Abstract
EUS-guided biliary drainage (EUS-BD) has been used as a salvage modality for relief of malignant biliary obstruction (MBO) after a failed ERCP. Multiple recent randomized controlled trials (RCTs) and observational studies have been published to assess the suitability of EUS-BD as a first-line modality for achieving palliative BD. We aimed to perform a systematic review and meta-analysis comparing primary EUS-BD versus ERCP for MBO. We searched PubMed, Medline, and Embase up to January 1, 2019, to identify RCTs and observational studies evaluating the efficacy and safety of primary EUS-BD (without a prior attempted ERCP) versus ERCP. Quality of RCTs and observational studies was assessed using Jadad and Newcastle–Ottawa scores, respectively. The outcomes of interest were technical success, clinical success, odds of requiring a repeat intervention, and procedure-related adverse events. Odds ratios (ORs) and standard mean difference were calculated for categorical and continuous variables, respectively. Meta-analysis was performed using the random effects model in RevMan 5.3 (the Cochrane Collaboration, the Nordic Cochrane Centre, Copenhagen, Denmark). Five studies (three RCTs and two observational studies) with 361 patients were included. Both procedures achieved comparable technical success (OR: 1.20 [0.44–3.24], I2 = 0%) and clinical success (OR: 1.44, confidence interval [CI]: 0.63–3.29, I2 = 0%). The overall adverse outcomes (OR: 1.59 [0.89–2.84]) did not differ between the two groups. In the ERCP group, 9.5% of patients developed procedure-related pancreatitis versus zero in the EUS group (risk difference = 0.08%, P = 0.02). There was no statistically significant difference in nonpancreatitis-related adverse events. The odds of requiring reintervention for BD (1.68 [0.76–3.73], I2 = 42%) did not differ significantly. The ERCP group had significantly higher odds of requiring reintervention due to tumor overgrowth (5.35 [1.64–17.50], I2 = 0%). EUS-BD has comparable technical and clinical success to ERCP and can potentially be used as a first-line palliative modality for MBO where expertise is available. ERCP-related pancreatitis which can cause significant morbidity can be completely avoided with EUS.
Keywords: EUS-guided biliary drainage, malignant biliary stricture, therapeutic EUS
INTRODUCTION
ERCP is the first-line modality for the management of malignant biliary obstruction (MBO). EUS-guided biliary drainage (BD) has been used as an alternative salvage modality for achieving BD in cases with failed ERCP or when ERCP is impracticable due to anatomic constraints imposed by prior foregut surgery or tumor invasion. High-quality evidence in terms of multiple meta-analyses of randomized controlled trials (RCTs) and observational studies already exist, establishing the superiority of EUS-BD over percutaneous transhepatic cholangiography and biliary drainage as a salvage modality after failed ERCP. However, the position of ERCP as the first-line modality for MBO has so for been unchallenged.[1] Multiple recent clinical trials and observational studies have spurred considerable interest in comparing EUS-BD with ERCP for the first-line management of MBO.[2,3,4,5,6]
Since it was first demonstrated in 2001,[7] EUS-BD has become an increasingly popular method of gaining biliary access. Increasing operator experience has led to greatly reduced rates of adverse events and has augmented clinical and technical success. The general principle of EUS-BD involves visualization of dilated intrahepatic or extrahepatic bile duct with EUS followed by puncturing them with a needle or lumen-apposing metal stent (LAMS). Either intrahepatic or extrahepatic approach can be used to gain biliary access. In the extrahepatic approach, the common bile duct is accessed through the duodenum. BD is achieved either by transluminal stenting (EUS-guided choledochoduodenostomy [CDS]) or by transpapillary stenting (the rendezvous technique). In the intrahepatic approach, the left lobe of the liver is accessed through the gastric wall. In this case, BD can be achieved via transluminal stenting (hepaticogastrostomy) or through transpapillary access via the rendezvous technique.[8]
We performed a systematic review and meta-analysis comparing primary EUS-BD (i.e., without a prior attempted ERCP) with ERCP on clinical and technical success and adverse events. We also looked into the differences of reintervention rates.
