Abstract
The clinical link between spatial and non-spatial attentional aspects in patients with hemispatial neglect is well known; in particular, an increase in alerting can transitorily help to allocate attention towards the contralesional side. In models of attention, this phenomenon is postulated to rely on an interaction between ventral and dorsal cortical networks, subtending non-spatial and spatial attentional aspects, respectively. However, the exact neural underpinnings of the interaction between these two networks are still poorly understood. In the present study, we included 80 right-hemispheric patients with subacute stroke (50% women; age range: 24–96), 33 with and 47 without neglect, as assessed by paper–pencil cancellation tests. The patients performed a computerized task in which they were asked to respond as quickly as possible by button-press to central targets, which were either preceded or not preceded by non-spatial, auditory warning tones. Reaction times in the two different conditions were measured. In neglect patients, a warning tone, enhancing activity within the ventral attentional ‘alerting’ network, could boost the reaction (in terms of shorter reaction times) of the dorsal attentional network to a visual stimulus up to the level of patients without neglect. Critically, using voxel-based lesion-symptom mapping analyses, we show that this effect significantly depends on the integrity of the right anterior insula and adjacent inferior frontal gyrus, i.e., right-hemispheric patients with lesions involving these areas were significantly less likely to show shorter reaction times when a warning tone was presented prior to visual target appearance. We propose that the right anterior insula and inferior frontal gyrus are a critical hub through which the ventral attentional network can ‘alert’ and increase the efficiency of the activity of the dorsal attentional network.
Keywords: neglect, warning tone, alerting, insula, inferior frontal gyrus (IFG)
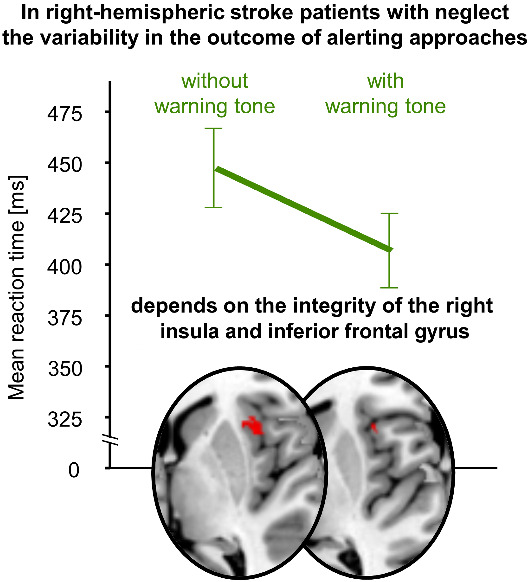
In right-hemispheric patients with neglect, the ability to benefit from non-spatial warning tones to shorten reaction times to visual stimuli depends on the integrity of the anterior insula and inferior frontal gyrus. Cazzoli et al. propose these regions as a hub through which the ventral attentional network ‘alerts’ its dorsal counterpart.
Graphical Abstract
Graphical Abstract.
