Abstract
Background and Objective:
EUS-guided-biliary drainage (EUS-BD) is an efficacious and safe option for patients who fail ERCP. EUS-BD is a technically challenging procedure. The aim of this study was to define the learning curve for EUS-BD.
Methods:
Consecutive patients undergoing EUS-BD by a single operator were included for a prospective registry over 6 years. Demographics, procedural information, adverse events, and follow-up data were collected. Nonlinear regression and CUSUM analyses were conducted for the learning curve. Technical success was defined as successful stent placement. Clinical success was defined as resolution of jaundice and/or at least a 30% reduction in the pretreatment bilirubin level within a week after placement or normalization of bilirubin within 30 days.
Results:
Seventy-two patients were included in the study (53% male, mean age 67 years). Technical success was achieved in 69 patients (96%). Clinical success was achieved in 59/69 patients (86%). Seven patients (10%) had adverse events including bleeding (n = 6) and liver abscess (n = 1). The median procedural time was 59 min (range 36–138 min). This was achieved at the 32nd procedure. Procedural durations were further reduced to 50 min and below after the 50th procedure in a nonlinear pattern. This suggests that procedural durations approach a potential plateau after 100 cases.
Conclusion:
Endoscopists experienced in EUS-BD are expected to achieve a reduction in procedural time over successive cases, with efficiency reached at 59 min and a learning rate of 32 cases. Continued improvement is demonstrated with additional experience, with mastery suggested after approximately 100 cases.
Keywords: biliary drainage, biliary stricture, CUSUM, ERCP, EUS-guided, EUS-guided biliary drainage, learning curve
INTRODUCTION
ERCP is the first-line therapy for pancreaticobiliary decompression with success rates >90%. However, failure can occur due to difficult patient anatomy caused by surgical alteration (Whipple, Roux-en-Y gastric bypass, and Billroth surgery), periampullary diverticulum, or malignancies that cause gastric outlet obstruction.[1,2,3,4,5,6,7] EUS-guided-biliary drainage (EUS-BD) is an alternative biliary drainage technique after unsuccessful ERCP. The procedure was first described in 2001 by Giovannini et al. and has since been validated as a safe and efficacious procedure in literature.[5,8,9] There are multiple approaches for EUS-BD, however the overall technique is similar, which involves direct access to the biliary tree via fine-needle aspiration needle, guidewire placement under endosonographic and fluoroscopic visualization, creation of a fistulous tract, and finally stent placement.[6]
Although a minimally invasive procedure, EUS-BD is a highly technical skill that requires specialized training. Adverse event rates have been reported to be between 3.5% and 38.6% due to bile leak, bleeding, cholangitis, perforation, sepsis and peritonitis, and stent migration.[10,11] According to the American Society of Gastrointestinal Endoscopy, proficiency in EUS recommends a minimum of 150 supervised cases to be performed, however no definition for proficiency has been established for EUS-BD. The aim of this study was to define the learning curve for EUS-BD.
METHODS
Study overview
Consecutive patients undergoing EUS-BD by a single endoscopist with expertise in therapeutic EUS and ERCP (MK) were included from a prospective registry over 6 years. The patient demographics, procedural information, adverse events, and follow-up data were collected. Technical success for EUS-BD was defined as successful biliary stent placement. Clinical success was defined as resolution of jaundice and/or at least a 30% decrease in pretreatment bilirubin level within a week after stent placement or normalization of bilirubin within 30 days of stent placement.
Procedural technique
All procedures were done with the patients under general anesthesia. All patients received antibiotics peri-procedurally. A linear echoendoscope was advanced into the stomach or duodenum. Biliary access was obtained either from the stomach into the intrahepatic (IH) biliary tree or from the duodenum into the extrahepatic (EH) biliary tree using an algorithm based on patient anatomy.[12] The biliary tree was accessed by a 19G fine-needle aspiration needle, after which a cholangiogram was obtained. A wire was then advanced into the biliary tree, transpapillary if feasible. Fistula tract creation was performed using a cautery followed by a dilating balloon, after which a stent was deployed. In one case, after the cholangiogram was obtained, the wire was unable to be advanced into the biliary tree. However, conventional ERCP was able to be performed using the cholangiogram.
