guided fine-needle biopsy using a novel franseen needle and a conventional end-cut type needle. Endosc Ultrasound. 2019;8:50–7. doi: 10.4103/eus.eus_11_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Hajj II, Wu H, Reuss S, et al. Prospective assessment of the performance of a new fine needle biopsy device for EUS-guided sampling of solid lesions. Clin Endosc. 2018;51:576–83. doi: 10.5946/ce.2018.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bang JY, Hebert-Magee S, Hasan MK, et al. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: Initial assessment. Dig Endosc. 2017;29:338–46. doi: 10.1111/den.12769. [DOI] [PubMed] [Google Scholar]
- 25.Bang JY, Hebert-Magee S, Navaneethan U, et al. Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2018;87:1432–8. doi: 10.1016/j.gie.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa T, Kawashima H, Ohno E, et al. Clinical impact of EUS-guided fine needle biopsy using a novel franseen needle for histological assessment of pancreatic diseases. Can J Gastroenterol Hepatol. 2019;2019:8581743. doi: 10.1155/2019/8581743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noda Y, Fujita N, Kobayashi G, et al. Diagnostic efficacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound-guided fine-needle aspiration. J Gastroenterol. 2010;45:868–75. doi: 10.1007/s00535-010-0217-5. [DOI] [PubMed] [Google Scholar]
- 28.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 29.Dougan SK. The pancreatic cancer microenvironment. Cancer J. 2017;23:321–5. doi: 10.1097/PPO.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 30.Whittle MC, Hingorani SR. Fibroblasts in pancreatic ductal adenocarcinoma: Biological mechanisms and therapeutic targets. Gastroenterology. 2019;156:2085–96. doi: 10.1053/j.gastro.2018.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung Ki EL, Lemaistre AI, Fumex F, et al. Macroscopic onsite evaluation using endoscopic ultrasound fine needle biopsy as an alternative to rapid onsite evaluation. Endosc Int Open. 2019;7:E189–94. doi: 10.1055/a-0770-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]