Introduction
Hemispatial neglect is defined according to lateralized attentional impairments, i.e., most commonly the inability to attend to the left, contralesional side of space after a right-hemispheric lesion (Heilman et al., 1993). Although far less studied, non-lateralized attentional impairments have also been described in hemispatial neglect patients (for a review, see Husain and Rorden, 2003). In particular, a relationship between alerting and the severity of hemispatial neglect symptoms was described, i.e., temporarily increasing the alerting level can help hemispatial neglect patients to transiently improve their attention allocation towards the contralesional side of space (for a review, see also Chandrakumar et al., 2019). However, the neural underpinnings of the interaction between lateralized and non-lateralized attentional functions in neglect patients are still under investigation. An influential model (Corbetta and Shulman, 2002, 2011) postulates two distinct neural networks subtending attentional control in the human brain. The dorsal network, comprising the superior parietal lobule and frontal eye field and their interconnecting white matter fibre tracts (for a recent review, see Lunven and Bartolomeo, 2017), subtends the voluntary allocation of attention to spatial locations or visual features, and is not lateralized, each hemisphere competing to direct attention towards the contralateral side of space. The ventral network, consisting of cortical regions around the temporo-parietal junction, the ventral frontal cortex, and their interconnecting white matter fibre tracts, is strongly lateralized towards the right hemisphere and subtends non-spatial attention aspects, such as alerting (Corbetta and Shulman, 2002, 2011). These two networks are thought to closely interact in order to guide attentional behaviour, i.e., the ventral network is thought to ‘alert’ the dorsal network in order to shift attention when unattended or unexpected visual stimuli occur (Corbetta and Shulman, 2011), thereby playing a circuit-breaker role (Corbetta and Shulman, 2002). However, the locus of this interaction, both at the anatomical and functional level, still remains a matter of debate. Correlational approaches by means of functional magnetic resonance imaging (fMRI) have suggested that the right middle frontal gyrus (He et al., 2007), as well as cortical areas around the inferior frontal junction (at the junction of the inferior frontal sulcus—separating the middle and the inferior frontal gyri—with the inferior precentral sulcus; Asplund et al., 2010) are active both in tasks engaging the ventral and the dorsal attentional network, and may thus play the role of a link between the two. The evidence coming from lesion studies is sparse. A single-case study in a patient with a right middle frontal gyrus resection (Japee et al., 2015) suggested the role of this region in flexibly modulating attention between endogenous and exogenous cues, functions typically subtended by both the dorsal and the ventral attentional network, respectively. Moreover, a qualitative lesion overlap analysis in nine individuals with hemispatial neglect, suggested that patients with damage to the right insula do not benefit from alerting in tasks requiring cognitive conflict resolution within the left, neglected space (Chica et al., 2012). However, to the best of our knowledge, no study to date investigated this issue by applying quantitative lesion-symptom mapping in a large group of right-hemispheric patients.
A possibility to address this important and unresolved issue is to apply a non-spatial, auditory warning tone, in order to temporarily increase the activity within the ventral network in a bottom-up fashion. This allows, in turn, to provide greater alerting input to the right-hemispheric dorsal network, improving its performance in detecting spatial, visual stimuli at an expected location, in a top-down fashion. Indeed, early studies have shown that—on a group level—auditory warning tones can temporarily improve visuospatial neglect deficits (Robertson et al., 1998). However, as suggested by the above-mentioned findings, it seems reasonable to assume that the positive alerting effects will substantially vary across patients, and will critically depend on whether the hub between ventral and dorsal attentional networks is intact.
In order to investigate this question, we applied non-spatial, auditory warning tones and measured the reaction time (RT) to visual stimuli in 80 right-hemispheric patients with subacute stroke, with and without neglect. Using lesion-symptom mapping analyses, we investigated in which patients a warning tone, enhancing activity within the ventral attentional ‘alerting’ network, would improve the performance of the dorsal attentional network in reacting to a visual stimulus. Finally, we assessed whether, and to what extent, the alerting level would go along with spatial biases, i.e., the severity of neglect.
Materials and methods
Patients
The data of 80 stroke patients (40 women, 40 men; age m = 65.94, SD = 16.51), who suffered from a first, right-hemispheric subacute stroke (63 ischaemia, 17 haemorrhage; days since event m = 21.50, SD = 14.92), were analysed.
Informed consent was obtained from all patients. The study was approved by the Ethics Committee of the states of Bern and Luzern, Switzerland and was performed according to the latest version of the Declaration of Helsinki.