Statistical analysis
Consecutive patients undergoing EUS-BD drainage by a single operator were included for statistical analyses from a prospective registry over 6 years. Demographics, procedural info, postprocedural follow-up data, and adverse events were collected. Nonlinear regression and CUSUM analyses were conducted for the learning curve using Stata 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
RESULTS
A total of 72 patients were included in this study: 53% male, mean age 67 years. Fifty-six patients (78%) had malignant obstruction, 16 (22%) had benign obstruction, and 3 had cholangitis from benign cause [Table 1]. Multiple approaches were performed for biliary stent placement: 32 IH anterograde, 24 EH rendezvous, and 16 hepaticogastrostomies, which also included an indirect rendezvous when EUS-BD provided a cholangiogram without advancement of the wire after which conventional ERCP was performed. Forty-two stents were transpapillary (61%) and 57 stents were metal (83%).
Table 1.
Demographics on the study population (n=72)
| Characteristics | N (%) |
|---|---|
| Age (years), mean (SD) | 67.1 (12.4) |
| Gender - male, n (%) | 38/72 (53) |
| Indication for EUS-BD, n (%) | |
| Malignant | 56 (78) |
| Benign | 16 (22) |
| Cholangitis | 3 (4) |
| Technical success, n (%) | 69/72 (96) |
| Approach, n (%) | |
| IH antegrade | 29/69 (42) |
| EH Rendezvous | 12/69 (17) |
| Hepaticogastrostomy | 16/69 (23) |
| Choledochoduodenostomy | 11/69 (16) |
| Other | 1/69 (1) |
| Stent placement, n (%) | |
| Transpapillary/transanastomotic | 42/69 (61) |
| Transluminal | 27/69 (39) |
| Stent type, n (%) | |
| Metal | 57/69 (83) |
| Plastic | 12/69 (17) |
| Adverse events, n (%) | 7/72 (10) |
| Bleeding - IR embolization | 3 |
| Bleeding - conservative | 2 |
| Bleeding - death | 1 |
| Liver abscess | 1 |
| Clinical success, n (%) | 59/69 (86) |
| Median procedural time (min) | 59 |
*All Benign. BD: Biliary drainage; IH: Intrahepatic; EH: Extrahepatic; IR: Interventional radiology.
Technical success was achieved in 69 patients (96%). Clinical success was achieved in 59/69 patients (86%): 28 patients had resolution of jaundice, 26 patients had improvement in bilirubin by 30%, and 5 patients had resolution of initial indication for biliary drainage. Of the ten patients who did not achieve clinical success, five were lost to follow-up or passed away <1 week postprocedure (unrelated to the procedure) and five had no change. Seven patients (10%) had adverse events. Six patients had peri-procedural bleeding, of which three required embolization by interventional radiology, one was managed with clips, one was managed with observation alone, and one died on the procedure table. One patient developed a liver abscess that was drained percutaneously.
The median procedural time was 59 min (range 36–138 min). The CUSUM chart demonstrated that a 59-min procedural time was achieved at the 32nd procedure, indicating efficiency after 32 procedures [Figure 1]. Procedural times further reduced after the 50th procedure, with durations remaining at 50 min or below (nonlinear regression P < 0.0001) [Figure 2]. This suggests that a potential plateau may be achieved after about 100 cases, indicating mastery.
Figure 1.
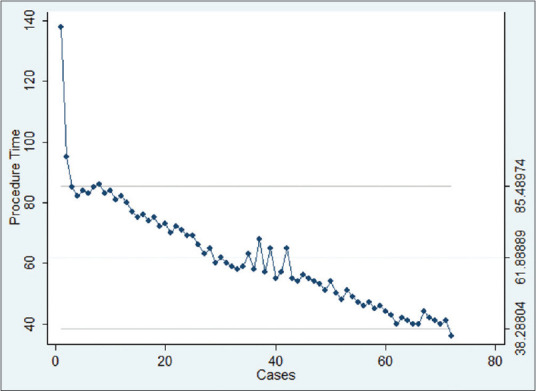
CUSUM chart
Figure 2.