METHODS
We followed the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” checklist and the “Meta-analysis of Observational Studies in Epidemiology” checklist to conduct this systematic review and meta-analysis.[9,10]
Search strategy
The search strategy was developed with the help of an experienced team of librarians. The initial search strategy was developed in PUBMED and subsequently translated to match headings and keywords for Embase, Cochrane Central Register of Controlled Trials, ISI Web of Science, and Scopus from January 2001 through January 1, 2019. We used the following keywords in combination: “EUS guided biliary drainage,” “choledochoduodenostomy,” “hepaticogastrostomy,” “malignant biliary obstruction,” “EUS-BD,” “EUS-HGS,” “ERCP,” “interventional EUS,” “therapeutic EUS” “transluminal biliary drainage,” and “biliary stent.” The search strategy accounted for plurals and spelling variations using appropriate wildcards. In addition to the above search strategy, we manually searched the bibliographies of retrieved articles (”backward snowballing”). We excluded articles not written in the English language. All results were downloaded to Zotero (version 5.0, 64 bit; open source program: https://www.zotero.org/), a bibliographic manager. Duplicate citations were removed.
Inclusion criteria
We included RCTs and observational studies that compared EUS-BD (without a prior attempted ERCP) with ERCP. In the EUS-BD group, EUS-CDS and EUS-HGS were both included. Cases of EUS-REN were excluded as some authors consider it to be simply EUS-assisted ERCP and can have overlapping complications to ERCP such as ERCP-associated pancreatitis.[11,12] Studies had to report at least two of the following: technical success (determined as stent placement determined endoscopically or radiographically), clinical success (defined as reduction in bilirubin by 50% at 2 weeks or 75% at 4 weeks and clinical resolution of MBO), and postprocedure adverse events (defined as adverse events occurring within 30 days of the procedure). We excluded studies that did not have a comparison arm of ERCP.
Data extraction
Structured data forms were constructed before data extraction. These forms consisted of study name and design, sample size and baseline demographics of the patient, etiology of MBO, types of EUS, types of stents used, clinical and technical success, adverse events, reintervention rates, and length of stay. Data were independently extracted by the two authors (GK and HS).
Quality assessment
We used the Newcastle–Ottawa Scale[13] for the assessment of the quality of observational studies and the Cochrane tool for the assessment of the quality of RCTs.[14] The Newcastle–Ottawa scale is a 9-point scale that judges the quality of observational studies based on three parameters: a selection of study groups, comparability of study groups, and the determination of exposure/outcomes for cohort/case–control studies. High-quality studies score more than seven on this scale, moderate quality studies score between 5 and 7, and low-quality studies score <5. The Cochrane tool for the assessment of risk of bias for RCTs is based on a number of domains: randomization and allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and an auxillary domain for important concerns not covered in the other domains (other bias).
Statistical analysis
Statistical analysis was performed tocompare predefined primary and secondary outcomes between ERCP and EUS group. Primary outcomes were defined as technical success, clinical success, and adverse events. Adverse events were further divided into procedure-related pancreatitis and nonpancreatitis-related adverse events. Secondary outcomes were procedure time and reintervention rates. Odds ratios (ORs) were calculated for categorical events, and risk differences were calculated for continuous events. These were pooled, and meta-analysis was performed using DerSimonian and Laird random effects model.[15] When studies included a zero event in either arm, we performed a continuity correction to include studies with zero events in the pooled analysis.[16,17]
Heterogeneity and publication bias
We used the I2 statistics and the Cochran's Q test (to assess for heterogeneity). We considered an I2 > 75% and P < 0.1 in the Cochran's Q test as indicative of high heterogeneity.[18] We visually inspected funnel plots to evaluate the risk of publication bias. Further tests were not carried out to assess heterogeneity as the power and specificity are limited when fewer than ten studies are included in the primary meta-analysis.
Statistical analysis was carried out using the Review Manager Version 5.3 for windows (the Cochrane Collaboration, the Nordic Cochrane Centre, Copenhagen, Denmark).
