Abstract
Background and Objective:
The widespread use of colonoscopy has led to an increasing number of subepithelial lesions (SELs) being detected in the lower gastrointestinal (GI) tract. This study aimed to analyze the utility of EUS and its role in the management of lower GI SELs.
Patients and Methods:
Records of all patients who were referred for lower EUS evaluation of a SEL at a tertiary center between 2007 and 2018 were retrospectively reviewed after IRB approval. Data collection included patient/lesion characteristics, technical details of procedure, and pathology results.
Results:
A total of 47 patients underwent EUS examinations for the evaluation of 49 suspected SEL in the lower GI tract (2 patients had 2 SELs each). Out of the 49 suspected lesions, the most frequent location was in the rectum (30/49, 61.2%). EUS showed extraluminal compression in 2 cases (2/49, 4.1%) and intraluminal lesions were identified in 40 cases (40/49, 81.6%). In 7 patients (7/49, 14.3%), no lesion could be identified by EUS. Twenty (20/49, 40.8%) SELs were malignant or had malignant potential. Twenty-six EUS-guided fine-needle aspirations (FNAs) and 14 EUS-core biopsies were performed. EUS-FNA alone was able to correctly diagnose 15/26 (57.7%) of the lower SELs. When EUS-guided fine needle biopsies (FNB) were performed during the same procedure, the final diagnosis was confirmed in 21/26 (80.8%) cases. There was only one procedure-related complication caused by use of narcotics.
Conclusion:
EUS-guided FNA/FNB are feasible and safe techniques for assessing lower GI SELs and provide valuable information regarding lesion characteristics and their malignant potential with high diagnostic accuracy.
Keywords: EUS, EUS-FNA, lower gastrointestinal tract, lower gastrointestinal, pathology, subepithelial lesions
INTRODUCTION
Subepithelial lesions (SELs) of the gastrointestinal (GI) tract can develop within any layer of the GI wall, from deep mucosa to serosa.[1] Typically, SELs appear as protruding lesions with a normal overlying mucosa and are found incidentally during endoscopic examinations.[2] SELs are not always intramural lesions, as they can also be caused by compression of extrinsic structures. The term SEL is preferred to the term “submucosal” tumor, which should be reserved for those lesions that arise from the submucosal layer.
The widespread use of colonoscopy has led to an increasing number of SELs being detected in the lower GI tract.[3] Most of these lesions are small (<2 cm in diameter) and found incidentally; however, SELs can present with bleeding, obstruction, or metastases, depending on tumor size, location, and histopathology.[2] The type of treatment and prognosis vary depending on the type of lesion, therefore making an accurate differential diagnosis is very important.
Due to the subepithelial location, endoscopic biopsies often fail to provide diagnostic tissue. Thus, further imaging and sampling techniques are used to characterize these lesions. Due to its high resolution, EUS is a useful imaging method for the evaluation of SELs.[4] Using EUS, the differential diagnosis is possible by documenting the originating GI wall layers and the associated echostructure, and cytological/histological confirmation can be obtained through EUS-guided tissue acquisition.[5]
Several studies have been published, mainly on SELs in the upper digestive tract.[6,7,8] Reports on the use of EUS in SELs of the lower GI tract are currently limited to mostly case reports and small case series. The aim of this study was to analyze the utility of EUS and EUS-guided tissue acquisition in the diagnosis and management of lower GI SELs.
PATIENTS AND METHODS
This is a single-center, retrospective, and descriptive study approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. Consecutive patients (>18 years old) who were referred for lower EUS evaluation of a SEL between 2007 and 2018 were identified from our clinical database. Data were retrospectively extracted from electronic medical records and included demographics, the location and size of the lesion by endoscopy, the layer of origin, echogenicity and size by EUS, the pathology result if EUS-guided tissue acquisition was performed and final diagnosis (based on follow-up visits or any further management such as endoscopic or surgical excision).
All EUS procedures were performed by four experienced endosonographers. For rectosigmoid lesions, EUS was performed using radial (GF-UE160-AL5) or linear echoendoscopes (GF-UCT180). For lesions located in the proximal colon, imaging was performed using 12MHz or 20MHz miniprobes that were passed through the biopsy channel of the colonoscope and examination was done with water immersion. For patients with rectosigmoid lesions undergoing flexible sigmoidoscopy with EUS, the approach at our institution was to prepare the patients with polyethylene glycol-electrolyte solution as for a standard colonoscopy, instead of just enemas.