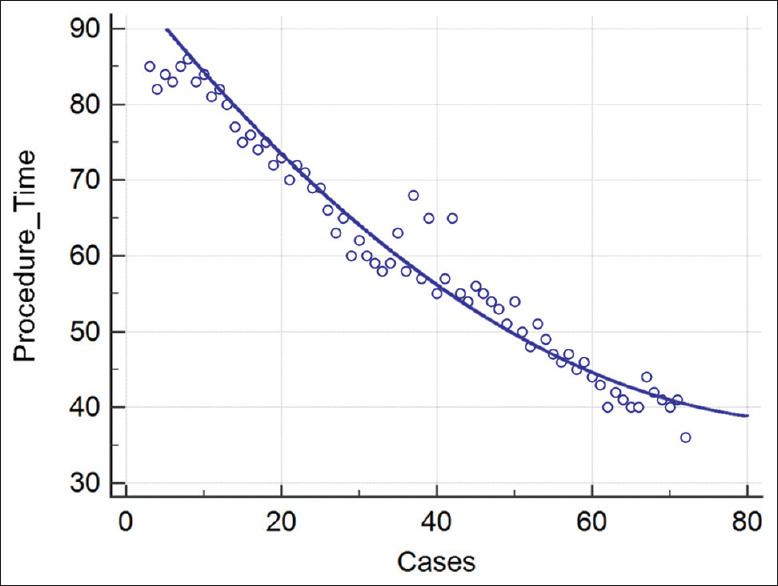
Nonlinear regression
DISCUSSION
Currently, there is little data showcasing the learning curve for EUS-BD since its introduction in 2001. However, literature has shown higher technical and clinical success rates and lower complication rates in more experienced endoscopists compared to early endoscopists. In a study comprising of 31 cases, the failure rate of EUS-BD was 38% during the first 3 years of training and 11% in the last 2 years of training.[13] In a Spanish national survey that included data from less experienced endoscopists performing EUS-BD, the technical success rate was 67.2%, which was lower than previously reported data in more experienced endoscopists.[14] In a single-center study on the cumulative experience of EUS-BD by a single operator for obstructive jaundice, there were five procedure-related deaths in the first fifty patients during the first 5 years, and there was one procedure-related death in the last 51 patients during the last 2 years among 101 patients in the 7-year study.[15] Because there is an observed learning curve, Hara et al. suggest endoscopists perform at least the first twenty cases under a mentor's supervision.[16] To better define the learning curve for EUS-BD, this study looked at the cumulative experience of a single operator over the course of 6 years.
The overall technical success rate for EUS-BD was 96%, which was comparable to previously reported success rates for EUS-guided hepaticogastrostomy (EUS-HGS) and EUS-guided choledochoduodenostomy (87% and 50%–100%, respectively) and higher than the pooled technical success rate of 89.18%.[10,17,18] Clinical success was achieved in 86% of patients, which is similar to the published clinical success rates in literature (87%–100%).[10,16] The adverse event rate was 10%, which was on the lower end of the reported adverse events in other studies (10%–30%).[5,14,16]
In this study, the single endoscopist's procedural times were correlated with the number of cases performed. On the CUSUM analysis, the single operator achieved efficiency with a procedural time of 59 min after the 32nd case. With cumulative experience, the procedural times eventually reduce in a nonlinear pattern after the 50th procedure, suggesting that there is a potential plateau and achievement of mastery of EUS-BD after approximately 100 cases. This was more than the number of procedures to achieve technical proficiency in Oh et al.'s study, which required 24 cases to reduce procedural times to 30.1 ± 13.1 min and 33 cases to stabilize for procedural times.[18] This difference can potentially be attributed to the fact that the study focused on EUS-HGS alone and did not include the other EUS-BD techniques, similar to the current study.
Factors that affect the procedural time and therefore the learning curve include patient factors and operator factors. Patient factors include surgically altered anatomy or complex biliary strictures that make it difficult for the guidewire to pass and insufficient IH bile duct dilatation that may make correct biliary puncture challenging.[15,17] There are multiple endoscopist- and procedure-related factors that contribute to the learning curve. Itoi et al. mentioned that although the bile duct appears close to the gastrointestinal wall under EUS, there is some displacement between the puncture site of the gastrointestinal wall and bile duct, potentially resulting in failure of the procedure.[19] The next and most challenging step is guidewire manipulation into the bile ducts, which requires identifying an appropriate plane and insertion into the biliary duct that may have an angular offset.[14,17] If the guidewire is advanced into the peripheral biliary tract, then dilation and stent placement cannot be performed properly.[20] Certain techniques may also be intrinsically more difficult than others, such as EUS-HGS compared to EUS because it requires a relatively stable scope position and a fixed biliary access point.[18] Given the complexity of EUS-BD, repeated number of procedures are needed to familiarize with the technical maneuvers involved.