RESULTS
Search results, quality, and baseline demographics of studies
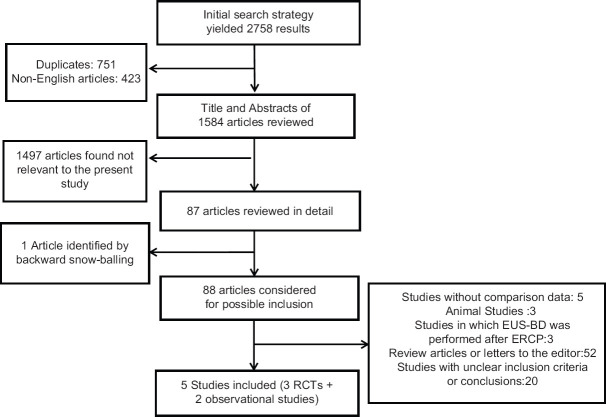
The initial search yielded 2758 results. After removing duplicates and screening out studies based on their title, 87 studies were selected for abstract review. After detailed abstract and bibliography review, one additional study was found for inclusion (”backward snowballing”) and 83 studies were excluded as they did not meet the eligibility criteria. There was one retrospective study that compared EUS-BD with prior attempted ERCP versus ERCP.[12] This study was excluded from our analysis. Eventually, a total of five studies (three RCTs and two observational studies) with 361 patients were included in this analysis. Details of the search strategy are illustrated in Figure 1.
Figure 1.
Study selection process for systematic review and meta-analysis
Out of the five studies included in this analysis, two were multicenter and three were single center [Table 1]. A total of 361 patients were included in this analysis: 190 in the ERCP group and 171 in the EUS group. In terms of the EUS modalities, four studies only included EUS-CDS, whereas one study included both EUS-CDS and EUS-HGS. With respect to the etiology of MBO, one of the studies included only pancreatic malignancies, whereas others included MBO resulting from all malignancies such as biliary, gastric, and malignant lymph nodes [Table 2]. Duodenal invasion was reported in three of the five studies and ranged from 23% to 44%. In the studies that it was reported, there was no statistically significant difference between the ERCP and the EUS group for having duodenal involvement. Only one study reported patients with anatomy altered by previous upper gastrointestinal (GI) surgery.[6] This study had one patient with Roux-en-y anatomy in the ERCP and the EUS group and two patients with Billroth-2 anatomies in the EUS group. Two studies in this analysis excluded patients with altered anatomy,[2,5] whereas the remaining two studies did not report about altered anatomy altogether. In terms of choice of the stent for EUS, all included studies used only self-expanding metal stents (SEMSs). None of the included studies used LAMSs. In terms of stent use in the ERCP group, all five studies only included metal stents. The studies used a different combination of covered, uncovered, and partially covered stents [Table 1].
Table 1.
Baseline characteristics and quality of included studies
| Study | Design | Males, n (%) | Age (years) | Overall survival (days) | EUS-BD modality | ERCP stent type | EUS stent | Quality of study |
|---|---|---|---|---|---|---|---|---|
| Bang | RCT | EUS: 33 (51.5) | 69.4 | 190 | EUS-CDS | Fully-covered SEMS | SEMS | Low quality (high risk of performance bias, low risk of selection, detection, attrition, or reporting bias) |
| ERCP: 34 (67.6) | 69.2 | 174 | ||||||
| Park | RCT | EUS: 14 (64.29) | 65.4 | 188 | EUS-CDS | Partially-covered SEMS | SEMS | Low quality (high risk of performance bias, low risk of selection, detection, attrition, or reporting bias) |
| ERCP: 14 (57.14) | 66.8 | 197 | ||||||
| Paik | RCT | EUS: 64 (64.06) | 64.8 | 144 | EUS-CDS and EUS-HGS | SEMS (uncovered, partially/fully covered) | SEMS | Low quality (high risk of performance bias, low risk of selection, detection, attrition, or reporting bias) |
| ERCP: 61 (42.62) | 68.4 | 178 | ||||||
| Nakai | Prospective cohort | EUS: 34 (53) | 79 | 249 | EUS-CDS | SEMS (unspecified covering) | SEMS | High quality |
| ERCP: 25 (48) | 69 | 216 | ||||||
| Kawakubo | Retrospective | EUS: 26 (30.8) | 71 | 296 | EUS-CDS | SEMS (uncovered, partially/fully covered) | SEMS | High quality |
| ERCP: 56 (53.6) | 68 | 156 |
RCT: Randomized controlled trials; SEMS: Self-expanding metal stents; BD: Biliary drainage; CDS: Choledochoduodenostomy; HGS: Hepaticogastrostomy.