Tasks
We analysed the results of a paper-pencil cancellation test [Random Shape Cancellation (Weintraub and Mesulam, 1985), Bells Test (Gauthier et al., 1989), Star Cancellation Test (Wilson et al., 1987), or Sensitive Neglect Test, SNT-Dual (Reinhart et al., 2016)] and of a subtest of a computerized, validated attention test battery [Testbatterie zur Aufmerksamkeitsprüfung (TAP); (Zimmermann and Fimm, 1993)]. For the paper–pencil cancellation tests, we computed the Center of Cancellation (CoC; Rorden and Karnath, 2010). The CoC assesses hemispatial neglect severity in cancellation tests by considering both the number and the spatial location of omissions, and it also enables the comparison of results of different tests in a standardized way (thereby, scores near 0 indicate a symmetrical pattern of omissions, scores near 1 or −1 the detection of only the rightmost or leftmost target, respectively). For the computerized assessment, the patients were presented with a central fixation dot of randomly varying duration (3000–5000 ms), which was then replaced by a central target (an ‘X’), to which they were asked to respond as quickly as possible by pressing a button with their right hand. The test encompassed both a phasic (the target was preceded by a warning tone, presented at a randomly determined time interval of 650–1240 ms) and a tonic (no warning tone) condition, which were tested in separate test blocks (4 test blocks entailing 20 trials each, in an ABBA design, starting with the tonic condition).
Statistical and lesion analyses
The trial was powered to test a significant effect size of f = 0.16 (Chandrakumar et al., 2019) at an alpha level of 0.05.
The results of the computerized assessment (in terms of mean RTs) were analysed by means of a mixed-model analysis of variance, with the between factor ‘group’ (hemispatial neglect, no hemispatial neglect) and the within factor ‘condition’ (with or without warning tone), followed by least significant difference-corrected post hoc tests. Moreover, we assessed the relationship between RTs and CoC results by means of Pearson's correlations. For all statistical tests, a significance level (alpha) of 5%, two-tailed, was used.
Finally, in order to assess the lesion-related determinants of the variability of the temporary decrease in RTs triggered by the warning tone, we performed voxel-based lesion-symptom mapping analyses by means of the non-parametric mapping software NPM (Rorden et al., 2007). Lesion delineation and normalization were performed with procedures identical to the ones used in our recent work (see e.g. Nyffeler et al., 2019). In order to quantify, in a single value, the extent by which the patients benefitted from the warning tone (i.e. in terms of shorter RTs), for each patient we calculated an RT quotient by the formula: mean RTs without warning tone/mean RTs with warning tone. Correspondingly, positive and higher values indicate a greater benefit from the warning tone (mean RTs shorter with than without warning tone). For voxel-based lesion-symptom mapping, the quotient values were entered as continuous predictor, applying the Brunner–Munzel test, considering only voxels lesioned in at least 20% of patients, adjusting the significance threshold by means of a family-wise error approach, and controlling for multiple comparisons using permutation-based thresholding with 4000 iterations.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Results
According to the corresponding CoC cut-off scores (see Rorden and Karnath, 2010), 33 right-hemispheric patients exhibited hemispatial neglect, while 47 did not.
In general, as shown in Fig. 1A, right-hemispheric patients with hemispatial neglect showed significantly higher RTs than the ones without hemispatial neglect [F(1,78) = 8.42, P = 0.005, η2 = 0.097], and the warning tone significantly ameliorated the performance, in terms of shorter RTs (F(1,78) = 19.83, P < 0.001, η2 = 0.203). Interestingly, however, there was a significant interaction between these two factors (F(1,78) = 9.42, P = 0.003, η2 = 0.108): a significant reduction in RTs by the warning tone was only observed in right-hemispheric patients with hemispatial neglect (P < 0.001), but not in the ones without hemispatial neglect (P = 0.28). Moreover, whereas right-hemispheric patients with hemispatial neglect showed significantly higher RTs than patients without hemispatial neglect in the absence of the warning tone (P = 0.013), this difference was not significant anymore with the warning tone (P = 0.12).
Figure 1.