CONCLUSION
Therapeutic EUS is a highly technical skill that requires specialized training. Proficiency of EUS can only be obtained through execution of numerous cases. In this study, it was determined that endoscopists experienced in EUS-BD are expected to achieve a reduction in procedural time over successive cases, with efficiency reached at 59 min and a learning rate of 32 cases. Continued improvement is demonstrated with additional experience, with mastery suggested after approximately 100 cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Khan MA, Akbar A, Baron TH, et al. Endoscopic ultrasound-guided biliary drainage: A systematic review and meta-analysis. Dig Dis Sci. 2016;61:684–703. doi: 10.1007/s10620-015-3933-0. [DOI] [PubMed] [Google Scholar]
- 2.Moole H, Bechtold ML, Forcione D, et al. A meta-analysis and systematic review: Success of endoscopic ultrasound guided biliary stenting in patients with inoperable malignant biliary strictures and a failed ERCP. Medicine (Baltimore) 2017;96:e5154. doi: 10.1097/MD.0000000000005154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coté GA, Singh S, Bucksot LG, et al. Association between volume of endoscopic retrograde cholangiopancreatography at an academic medical center and use of pancreatobiliary therapy. Clin Gastroenterol Hepatol. 2012;10:920–4. doi: 10.1016/j.cgh.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Itoi T, Dhir V. EUS-guided biliary rendezvous: Slow, hesitant, baby steps forward. Gastrointest Endosc. 2016;83:401–3. doi: 10.1016/j.gie.2015.05.049. [DOI] [PubMed] [Google Scholar]
- 5.Minaga K, Kitano M. Recent advances in endoscopic ultrasound-guided biliary drainage. Dig Endosc. 2018;30:38–47. doi: 10.1111/den.12910. [DOI] [PubMed] [Google Scholar]
- 6.Baron TH, Petersen BT, Mergener K, et al. Quality indicators for endoscopic retrograde cholangiopancreatography. Am J Gastroenterol. 2006;101:892–7. doi: 10.1111/j.1572-0241.2006.00675.x. [DOI] [PubMed] [Google Scholar]
- 7.Enochsson L, Swahn F, Arnelo U, et al. Nationwide, population-based data from 11,074 ERCP procedures from the Swedish Registry for Gallstone Surgery and ERCP. Gastrointest Endosc. 2010;72:1175–84. doi: 10.1016/j.gie.2010.07.047. 1184e1-3. [DOI] [PubMed] [Google Scholar]
- 8.Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest Endosc. 2016;83:1218–27. doi: 10.1016/j.gie.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Ogura T, Higuchi K. Technical tips of endoscopic ultrasound-guided choledochoduodenostomy. World J Gastroenterol. 2015;21:820–8. doi: 10.3748/wjg.v21.i3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baars JE, Kaffes AJ, Saxena P. EUS-guided biliary drainage: A comprehensive review of the literature. Endosc Ultrasound. 2018;7:4–9. doi: 10.4103/eus.eus_105_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyberg A, Desai AP, Kumta NA, et al. EUS-guided biliary drainage after failed ERCP: A novel algorithm individualized based on patient anatomy. Gastrointest Endosc. 2016;84:941–6. doi: 10.1016/j.gie.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Attasaranya S, Netinasunton N, Jongboonyanuparp T, et al. The spectrum of endoscopic ultrasound intervention in biliary diseases: A single center's experience in 31 cases. Gastroenterol Res Pract. 2012;2012:680753. doi: 10.1155/2012/680753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest Endosc. 2012;76:1133–41. doi: 10.1016/j.gie.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Poincloux L, Rouquette O, Buc E, et al. Endoscopic ultrasound-guided biliary drainage after failed ERCP: Cumulative experience of 101 procedures at a single center. Endoscopy. 2015;47:794–801. doi: 10.1055/s-0034-1391988. [DOI] [PubMed] [Google Scholar]
- 16.Hara K, Yamao K, Mizuno N, et al. Endoscopic ultrasonography-guided biliary drainage: Who, when, which, and how? World J Gastroenterol. 2016;22:1297–303. doi: 10.3748/wjg.v22.i3.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwashita T, Doi S, Yasuda I. Endoscopic ultrasound-guided biliary drainage: A review. Clin J Gastroenterol. 2014;7:94–102. doi: 10.1007/s12328-014-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh D, Park DH, Song TJ, et al. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol. 2017;10:42–53. doi: 10.1177/1756283X16671671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoi T, Sofuni A, Itokawa F, et al. Endoscopic ultrasonography-guided biliary drainage. J Hepato Biliary Pancreat Sci. 2010;17:611–6. doi: 10.1007/s00534-009-0196-1. [DOI] [PubMed] [Google Scholar]
- 20.Ogura T, Higuchi K. Technical tips for endoscopic ultrasound-guided hepaticogastrostomy. World J Gastroenterol. 2016;22:3945–51. doi: 10.3748/wjg.v22.i15.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]