Table 2.
Tumor characteristics
| Study | Etiology of obstruction in EUS group (n) | Etiology of obstruction in ERCP group (n) | Altered anatomy | Curative resection | Chemotherapy | Duodenal invasion |
|---|---|---|---|---|---|---|
| Bang | Pancreatic cancer: 34 | Pancreatic cancer: 33 | Excluded | EUS: 5 | EUS: 25 | Not reported |
| ERCP 5 | ERCP: 23 | |||||
| Park | Pancreatic cancer: 14 | Pancreatic cancer: 12 | Excluded | Excluded | Not reported | Not reported |
| Malignant lymphadenopathy: 2 | ||||||
| Paik | Pancreatic cancer: 40 | Pancreatic cancer: 38 | EUS group: | None | EUS: 37 | EUS: 28.1% |
| Cholangiocarcinoma: 8 | Cholangiocarcioma: 3 | Roux-en- y 1 | ERCP: 26 | ERCP: 24.6% | ||
| Gallbladder cancer: 4 | Gallbladder cancer: 4 | Billroth II 3 | ||||
| Ampullary cancer: 3 | Ampullary cancer: 5 | ERCP group: | ||||
| Gastric cancer: 2 | Gastric cancer: 4 | Roux-en-y 1 | ||||
| Duodenal cancer: 1 | Duodenal cancer: 2 | |||||
| Hepatocellular carcinoma: 1 | Others: 8 | |||||
| Others: 2 | ||||||
| Nakai | Pancreatic cancer: 28 | Pancreatic cancer: 21 | Not reported | Not reported | Not reported | EUS: 41% |
| Biliary tract cancer: 2 | Biliary tract cancer: 2 | ERCP: 44% | ||||
| Metastatic lymphadenopathy: 2 | ||||||
| Kawakubo | Pancreatic cancer: 25 | Pancreatic cancer: 43 | Not reported | EUS 1, ERCP 3 | EUS: 20 | EUS: 23% |
| Other: 1 | Other: 13 | ERCP: 36 | ERCP: 32% |
Quality
All the included RCTs carried a high risk of performance bias as they were the endoscopist performing the procedure were not blinded. There was a low risk of detection, attrition, reporting, or selection bias. Based on the Newcastle–Ottawa Scale, both the included observational studies were of high quality. Quality of the included studies is summarized in Table 1.
Meta-analysis
Technical success
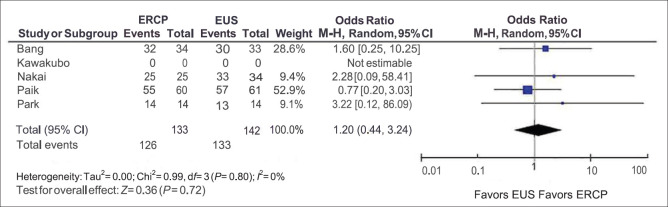
Technical success was included in four of the five studies. The observed technical success was 94.73% in the ERCP group and 93.67% in the EUS group [Figure 2]. There was no difference in the observed technical success with the pooled ORs being 1.20, and the 95% confidence interval (CI) was 0.44–3.24 with I2 = 0% and the P value for Cochran's Q was 0.72 ruling out heterogeneity. The funnel plot was asymmetric on visual inspection. However, objective tests for publication bias could not be carried out due to the low number of studies.
Figure 2.
Technical success
A subgroup analysis of RCTs also revealed no difference in the technical success between ERCP and EUS (OR = 1.12; 95% CI = 0.40–3.19; I2 = 0%, Cochran's Q test P = 0.66).