Procedures were performed with patients under deep sedation using propofol according to standard anesthesia care guidelines. EUS-guided FNA and/or fine needle biopsies (FNB) were used for tissue acquisition. All patients undergoing EUS-FNA/FNB received periprocedural antibiotics.
Statistical analysis
Descriptive statistical analysis was performed. The distribution of continuous variables was summarized by means and standard deviations. The distribution of categorical variables was summarized using frequencies and percentages. The Fisher's exact test or the Chi-square was conducted to evaluate associations between categorical variables and a P < 0.05 was considered statistically significant. The statistical analysis was carried out using SPSS Statistics software (version 24.0; IBM Corporation, Armonk, NY, USA).
RESULTS
A total of 47 patients (30 women, 17 men), with a median age of 59 years (range 33–79 years) underwent EUS evaluation of 49 suspected SELs in the lower GI tract. Most patients were referred for EUS because of abnormal findings on other imaging methods (34/47, 72.3%) or as follow-up examination for a previous/suspicious malignancy (13/47, 27.7%).
There were 26 EUS assessments done with a linear echoendoscope and 22 EUS assessments done with a radial echoendoscope. Five of these EUS assessments required the use of both linear and radial echoendoscopes within the same procedure. The remaining 4 EUS assessments were performed using a through-the-scope mini-probe.
Standard colonoscopy was performed in 26 (55.3%) cases, while 21 (44.7%) patients underwent flexible sigmoidoscopy.
Main characteristics of the subepithelial lesions
The main characteristics of the SELs detected on EUS in the lower GI tract are summarized in Table 1. The lesions were located most frequently in the rectum (30/49, 61.2%).
Table 1.
Characteristics of the subepithelial lesions detected by lower EUS
| n (%) | |
|---|---|
| SEL characteristics (n=49) | |
| Intramural lesion | 40 (81.6) |
| Extrinsic compression | 2 (4.1) |
| No lesion detected | 7 (14.3) |
| Location (n=49) | |
| Anal canal/anorectal junction | 4 (8.2) |
| Rectum | 30 (61.2) |
| Sigmoid colon | 6 (12.2) |
| Transverse colon | 4 (8.2) |
| Cecum | 2 (4.1) |
| Appendiceal orifice | 3 (6.1) |
| Size (n=49) | |
| <1 cm | 11 (22.4) |
| 1–2 cm | 16 (32.7) |
| 2 cm | 22 (44.9) |
| Final diagnosis *(n=49) | |
| No lesion | 7 (14.3) |
| Rectal Adenocarcinoma | 2 (4.1) |
| Squamous cell carcinoma | 3 (6.1) |
| Other malignant tumors | 3 (6.1) |
| GIST | 4 (8.2) |
| Lymphoma | 2 (4.1) |
| Lipoma | 3 (6.1) |
| Leiomyoma | 5 (10.2) |
| Benign cyst | 4 (8.2) |
| Endometriosis | 2 (4.1) |
| Neuroendocrine tumor | 6 (12.3) |
| Rectal varices | 1 (2.0) |
| Reactive colonic changes – ulcers, abscesses, inflammation | 3 (6.1) |
| Ovarian serous cystadenofibroma | 1 (2.0) |
| Acute appendicitis | 3 (6.1) |
*Final diagnosis was established by the histopathologic examination of the surgically resected specimen, based on the results of other diagnostic investigations, or clinical follow-up. GIST: Gastrointestinal stromal tumors; SEL: Subepithelial lesions.
Forty-nine suspected SELs were referred for EUS evaluation. Two patients were found to each have 2 SELs: One patient had a lipoma located in the distal transverse colon and a cystic lymphangioma in the proximal transverse colon, while the other patient had two sigmoid SELs diagnosed as endometriosis.
EUS showed extraluminal compression in 2 cases (2/49, 4.1%) and intraluminal lesions were identified in 40 cases (40/49, 81.6%). In 7 patients (7/49, 14.3%), the normal GI wall was displayed in 5 layers and no intramural lesion or extrinsic compression could be identified by EUS. In 4 of these patients, lesions were not even visualized grossly by endoscopy (these patients likely had a looped colonoscope pushing on a proximal portion of the colon at the time of index colonoscopy); whereas in the remaining three patients, the final diagnosis was low grade neuroendocrine tumor. These lesions were identified endoscopically, but the size was too small (3-4 mm) and an abnormality could not be detected on EUS.
