Abstract
For a definitive diagnosis of fine-needle aspiration (FNA)/biopsy, one of the reliable techniques to determine the adequacy and accuracy rapid on-site evaluation (ROSE) of cytological samples is preferable. Because of the lack of trained pathologists, alternatives have to be explored. This study is primarily conducted to determine the diagnostic sensitivity and specificity of full-field optical coherence tomography (FF-OCT) and secondarily to evaluate the possibility of FF-OCT differentiating different types of pancreatic diseases. The diagnostic coherence of FF-OCT by a trained assistant (endoscopist) and trained pathologist is also compared. This is a single-center, prospective, observation trial. Eighty patients would be enrolled in the study. The tissue samples acquired by endoscopic ultrasound fine-needle biopsy (EUS-FNB) would be imaged by the FF-OCT system, interpreted by a trained endoscopist and a pathologist. The results of the image interpretation would be verified with histological findings. This study determines the diagnostic capability of FF-OCT as a ROSE technique while performing EUS-FNB, and whether endoscopists can implement the assessment.
Keywords: diagnostic yield, EUS, fine-needle biopsy, full-field optical coherence tomography, macroscopic on-site evaluation
INTRODUCTION
EUS-guided fine-needle biopsy (EUS-FNB) is a widely used, reliable, and safe method for diagnosing pancreatic diseases, especially malignant diseases.[1] Some meta-analytic studies[2,3] have confirmed that rapid on-site evaluation (ROSE) is capable of improving the adequacy rates and elevating the diagnostic accuracy of EUS-FNA of solid pancreatic lesions. Nevertheless, ROSE is unavailable because of the inadequacy of trained pathologists. Several centers have performed macroscopic on-site evaluation (MOSE) studies to predict the diagnostic adequacy for addressing this issue.[4,5,6,7,8] However, 22G and 19G FNA or FNB needles were utilized in these trials. The inner diameter of the 25G needle is smaller than that of 22G and 19G needles; therefore, the tissue samples obtained from the help of a 25G needle are smaller than those using different sized needles. MOSE for 25G FNB needle needs detailed estimation.[9]
A promising new tomographic technology improved dramatically in recent years, especially useful in vivo and in vitro detection and imaging of biological tissues is full-field optical coherence tomography (FF-OCT). This technology has been extensively used in imaging several organs such as resected pancreatic cancer specimens,[10] cartilage,[11] prostate biopsy cores,[12] skin,[13] lymph nodes,[14] and other organs.[15]
The field of digestive endoscopy is also being aided by the OCT system. Some earlier studies have detailed information about the device.[16,17] This system is used as an accessory device of the endoscope to carry out real-time endoscopic imaging by introducing it through the working channel of a routine duodenoscope or gastroscope. Nevertheless, as the pancreas cannot be directly observed through endoscopy, the OCT system cannot be used directly in EUS. Therefore, it is used for imaging tissue samples through EUS-FNB in vitro. Preliminarily, a prospective study was performed by Grieve et al.[18] to confirm the consistency of diagnosis between FF-OCT images and the final histological diagnosis. Nonetheless, only eight of the small sample size of 14 patients who participated in this study had solid pancreatic lesions. Hence, the diagnostic consistency of the FF-OCT system requires authentication through prospective studies comprising a higher number of participants.
Study design
Ethical considerations
The study was approved by Shanghai Changhai Hospital Ethics Committee (approval number: CHEC2019-108). The study protocol has been registered on ClinicalTrials.gov (ID: NCT04153318). Informed consent would be acquired from all enrolled patients or their closest relatives along with authorization. Totally, eighty patients would be enrolled in this study from the Changhai Hospital in China. The study complies with the Declaration of Helsinki and the principles of Good Clinical Practice guidelines.
Objectives
The primary objective of the trial is to ascertain the diagnostic sensitivity and specificity of FF-OCT. The secondary objectives are as follows: (1) to evaluate the possibility of FF-OCT differentiating different types of pancreatic diseases (2) and to compare the diagnostic coherence of FF-OCT by a trained endoscopist and a trained pathologist.
Eligibility
Patients who are scheduled to receive EUS-FNB, target is solid pancreatic lesion, and who signed the informed consent letter were included in the study. However, patients who failed to obtain tissue samples by EUS-FNB and have the inability or refused to provide signed informed consent were excluded from the study.
Blinding
The samples will not be randomized in this study.
The pathologist and endoscopist who would diagnose the FF-OCT images and the pathologist who conducts the final diagnosis of the tissue samples would be blind to each other's diagnoses.