Clinical success
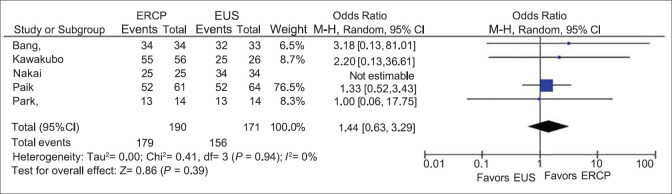
All five included studies reported the clinical success of EUS and ERCP. The overall clinical success rate was 94.21% in the ERCP group and 91.23% in the EUS group [Figure 3]. There was no statistically significant difference between the two groups, with the pooled OR being 1.44 and 95% CI being 0.63–3.29. Cochran's Q test and I2 test failed to reveal any heterogeneity. (I2 = 0%, Cochran's Q test P = 0.66).
Figure 3.
Clinical success
A subgroup analysis of RCTs also revealed no difference in the clinical success between ERCP and EUS. (OR = 1.38; 95% CI = 0.58–3.29, I2 = 0%, Cochran's Q test P = 0.66).
Adverse outcome
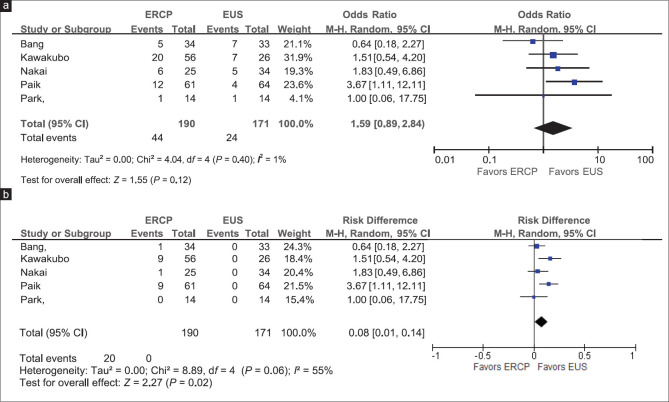
The rate of procedure-related adverse outcomes in the EUS group was 15.2%, whereas that in the ERCP group was 22.3% [Figure 4a]. There was no difference between the overall adverse outcomes of the two groups (OR: 1.59; 95% CI: 0.89–2.84) with I2 = 0% and the P value for Cochran's Q was 0.40, suggesting low heterogeneity). We further looked into procedure-related pancreatitis and nonpancreatitis-related adverse outcomes [Table 3].
Figure 4.
Adverse events. (a) Overall adverse events. (b) Procedure-related pancreatitis
Table 3.
Adverse events
| Study | Modality | Adverse events, n (%) | Cholecystitis (n) | Pancreatitis (n) | Liver abscess (n) | Fever (n) | Abdominal pain (n) | Peritonitis (n) | Cholangitis (n) | Pneumoperitoneum (n) | Stent migration (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bang | EUS-BD | 7 (21.2) | 1 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 0 |
| ERCP | 5 (14.7) | 0 | 1 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | |
| Park | EUS-BD | 0 (0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ERCP | 0 (0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Paik | EUS-BD | 4 (6.3) | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 |
| ERCP | 12 (19.7) | 2 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Nakai | EUS-BD | 5 (15 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| ERCP | 6 (24) | 4 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Kawakubo | EUS-BD | 7 (26.9) | 3 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 0 |
| ERCP | 20 (35.7) | 3 | 9 | 0 | 3 | 5 | 0 | 1 | 0 | 0 |
BD: Biliary drainage
The rate of pancreatitis in the ERCP group was 9.5%, whereas that in the EUS group was 0% [Figure 4b]. The risk difference between the two groups was 8% (95% CI: [1%–14%]), which was statistically significant. The OR for having nonpancreatitis-related adverse events of ERCP over EUS was 0.74 (95% CI: 0.39–1.39), which was not significant. No publication bias was detected in the funnel plots.
Reintervention rates and tumor overgrowth
The rate of reintervention was 22.6% in the ERCP group, whereas that in the EUS group was 15.2% [Figure 5]. While the rate of reintervention was lower in the EUS group, it did not reach statistical significance (OR = 1.68, 95% CI = 0.76–3.73). The ERCP group had higher odds of requiring reintervention due to tumor overgrowth (OR = 5.35, 95% CI: 1.64–17.50). The ERCP group also had higher odds of requiring reintervention due to stent blockage. However, it did not achieve statistical significance (OR: 2.11, 95% CI: 0.95, 4.67). The most common causes of reintervention were stent blockage either due to tumor overgrowth/ingrowth or by other causes such as food debris or sludge. The causes of reintervention are listed in Table 4.