Subepithelial lesions with malignant potential
Twenty (20/49, 40.8%) SELs included in the analysis were malignant or had malignant potential.
Rectal carcinoid tumors
Six SELs (6/49, 12.2%) were diagnosed with rectal carcinoid tumor. Three of these tumors were identified only endoscopically and were treated radically by endoscopic resection. Three carcinoid tumors were detected on EUS, and seen as hypoechoic lesions, well-defined, involving the deep mucosa and/or submucosa. One of these patients, with a history of high-grade neuroendocrine tumor of the anorectal junction which was resected, had computed tomography (CT) imaging evidence of rectal thickening and underwent EUS for further evaluation. This revealed hypoechoic thickening of the rectal wall with extension through the muscularis propria and possible infiltration into surrounding mesorectum, highly suspicious for recurrence. The patient underwent chemoradiation and follow-up investigations revealed no recurrence.
Endoscopic biopsies were diagnostic in all the six cases of rectal carcinoids tumors included in our analysis; no EUS-guided FNA or FNB was performed in these cases.
Recurrent gastrointestinal stromal tumors
Four (4/49, 8.2%) of the SELs included in our analysis were diagnosed with recurrent GIST. On EUS, the lesions were hypoechoic and in continuity with muscularis propria. In two patients, the lesions were not detected on sigmoidoscopy which revealed only a scar at a previous polypectomy site. EUS identified in both cases a hypoechoic lesion at the site of the scar. One lesion arised only from the musculais propria, while another lesion involving the muscularis propria was seen to extend further into the perirectal fat. Endoscopic biopsies were nondiagnostic in all cases, showing only reactive changes in the colonic mucosa. EUS-guided FNA and/or FNB with immunohistochemical (IHC) studies confirmed the diagnosis of recurrent GIST. However, EUS-FNA was nondiagnostic in one case. A 51-year-old patient with a history of rectosigmoid GIST who underwent endoscopic mucosal resection, was referred for flexible sigmoidoscopy with EUS to rule out residual disease. The initial EUS-FNA of the previous polypectomy site was nondiagnostic. Three months later, the patient underwent a repeat EUS-guided FNA and FNB. Immunostains, performed on cell block sections with appropriate controls, showed the spindle cells to be positive for CD117, DOG1 and negative for SMA, confirming the diagnosis of GIST [Figure 1].
Figure 1.
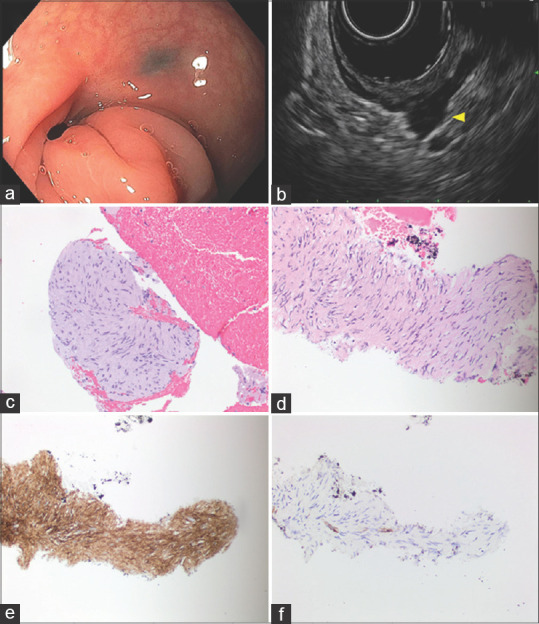
Gastrointestinal stromal tumor. (a) Endoscopic findings: Flat scar at 13 cm with adjacent tattoos in tortuous rectosigmoid junction; (b) EUS: Hypoechoic mass was found in the perirectal space extending away from lumen but arising from the thickened MP layer; (c) H and E stained biopsy; (d) H and E stained cell block section of the FNA; (e) immunoperoxidase stain for DOG1 is positive in the tumor cells; (f) immunoperoxidase stain for SMA is negative in the tumor cells
Recurrent rectal adenocarcinoma
Recurrence of rectal adenocarcinoma was found in two patients (2/49, 4.1%). EUS images showed hypoechoic, heterogeneous lesions, with irregular margins, involving deep mucosa and submucosa, and even invading through the muscularis propria into the perirectal fat in one case [Figure 2]. Endoscopic biopsies revealed adenocarcinoma in one case, while for the other, the endoscopic biopsies were not diagnostic, thus EUS-guided FNA using a 25-gauge needle was performed and revealed adenocarcinoma in a background of necrosis.