METHODS
Technique for EUS-fine-needle biopsy
An experienced endoscopist would perform all the procedures with the help of a linear-array echoendoscope (EG-580UT; Fuji Film, Tokyo, Japan), and a 22G or a 25G ProCore™ needle (Cook Medical, National Technology Park, Limerick, Ireland). Patients would be made to lie in the left lateral decubitus position and administered anesthesia-assisted sedation using intravenous propofol (2.0–2.5 mg/kg for initialization and then 8–10 mg/kg/h for maintenance)[19] immediately before the procedure. Continuous monitoring of the vital signs will be performed throughout the procedure. The EUS-FNB procedure will be performed in the following way. The lesion would be localized and the echoendoscope would be positioned. The lesion would be traced in the needle path following the insertion of the needle into the echoendoscope. Suction would be applied under real-time ultrasound guidance, and the needle is moved forward and backward within the lesion twenty times. The suction technique would be applied based on the endoscopist's experience and the lesion's characteristics.
Full-field-optical coherence tomography system and sample preparation
The new FF-OCT system (Light-CT Scanner, LLTech SAS, Paris, France) has been upgraded in recent years, which is a combination of OCT and dynamic cell imaging. The principle of dynamic cell imaging involves virtual coloring of cells as per their various metabolites: the signals are then integrated into various frequency bands to measure the dynamic signals from different objects. The corresponding values are provided in the hue, saturation, and value color space, from which the center frequency of each voxel represents the gray scale, the spectral width represents the saturation, and the wave amplitude represents the degree. The following are the parameters: (1) the axial and transverse resolutions of the system are 1 μm and 1.5 μm, respectively; (2) the field of view is 1.27 mm × 1.27 mm; (3) the depth of penetration is 200 μm to 1 mm; (4) the imaging speed is 1 min/cm2; and (5) the maximum sample size is 27 mm diameter and 5 mm height.
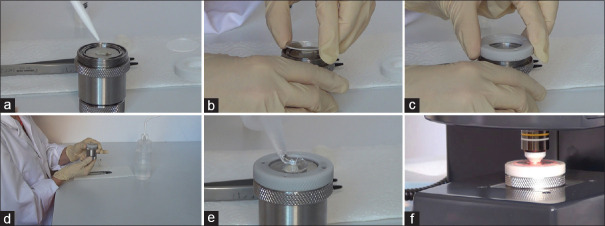
The system would be installed at the digestive endoscopic center of Changhai Hospital. The tissue samples accumulated with the help of EUS-FNB would be immediately transferred on to the sample holder, which may be used several times.[18] About 1–2 mL of normal saline will be dropped onto the holder, which would then be covered using a glass slide. Thereafter, the holder would be positioned at the optical capture unit and the height would be adjusted. The tissue sample is to be flattened against the glass to capture an image across the entire sample area. Care is to be taken while adjusting the height of the sample, as too much pressure on the tissue laid by the slides may damage the sample.[18] Thereafter a suitable volume of optical fluid, which is similar to the oil used in high-power microscopes to reduce the refraction of light, is added to the glass surface. The system is now ready for imaging for about 1 min. Once imaging is carried out, the tissue samples would be stored in a formalin vial. The sample would then be transported to the pathology department for paraffin embedding and hematoxylin and eosin staining. The detailed steps are shown in Figure 1.
Figure 1.
Illustration of sample processing. (a) add 1–2 mL of saline on the sample holder; (b) place the tissue sample on the holder and cover the slide; (c) close the sample holder; (d) adjust the height of holder; (e) add the optical fluid on the glass surface; (f) perform full-field-optical coherence tomography imaging
Diagnostic criteria
Considering that there is no diagnostic criteria for FF-OCT imaging of pancreatic lesions, we used ROSE's diagnostic criteria to judge the benign and malignant nature of FF-OCT imaging.[20] The diagnostic criteria are focusing on four diagnostic cytological features of pancreatic carcinoma: (i) nuclear enlargement (if more than two red blood cells); (ii) anisonucleosis (variation in nuclear size greater than four times within the same epithelial group); (iii) nuclear crowding/overlapping/three dimensionality; and (iv) nuclear membrane irregularity. The final diagnosis is classified as G1–G3: G1: does not meet any of the above criteria; G2: meet 1–2 criteria; and G3: meet 3–4 criteria. G1 is classified as benign, G2 is associated with atypical, while G3 is considered malignant.
Data collection
Data collection would be conducted in three stages.
Before recruiting the patients to the study, the following data would be collected: baseline characteristics such as age, gender, height, and weight; symptoms including pain, jaundice, and weight loss; laboratory data including complete blood count, international normalized ratio, bilirubin, and carbohydrate antigen 19-9; and imaging data available prior to EUS-FNB.
During the procedure, lesion location and size, the number of needle passes that yield the tissue sample, preliminary evaluation of FF-OCT imaging by trained endoscopist, and the time from the sample acquisition to FF-OCT imaging would be collected.