Figure 5.
Reintervention rate. (a) Overall reinterventions. Reinterventions due to (b) stent blockage, (c) tumor overgrowth, (d) stent migration
Table 4.
Reinterventions
| Study | Modality | Reinterventions (n) | Nontumor-related mechanical obstruction (n) | Tumor overgrowth (n) | Stent migration (n) | Acute cholecystitis (n) | Acute cholangitis (n) | Bleeding (n) | Biloma (n) | Unknown (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bang | EUS-BD | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ERCP | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Park | EUS-BD | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ERCP | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Paik | EUS-BD | 10 | 6 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| ERCP | 26 | 14 | 9 | 1 | 1 | 0 | 0 | 1 | 0 | |
| Nakai | EUS-BD | 10 | 4 | 0 | 6 | 0 | 0 | 0 | 0 | 0 |
| ERCP | 9 | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Kawakubo | EUS-BD | 5 | 0 | 0 | 2 | 0 | 3 | 0 | 0 | 0 |
| ERCP | 7 | 4 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
BD: Biliary drainage.
Chemotherapy and curative resection
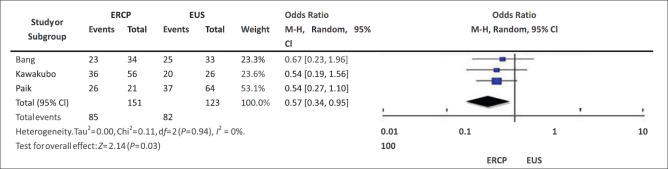
The administration of postprocedure chemotherapy was reported in three studies.[2,4,6] 66.67% of patients in the EUS group and 56.3% of patients in the ERCP group received systemic chemotherapy [Figure 6]. The pooled OR was 0.57 with 95% CI being 0.34–0.95, indicating that the higher proportion of patients receiving chemotherapy in the EUS group was statistically significant. Only two studies included patients who were eligible for undergoing surgical resection. In those studies, six patients in the EUS group and eight patients in the ERCP group received pancreatoduodenectomy. Only one study evaluated the quality of life score. The study finds that there was a lesser decline in quality of life at 12 weeks in the EUS cohort than the ERCP cohort.
Figure 6.
Chemotherapy administration
DISCUSSION
To our knowledge, this is the first meta-analysis to compare EUS without a prior attempted ERCP with ERCP for MBO. In this analysis, we found that EUS achieved comparable technical success and comparable success when avoiding the risk of procedure-related pancreatitis associated with ERCP. Further, it was found that the risk of reinterventions and the overall outcomes were comparable in both the groups.
In the current clinical practice, EUS-BD is only considered an alternative modality after a failed ERCP. It is well established that a difficult ERCP is associated with considerably higher complications. Studies have shown that pancreatitis rates are <3% if cannulation is achieved within 5 min and are >10% if it takes more than 10 min to achieve cannulation or with more than ten attempts.[19,20] There are many factors that could potentially predict difficulty in selective biliary cannulation (SBC). These include prior upper GI surgery or malignant infiltration of the duodenum and the papilla. Malignant distal biliary obstruction can itself preclude to difficult SBC. In this study, 24.6%–44% of patients in the ERCP group had duodenal involvement of the tumor. While none of the studies reported the number of patients who had difficult biliary cannulation, it is possible that a large proportion of the included patients were at high risk of having difficult biliary cannulation.
The pooled rate of procedure-related pancreatitis was 9.5% (ranged from 0% to 16%), which is in line with the previously published data.[21] Procedure-related pancreatitis has a mortality rate of 0.7% and is associated with significant morbidity. The estimated cost for ERCP-related pancreatitis was about 150 million US Dollars.[22] The risk of procedure-related pancreatitis can completely be avoided by EUS-BD. There was no difference between the two groups in nonpancreatitis complications. The most common causes of nonpancreatitis complications were abdominal pain and acute cholecystitis; the incidence of each was similar in both groups.