Figure 2.
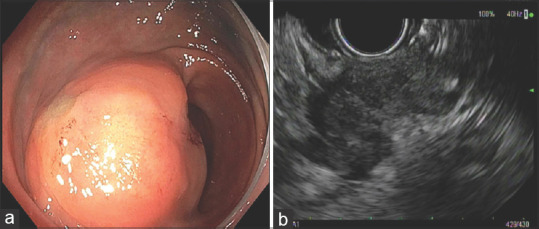
Recurrent rectal adenocarcinoma. (a) Endoscopic findings: Moderate sized, firm, subepithelial mass with smooth mucosa in the rectosigmoid region; (b) EUS findings: Hypoechoic mass within the sigmoid/rectal wall, with intact mucosa. The mass was involving the muscularis propria, submucosa. The mass had an infiltrating appearance and appeared to be invading into perirectal fat
Anal squamous cell carcinoma
Three SELs (6.1%) were diagnosed as anal squamous cell carcinoma (SCC). Two patients with a history of anal SCC had received chemoradiation and were referred for a flexible sigmoidoscopy with EUS to rule out recurrent disease. The sigmoidoscopy identified in both cases an anal SEL. EUS further revealed hypoechoic lesions with the loss of all GI wall layers in one case, while the lesion was located in the submucosa and muscularis propria for the other case. EUS-guided FNAs of the anal lesions showed only reactive changes in one case, but the endoscopic biopsies confirmed the diagnosis.
Another patient, without a prior diagnosis of malignancy, underwent a screening colonoscopy showing an anal SEL. EUS was further performed and an irregular, hypoechoic lesion confined to the muscularis propria was identified. EUS-guided FNA with a 25G needle revealed SCC.
Adenocarcinoma arising from endometriosis
A 46-year-old patient underwent lower EUS for the evaluation of a pelvic mass detected on CT. A large, perirectosigmoid mass, contiguous with muscularis propria was identified; the mass was hypoechoic with large anechoic areas [Figure 3]. Transrectal EUS-FNA with a 25G needle was performed and cytological examination was interpreted as suspicious, revealing tumor areas with papillary architecture. Immunohistochemical studies were required to further characterize this tumor. FNB was performed with a 19G EUS core biopsy needle and it revealed adenocarcinoma, likely of Müllerian origin. The patient underwent tumor reductive surgery and the final diagnosis was adenocarcinoma arising from endometriosis.
Figure 3.
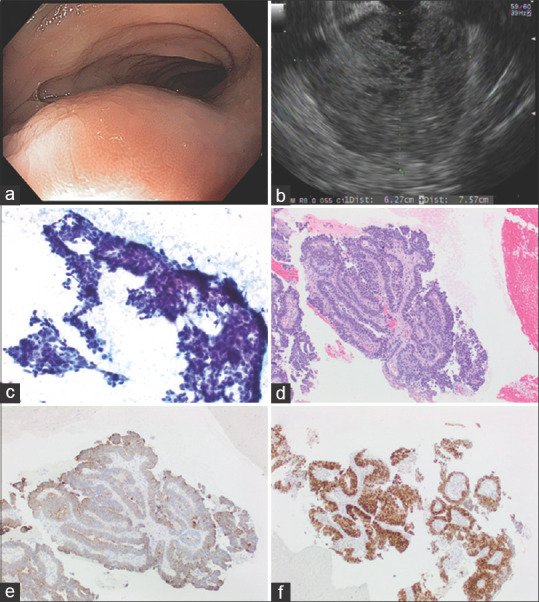
Adenocarcinoma arising from endometriosis (a) Endoscopic findings: Smooth subepithelial compression without any intraluminal disease process. (b) EUS Findings: A large, 7.57 cm by 6.27 cm perirectosigmoid mass was seen that appeared to be contiguous with muscularis propria of the rectum in some views. The mass was hypoechoic but with cystic (anechoic) areas; (c) EUS-FNA sample: Papanicolaou stained direct smear; (d) H and E stained biopsy; (e) immunoperoxidase stain for cytokeratin 7 is positive in the tumor cells; (f) immunoperoxidase stain for estrogen receptor is positive in the tumor cells
Rectal metastasis
A 63-year-old patient with a history of poorly-differentiated signet ring gastric cancer with linitis plastica, presented for changes in bowel movements and underwent colonoscopy which revealed indurated, edematous and nodular rectal mucosa, with endoscopic biopsy negative for malignancy. The patient underwent EUS which showed circumferential thickening of the rectal wall. EUS-guided FNA and core biopsies were performed, but without evidence of malignancy. Surgical tru-cut biopsies were performed under anesthesia and the final diagnosis was signet-ring cell carcinoma with linitis of the rectum consistent with gastric metastases.