Post-EUS-FNB procedure, the following data are recorded: operation-related complications, cytological findings, the diagnosis of FF-OCT imaging and conventional histology by trained pathologists, and the outcomes of surgical pathological examinations, if available.
Statistical analysis
The baseline characteristics of patients are presented without statistical comparison. Descriptive statistics for continuous variables are presented as means and standard deviations or medians and ranges. Categorical variables are expressed as counts and percentages. Logistic regression is applied to assess the sensitivity of FF-OCT imaging, adjusted for age, sex, lesion location within the pancreas, lesion size, and needle type used. The Chi-square test or Fisher's exact test (if at least one of the values in the cells of the contingency table is <5) is applied to compare the sensitivity and accuracy of different types of pancreatic mass, and to compare the differences between the FF-OCT diagnoses made by the pathologist and the endoscopist. All statistical analyses were performed using IBM SPSS Statistics v22.0 software (SPSS Inc., Chicago, IL, USA).
DISCUSSION
The major aim of this study is to verify the technical feasibility of FF-OCT system for tissue samples obtained by the EUS-FNB technique and to verify the time needed for imaging. Owing to the inadequacy of pathologists, the secondary objective is to investigate whether the FF-OCT system could be used for on-site histological evaluation by trained endoscopists at centers where ROSE is unavailable. Furthermore, as our center also receives patients with several types of pancreatic solid lesions other than pancreatic ductal adenocarcinoma, this study would also analyze the diagnostic capabilities of FF-OCT imaging for different types of pancreatic lesions.
On the basis of the clinical experience of our center, MOSE has limitations to a certain extent. It is often difficult to distinguish the actual core tissue sample because of blood contamination through MOSE. Certain visual solid samples initially identified as tissue samples were later identified as blood clots after histological examination. A recent systematic review determined the OCT system to exhibit promising results in detecting the tumors in multiple organs, both in vivo and in vitro.[15] Thus, the OCT system is believed to be a more reliable technique for ROSE, which contributes to yield a higher diagnostic rate of EUS-FNB.
As concerns, the time efficiency that needed to perform ROSE for the sample acquired from each needle pass is about 2 min, whereas the estimated time taken for sample preparation of FF-OCT is 1–2 min, which is comparable.
The 2017 European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline[9] recommended utilizing 25G or 22G FNA or FNB needles for routine EUS-guided sampling of pancreatic solid masses (high-quality evidence, strong recommendation). When the primary aim of sampling is to acquire a core tissue specimen, ESGE suggests using 19G FNA or FNB or 22G FNB needles (low-quality evidence, weak recommendation). Owing to the large inner diameter and poor flexibility of the 19G needle, it turns out to be difficult to puncture the lesions located in the pancreatic head and uncinate. Therefore, in this study, 22G and 25G FNB needles would be used. The diameter of the tissue samples acquired using 22G or 25G EUS-FNB needle, which is also considered to be the height while imaging, is about 1–2 mm. This is suitable for full-thickness imaging utilizing the FF-OCT system, which selects the photon interference signal scattered from a specific plane below the surface of the sample.
The only relative study conducted earlier found that two samples acquired by 25G needle were lost due to the additional steps of FF-OCT.[18] Tissue damage occurred when it was pressed against the coverslip. Histological examination showed that these tissue samples had disintegrated into multiple fragments; however, the histological analysis was not affected. If the tissue sample obtained by the 25G ProCore™ needle is unsuitable for imaging, or a loss of tissue material occurs as in the earlier study in more than five cases, a 22G ProCore™ needle would be utilized in the rest of the cases in the study.
Grieve et al.[18] too found the success of FF-OCT imaging to be rather related to the lesion type; however, due to the less number of patients participated in the study, it was inconclusive. In an earlier retrospective study conducted at our center,[21] in addition to pancreatic ductal adenocarcinoma, several other diseases including pancreatic neuroendocrine tumor, intraductal papillary mucinous neoplasm, lymphoma, adenosquamous carcinoma, and acinar cell carcinoma were also found, which may verify this deduction. Thus, this trial applies to a wider range and can explore the diagnostic role of FF-OCT in specific pancreatic tumors.
Trial status
Patient enrollment would likely start in October 2020 and end by December 2020.
Trial registration
ClinicalTrials.gov, NCT04153318.