The pooled reintervention rates were 15.6% in the EUS group and 22.6% in the ERCP group which did not achieve statistical significance. However, the causes for requiring reintervention were different in both the groups. The ERCP group had statistically significant higher odds of requiring reintervention due to tumor overgrowth, whereas the EUS group had significantly higher odds of needing a reintervention due to stent migration. Other causes necessitating reintervention were acute cholangitis, cholecystitis, biloma, and bleeding. The incidence of these was very low for any meaningful comparison between the groups. All studies used only SEMS in the ERCP group. Stent coverage can influence reintervention rates. Covered metal stents have higher rates of stent migration and sludge formation, whereas uncovered metal stents have higher rates of tumor overgrowth/ingrowth.[23] The studies included in this analysis used a different combination of covered, uncovered, and partially covered SEMS in the ERCP group. It is unclear if choice of stents could have explained the difference in the reintervention rates of the EUS group and the ERCP group.
Only two studies included patients eligible for curative resection.[2,4] A total of six patients in the EUS group and eight patients in the ERCP group received pancreatoduodenectomy, and the authors reported no difference in outcomes between the two groups. A transduodenal stent is unlikely to impede surgery as the site of puncture is a part of the operative specimen.[2] However, the number of cases undergoing curative resection is very small to draw definitive conclusions. In a recently published case series, five patients underwent EUS-CDS using LAMS. Each of the five patients subsequently underwent pylorus-preserving pancreatoduodenectomy.[24] Large multicenter trials are needed to assess the suitability of EUS-BD as a bridge to surgery.
A higher proportion of patients received systemic chemotherapy in the EUS group and the ERCP group. Further, none of the five studies reported a difference in the overall survival. Change in the quality of life scores was reported in only one study,[6] and it was found that patients in the EUS group had lesser deterioration in the quality of life at 12 weeks in the EUS cohort than in the ERCP cohort. More trials would be necessary to definitively conclude the superiority of EUS over ERCP in terms of changes in quality of life.
Despite the findings in this study, it is worth stating that ERCP remains the standard of care for achieving biliary access and decompression in patients with MBO. Furthermore, it should be noted that EUS-BD is a procedure that requires considerable expertise in both EUS and ERCP techniques to be performed safely and successfully. There is no standardized approach in terms of the technical aspects. There are limited devices dedicated to performing EUS-BD. Finally, unlike ERCP, where repeat cannulation attempts or alternate cannulation methods can be performed when the initial attempt fails, repeat attempts at EUS-BD in a single setting may result in a higher risk of severe complications such as bile leak, infection, and bleeding. Thus, further advances in endoscopic equipment and knowledge regarding technique are needed before EUS-BD can become a widely accepted primary intervention.
The strengths of this study included well-defined inclusion criteria, detailed assessment of adverse outcomes subtypes, and causes of reintervention. Further, there was very low heterogeneity in all assessed outcomes. The study had some important limitations as well. Publication bias could not be assessed by an objective test like the Egger's test as it has low power when the number of included studies is fewer than 10. Each of the three included RCTs are affected by performance bias as the endoscopist could not be blinded. Further, all studies were carried out by expert endoscopist in high-volume EUS centers. Further, four of the five studies were carried out in Japan or South Korea. The technology used in one of the studies, the one step stent introducer, is not available in the US.[6] Hence, caution should be exercised in extrapolating the findings of the study to other setups.