Solitary fibrous tumor
A solitary fibrous tumor was found in one patient. The EUS showed a heterogeneous mass, with irregular margins, hypoechoic with hyperechoic areas, involving muscularis propria EUS-guided FNA and FNB confirmed the diagnosis [Figure 4].
Figure 4.
Solitary fibrous tumor (a). Endoscopic findings: A large sub-epithelial mass found in the distal rectum measuring 5 cm in length (b) EUS: A hypoechoic, hyperechoic, and heterogeneous mass were found in the right-lateral perirectal space. The endosonographic borders were well-defined. The mass measured 50 mm (in maximum width) by 41 mm (in maximum thickness). The mass appeared to be contiguous with the muscularis propria suggesting that it is arising from the MP or invading it. (c) Papanicolaou stained FNA direct smear; (d) H and E stained biopsy; (e) immunoperoxidase stain for STAT6 is positive in the tumor cells; (f) immunoperoxidase stain for CD34 is positive in the tumor cells
Lymphoma
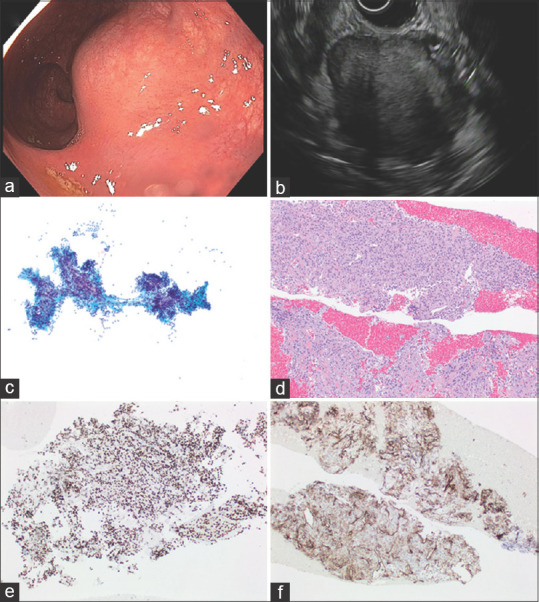
Two SELs (2/49, 4.1%) were diagnosed as lymphoma. EUS showed hypoechoic homogenous lesions, both larger than 30 mm. One lesion involved all rectal wall layers and endoscopic biopsies revealed mantle cell lymphoma, blastoid variant. The other lesion was located in the perirectal region [Figure 5], with nondiagnostic endoscopic biopsies. For this case, both EUS-FNA (19G needle) and EUS-FNB (22G needle) were performed. Furthermore, EUS-guided through the needle forceps biopsy was done with a Moray forceps passed through the 19G needle.
Figure 5.
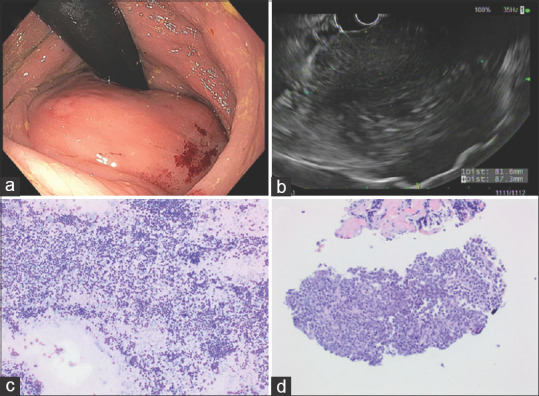
Lymphoma (a) endoscopic findings: A subepithelial (with smooth mucosal surface) partially obstructing large mass was found in the rectum. (b) EUS: A hypoechoic and heterogeneous mass was found in the perirectal space. The mass was visualized endosonographically with the probe positioned at 0.5 cm (from the anal verge). The endosonographic borders were irregular; (c) Romanowsky stained FNA direct smear; (d) H and E stained cell block section of the FNA. Flow cytometric analysis demonstrated aberrant monoclonal B-cell population consistent with lymphoma
Cytological specimens showed mixed small and large atypical lymphocytes, and flow cytometry analysis revealed two aberrant B-cell populations, supporting the diagnosis of B-cell lymphoma. Histologic sections showed multiple small fragments of tissue extensively replaced by lymphoma associated with marked sclerosis. The presence of extensive sclerosis and crush artifact limited optimal evaluation of cytological features. Immunohistochemical studies showed the lymphoma cells were positive for CD20, BCL6, and BCL2. The CD21 stain highlighted the presence of disrupted follicular dendritic meshworks. The neoplasm had a Ki-67 proliferation rate of ~10%. The overall morphologic features and immunohistochemical findings were diagnostic of low grade follicular lymphoma with sclerosis.