Financial support and sponsorship
This study was funded by Construction of Shanghai Pancreatic Diseases Medical Center (Grant No. 2017ZZ01009).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 2.Matynia AP, Schmidt RL, Barraza G, et al. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: A systematic review and meta-analysis. JGastroenterol Hepatol. 2014;29:697–705. doi: 10.1111/jgh.12431. [DOI] [PubMed] [Google Scholar]
- 3.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwashita T, Yasuda I, Mukai T, et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: A single-center prospective pilot study (MOSE study) Gastrointest Endosc. 2015;81:177–85. doi: 10.1016/j.gie.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 5.Oh D, Seo DW, Hong SM, et al. The impact of macroscopic on-site evaluation using filter paper in EUS-guided fine-needle biopsy. Endosc Ultrasound. 2019;8:342–7. doi: 10.4103/eus.eus_34_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ, Jung YS, Park JH, et al. Endosonographer's macroscopic evaluation of EUS-FNAB specimens after interactive cytopathologic training: A single-center prospective validation cohort study. Surg Endosc. 2016;30:4184–92. doi: 10.1007/s00464-015-4727-3. [DOI] [PubMed] [Google Scholar]
- 7.Leung Ki EL, Lemaistre AI, Fumex F, et al. Macroscopic onsite evaluation using endoscopic ultrasound fine needle biopsy as an alternative to rapid onsite evaluation. Endosc Int Open. 2019;7:E189–94. doi: 10.1055/a-0770-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masutani H, Okuwaki K, Kida M, et al. On-site stereomicroscope quality evaluations to estimate white core cutoff lengths using EUS-FNA biopsy sampling with 22-gauge needles. Gastrointest Endosc. 2019;90:947–56. doi: 10.1016/j.gie.2019.08.033. [DOI] [PubMed] [Google Scholar]
- 9.Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline-March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 10.van Manen L, Stegehuis PL, Fariña-Sarasqueta A, et al. Validation of full-field optical coherence tomography in distinguishing malignant and benign tissue in resected pancreatic cancer specimens. PLoS One. 2017;12:e0175862. doi: 10.1371/journal.pone.0175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pailhé R, Mounier A, Boisson B, et al. Qualitative and quantitative assessment of cartilage degeneration using full-field optical coherence tomography ex vivo. Osteoarthritis Cartilage. 2018;26:285–92. doi: 10.1016/j.joca.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Lopater J, Colin P, Beuvon F, et al. Real-time cancer diagnosis during prostate biopsy: Ex vivo evaluation of full-field optical coherence tomography (FFOCT) imaging on biopsy cores. World J Urol. 2016;34:237–43. doi: 10.1007/s00345-015-1620-6. [DOI] [PubMed] [Google Scholar]
- 13.Dalimier E, Salomon D. Full-field optical coherence tomography: A new technology for 3D high-resolution skin imaging. Dermatology. 2012;224:84–92. doi: 10.1159/000337423. [DOI] [PubMed] [Google Scholar]
- 14.Grieve K, Mouslim K, Assayag O, et al. Assessment of sentinel node biopsies with full-field optical coherence tomography. Technol Cancer Res Treat. 2016;15:266–74. doi: 10.1177/1533034615575817. [DOI] [PubMed] [Google Scholar]
- 15.van Manen L, Dijkstra J, Boccara C, et al. The clinical usefulness of optical coherence tomography during cancer interventions. JCancer Res Clin Oncol. 2018;144:1967–90. doi: 10.1007/s00432-018-2690-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poneros JM, Brand S, Bouma BE, et al. Diagnosis of specialized intestinal metaplasia by optical coherence tomography. Gastroenterology. 2001;120:7–12. doi: 10.1053/gast.2001.20911. [DOI] [PubMed] [Google Scholar]
- 17.Poneros JM, Tearney GJ, Shiskov M, et al. Optical coherence tomography of the biliary tree during ERCP. Gastrointest Endosc. 2002;55:84–8. doi: 10.1067/mge.2002.120098. [DOI] [PubMed] [Google Scholar]
- 18.Grieve K, Palazzo L, Dalimier E, et al. A feasibility study of full-field optical coherence tomography for rapid evaluation of EUS-guided microbiopsy specimens. Gastrointest Endosc. 2015;81:342–50. doi: 10.1016/j.gie.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 19.Wang KX, Jin ZD, Du YQ, et al. EUS-guided celiac ganglion irradiation with iodine-125 seeds for pain control in pancreatic carcinoma: A prospective pilot study. Gastrointest Endosc. 2012;76:945–52. doi: 10.1016/j.gie.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi T, Ishiwatari H, Yoshida M, et al. Rapid on-site evaluation by endosonographer during endoscopic ultrasound-guided fine needle aspiration for pancreatic solid masses. JGastroenterol Hepatol. 2013;28:656–63. doi: 10.1111/jgh.12122. [DOI] [PubMed] [Google Scholar]
- 21.Zhou W, Gao L, Wang SM, et al. Comparison of smear cytology and liquid-based cytology in EUS-guided FNA of pancreatic lesions: Experience from a large tertiary center. Gastrointest Endosc. 2020;91:932–42. doi: 10.1016/j.gie.2019.10.033. [DOI] [PubMed] [Google Scholar]