CONCLUSIONS
EUS-BD and ERCP have similar technical and clinical success. The risk of procedure-related pancreatitis is absent in EUS-BD. EUS-BD can potentially be used as the first-line palliative modality for MBO in selected cases where the expertise is available. It would mainly be useful in cases with anticipated difficult biliary cannulation. Additional trials are needed to establish the role of EUS-BD as a bridge to surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
N. A. Kumta: Consulting for Olympus. Consulting and teaching for Boston Scientific and Apollo Endosurgery.
C. J. DiMaio: Consulting and teaching for Boston Scientific and Medtronic.
REFERENCES
- 1.Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904–14. doi: 10.1016/j.gie.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 2.Bang JY, Navaneethan U, Hasan M, et al. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: A randomized trial (with videos) Gastrointest Endosc. 2018;88:9–17. doi: 10.1016/j.gie.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Nakai Y, Isayama H, Kawakami H, et al. Prospective multicenter study of primary EUS-guided choledochoduodenostomy using a covered metal stent. Endosc Ultrasound. 2019;8:111–7. doi: 10.4103/eus.eus_17_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawakubo K, Kawakami H, Kuwatani M, et al. Endoscopic ultrasound-guided choledochoduodenostomy vs. transpapillary stenting for distal biliary obstruction. Endoscopy. 2016;48:164–9. doi: 10.1055/s-0034-1393179. [DOI] [PubMed] [Google Scholar]
- 5.Park JK, Woo YS, Noh DH, et al. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: Prospective randomized controlled study. Gastrointest Endosc. 2018;88:277–82. doi: 10.1016/j.gie.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Paik WH, Lee TH, Park DH, et al. EUS-guided biliary drainage versus ERCP for the primary palliation of malignant biliary obstruction: A multicenter randomized clinical trial. Am J Gastroenterol. 2018;113:987–97. doi: 10.1038/s41395-018-0122-8. [DOI] [PubMed] [Google Scholar]
- 7.Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 8.Khan MA, Akbar A, Baron TH, et al. Endoscopic ultrasound-guided biliary drainage: A systematic review and meta-analysis. Dig Dis Sci. 2016;61:684–703. doi: 10.1007/s10620-015-3933-0. [DOI] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting.Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwashita T, Lee JG, Shinoura S, et al. Endoscopic ultrasound-guided rendezvous for biliary access after failed cannulation. Endoscopy. 2012;44:60–5. doi: 10.1055/s-0030-1256871. [DOI] [PubMed] [Google Scholar]
- 12.Dhir V, Itoi T, Khashab MA, et al. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest Endosc. 2015;81:913–23. doi: 10.1016/j.gie.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 13.Ottawa Hospital Research Institute. [Last accessed on 2019 Mar 24]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 14.Cochrane Handbook for Systematic Reviews of Interventions. [Last accessed on 2019 Mar 24]. Available from: http://handbook-5-1.cochrane.org/
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–75. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 17.Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7:5. doi: 10.1186/1471-2288-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.What is Heterogeneity? [Last accessed on 2019 Mar 27]. Available from: https://handbook-5-1.cochrane.org/chapter_9/9_5_1_what_is_heterogeneity.htm .
- 19.Bailey AA, Bourke MJ, Williams SJ, et al. A prospective randomized trial of cannulation technique in ERCP: Effects on technical success and post-ERCP pancreatitis. Endoscopy. 2008;40:296–301. doi: 10.1055/s-2007-995566. [DOI] [PubMed] [Google Scholar]
- 20.Halttunen J, Meisner S, Aabakken L, et al. Difficult cannulation as defined by a prospective study of the Scandinavian Association for Digestive Endoscopy (SADE) in 907 ERCPs. Scand J Gastroenterol. 2014;49:752–8. doi: 10.3109/00365521.2014.894120. [DOI] [PubMed] [Google Scholar]
- 21.Kochar B, Akshintala VS, Afghani E, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: A systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81:143–9. doi: 10.1016/j.gie.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 22.Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. NEngl J Med. 2012;366:1414–22. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tringali A, Hassan C, Rota M, et al. Covered vs. uncovered self-expandable metal stents for malignant distal biliary strictures: A systematic review and meta-analysis. Endoscopy. 2018;50:631–41. doi: 10.1055/s-0043-125062. [DOI] [PubMed] [Google Scholar]
- 24.Fabbri C, Fugazza A, Binda C, et al. Beyond palliation: Using EUS-guided choledochoduodenostomy with a lumen-apposing metal stent as a bridge to surgery. A case series. JGastrointestin Liver Dis. 2019;28:125–8. doi: 10.15403/jgld.2014.1121.281.eus. [DOI] [PubMed] [Google Scholar]