Benign subepithelial lesions
Cystic lesions
Cystic SELs were found in four patients (4/49, 8.2%): One duplication cyst and three cystic lymphangiomas. One lesion was located in the appendiceal orifice, one in the cecum and two in the transverse colon. EUS characteristics were enough for establishing the diagnosis. The cystic lymphangioma were seen on EUS as anechoic lesions with septal structures in the submucosal layer. The duplication cyst appeared as a round anechoic compressible structure located within the submucosa, without any septations or mural nodes.
Lipoma
EUS detected lipoma in three SELs (3/49, 6.1%). The lesions were located in the submucosa and appeared homogeneous, hyperechoic with distinct borders. The EUS features were characteristic of lipoma and thus further investigation with EUS-FNA was not required.
Leiomyoma
Five SELs (5/49, 10.2%) were diagnosed as leiomyoma. The lesions were visualized on EUS as homogenous, hypoechoic masses with regular borders located in the muscularis propria. EUS-guided FNA and/or FNB with IHC studies confirmed the diagnosis in four cases. One patient had a small SEL in the transverse colon that was evaluated with a 20 mHz EUS miniprobe and the findings were most consistent with a small leiomyoma. The lesion was followed with annual colonoscopy and EUS, showing stable lesion over time.
Endometriosis
Endometriosis was found in one patient. EUS revealed two SELs (2/49, 4.1%) hypoechoic lesions arising from the muscularis propria of the transverse colon, and EUS-guided FNA and FNB showed endometriosis [Figure 6].
Figure 6.
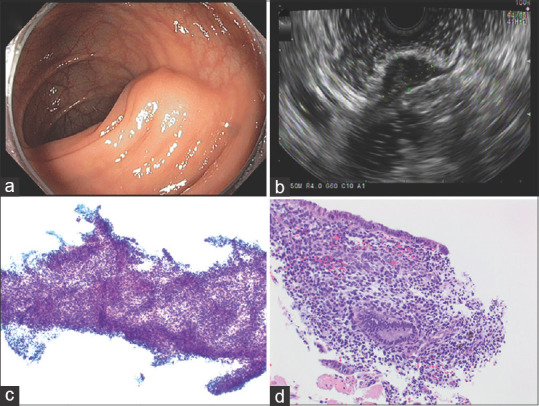
Endometriosis. (a) Endoscopic findings: 1.5–2 cm subepithelial mass in the sigmoid; (b) EUS: 1.4 cm hypoechoic lesion arising from the muscularis propria (c) Papanicolaou stained FNA direct smear; (d) H and E stained biopsy
Rectal varices
Rectal varices were found in one patient. EUS showed multiple anechoic, tubular structures in the submucosa.
EUS-guided tissue acquisition
Twenty-six EUS-guided FNA were performed. Two lesions were sampled twice during the same procedure due to insufficient cellularity of the first FNA-specimen. Three EUS-FNA samples (3/26, 11.5%) were nondiagnostic. Five samples (5/26, 19.2%) were considered suspicious and EUS-FNB was further performed during the same procedure and the final diagnosis was confirmed in all those cases.
EUS-guided FNA alone was able to correctly diagnose 15/26 (57.7%) of the lower SELs. When EUS-FNB was performed during the same procedure, the final diagnosis was confirmed in 21/26 (80.8%) cases.
Fourteen EUS-guided FNB of the lower GI tract SELs were performed, and the final diagnosis was positively confirmed in 13 cases (92.9%).
Adverse events related to the EUS-guided tissue acquisition procedure were reported in only one case. After the procedure, the patient had anal pain requiring significant amount of analgesia. After the administration of narcotics, he developed hypoxemia and altered mental status. He was transferred to the ICU for further evaluation and correction of his hypercarbic respiratory failure secondary to opiate toxicity, which was reversed with naloxone administration and noninvasive positive pressure ventilation. The patient was discharged from ICU the following day.
DISCUSSION
The rapidly increase in the use of colonoscopy performed for GI symptoms or cancer screening has led to an increasing number of SELs being detected in the lower GI tract. When a SEL is found during colonoscopy and it is determined that further examination is required, EUS might be regarded as an ideal method. Reports on the use of EUS in colorectal SELs are currently limited. To the best of our knowledge, this is the largest cumulative cohort study regarding the clinical impact of EUS and EUS-tissue acquisition in the evaluation of SELs in the lower GI tract. EUS proved to be a safe and accurate method as it provided valuable information regarding the location of the lesions (intramural or extramural), echogenic characteristics, and their malignant potential.
When a subepithelial compression is seen on a colonoscopy, it is crucial to exactly establish the etiology of the abnormality, because each type of SEL has a different treatment, follow-up and prognosis. SELs that may be found during colonoscopy includes a broad spectrum of intramural lesions and extrinsic compressions. Hwang et al.[9] showed that endoscopic observation can distinguish between intramural lesions and extramural compression with 89% accuracy. However, extramural lesions were mistaken for intramural lesions in many cases. By investigating the relationship between the GI wall and the location of the tumor using EUS, it is possible to more accurately differentiate between extramural compressions and intramural tumors.[3] If the subepithelial compression is due to a normal adjacent structure or an organ as an anatomic variant, no further work-up is needed. In other cases, the work-up of the extramural lesions is based on the clinical indications. In our study, EUS showed extraluminal compression in two cases. Findings included a perirectal cystic lesion without any typical appearance; given the stability in size during follow-up, the lesion was thought to be benign. Differential considerations included endometrioma, hindgut cyst or ovarian remnant syndrome, given the patient's history of the right oophorectomy. The other patient was found to have angiomyxoma and underwent preoperative chemoradiation followed by surgical resection.
Based on EUS observations, it is easy to diagnose intramural cystic lesions or lipomas without any further tests including biopsies. Follow-up is required only in selected patients, without any invasive or aggressive intervention.
On the other hand, when a hypoechoic SEL is seen in the submucosa or muscularis propria, the differential diagnosis may include GIST, leiomyoma, leiomyosarcoma, or schwannoma. The diagnostic accuracy for third and fourth layer tumors by EUS alone is only around 50%.[9] Therefore, EUS-FNA and EUS-FNB play an important role in distinguishing between these lesions. The average diagnostic accuracy rate of EUS-FNA ranges from 60% to 80% in SELs.[10] The diagnostic accuracy rate of EUS-FNA alone for lower GI tract SELs in our study was in line with these results (58%). Due to the small number of cells obtained by aspiration, the samples are not always adequate for IHC analysis. IHC markers are essential for the differential diagnosis of the GI mesenchymal tumors. For example, the most sensitive and specific immunohistochemistry marker for GIST is CD117/c-kit while other standard markers are platelet-derived growth factor receptor-alpha, CD34 and Discovered On GIST-1 (DOG-1).[11,12] Leiomyomas demonstrate positive staining with α-smooth muscle actin and desmin protein, while S100 protein is positive in schwannomas.[13] Accordingly, EUS-FNB may overcome the limitations of EUS-FNA in procuring sufficient core tissue specimens.[14,15] Needles specifically designed to acquire core biopsy specimens are available in different gauges (19G, 22G, and 25G). Kim et al.[15] reported that the yield of FNB by using a 22G core biopsy needle (75%) was significantly greater than the yield of a 22G FNA needle (20%) for the evaluation of SELs. In this study, EUS-FNB was 92% accurate in predicting the diagnosis compared to FNA, which achieved a correct diagnosis only in 58% of cases. Moreover, both EUS-FNA and FNB were performed in eight cases and core biopsies were able to correctly diagnose six samples which were initially nondiagnostic or interpreted as suspicious using the material obtained by EUS-FNA.
EUS-guided tissue acquisition was performed in 45% of the patients included in our study and only one procedure-related adverse event was reported. Levy et al.[16] conducted a prospective study including 563 patients who underwent lower GI EUS with FNA and showed that the procedure was associated with a high complication rate, and in particular serious Grades 3–4 adverse events. However, according to our results, EUS-guided tissue acquisition of the lesions located in the lower GI tract was a safe procedure even if more than 55% of the patients underwent additional procedures such as endoscopic biopsies or polypectomies at the time of the EUS-FNA. All the patients who underwent EUS-FNA/FNB at our institution received periprocedural antibiotics.
Limitations of our study should be acknowledged. It was a conducted retrospectively in a single tertiary cancer center and the results may not reflect the outcomes of other institutions. However, to the best of our knowledge, this is the largest cumulative cohort study regarding the clinical impact of EUS in the evaluation of SELs in the lower GI tract.
CONCLUSION
EUS is a safe and accurate method as it provides valuable information regarding the location (intramural or extramural), echogenic characteristics and malignant potential of the SELs located in the lower GI tract. EUS-guided FNA or core biopsies are feasible and safe and can be used to obtain tissue from SELs located deeper to the mucosa, with a high diagnostic accuracy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Polkowski M, Butruk E. Submucosal lesions. Gastrointest Endosc Clin N Am. 2005;15:33–54, viii. doi: 10.1016/j.giec.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Standards of Practice Committee. Faulx AL, Kothari S, et al. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc. 2017;85:1117–32. doi: 10.1016/j.gie.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Kim TO. Colorectal subepithelial lesions. Clin Endoscopy. 2015;48:302. doi: 10.5946/ce.2015.48.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cârţână ET, Gheonea DI, Săftoiu A. Advances in endoscopic ultrasound imaging of colorectal diseases. World J Gastroenterol. 2016;22:1756–66. doi: 10.3748/wjg.v22.i5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landi B, Palazzo L. The role of endosonography in submucosal tumours. Best Practice Res Clin Gastroenterol. 2009;23:679–701. doi: 10.1016/j.bpg.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Baysal B, Masri OA, Eloubeidi MA, et al. The role of EUS and EUS-guided FNA in the management of subepithelial lesions of the esophagus: A large, single-center experience. Endosc Ultrasound. 2017;6:308–16. doi: 10.4103/2303-9027.155772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakamoto H, Kitano M, Kudo M. Diagnosis of subepithelial tumors in the upper gastrointestinal tract by endoscopic ultrasonography. World J Radiol. 2010;2:289–97. doi: 10.4329/wjr.v2.i8.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen EF, Arnott ID, Plevris J, et al. Endoscopic ultrasonography in the diagnosis and management of suspected upper gastrointestinal submucosal tumours. Br J Surg. 2002;89:231–5. doi: 10.1046/j.0007-1323.2001.02002.x. [DOI] [PubMed] [Google Scholar]
- 9.Hwang JH, Saunders MD, Rulyak SJ, et al. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202–8. doi: 10.1016/s0016-5107(05)01567-1. [DOI] [PubMed] [Google Scholar]
- 10.Moon JS. Endoscopic ultrasound-guided fine needle aspiration in submucosal lesion. Clin Endoscopy. 2012;45:117. doi: 10.5946/ce.2012.45.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kida M, Kawaguchi Y, Miyata E, et al. Endoscopic ultrasonography diagnosis of subepithelial lesions. Dig Endosc. 2017;29:431–43. doi: 10.1111/den.12854. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT) Mod Pathol. 2000;13:1134–42. doi: 10.1038/modpathol.3880210. [DOI] [PubMed] [Google Scholar]
- 13.Turner MS, Goldsmith JD. Best practices in diagnostic immunohistochemistry: Spindle cell neoplasms of the gastrointestinal tract. Arch Pathol Lab Med. 2009;133:1370–4. doi: 10.5858/133.9.1370. [DOI] [PubMed] [Google Scholar]
- 14.Wahnschaffe U, Ullrich R, Mayerle J, et al. EUS-guided Trucut needle biopsies as first-line diagnostic method for patients with intestinal or extraintestinal mass lesions. Surg Endosc. 2009;23:2351–5. doi: 10.1007/s00464-009-0345-2. [DOI] [PubMed] [Google Scholar]
- 15.Kim GH, Cho YK, Kim EY, et al. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonography-guided subepithelial tumor sampling. Scand J Gastroenterol. 2014;49:347–54. doi: 10.3109/00365521.2013.867361. [DOI] [PubMed] [Google Scholar]
- 16.Levy MJ, Abu Dayyeh BK, Fujii LL, et al. Prospective evaluation of adverse events following lower gastrointestinal tract EUS FNA. Am J Gastroenterol. 2014;109:676–85. doi: 10.1038/ajg.2013.479. [DOI] [PubMed] [Google Scholar]