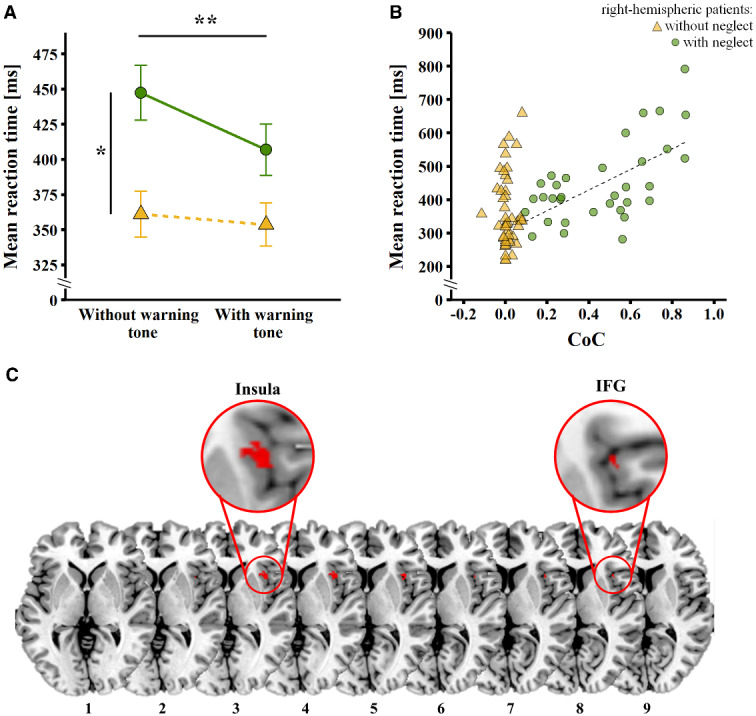
Results of behavioural data and lesion-symptom mapping analyses. (A) Mean RTs (in ms) in the computerized assessment, i.e., reacting to a visual stimulus without (left hand side) and with (right hand side) a prior warning tone, in right-hemispheric patients with (green dots) and without (yellow triangles) hemispatial neglect. The error bars represent the standard error of the mean. Asterisks depict significant post hoc tests (*P < 0.05; **P < 0.001). (B) Scatter plot depicting mean RTs (in ms) in the computerized assessment without warning tone (y-axis) against the CoC (x-axis), in right-hemispheric patients with (green dots) and without (yellow triangles) hemispatial neglect. The dotted line represents the significant correlation between the two variables observed in patients with hemispatial neglect. (C) Results of the voxel-based lesion-symptom mapping analysis. Right-hemispheric patients with lesions involving the right anterior insula and the adjacent right IFG were significantly less likely to show shorter RTs when a warning tone was presented prior to visual target appearance in the computerized assessment. The significant lesion cluster (123 voxels, 0.12 cc) is depicted in red (significance level P < 0.05, based on the Brunner-Munzel test, family-wise error-corrected, 4000 permutations) on the ch2bet template, as available in MRIcron (http://www.mccauslandcenter.sc.edu/crnl/chris-rordens-neuropsychology-lab), and in the magnifying lens circles. The axial slices are oriented according to the neurological convention. The z-position of each axial slice, in Montreal Neurological Institute coordinates, is indicated by the numbers at the bottom of each slice.
As shown in Fig. 1B, in right-hemispheric patients with hemispatial neglect there was a strong, positive correlation between RTs and hemispatial neglect severity (i.e. the higher the RTs, the more severe the hemispatial neglect) when no warning tone was presented (r = 0.622, P < 0.001); this was not the case in right-hemispheric patients without hemispatial neglect (r = 0.037, P = 0.807). A similar pattern was observed when a warning tone was presented, i.e., a significant, although weaker, correlation in right-hemispheric patients with hemispatial neglect (r = 0.495, P = 0.003), and no significant correlation in right-hemispheric patients without hemispatial neglect (r = 0.132, P = 0.378).
As shown in Fig. 1C, the extent by which the patients benefitted from the warning tone, as reflected by the RT quotient, was significantly affected by lesions involving the right anterior insula and the adjacent right inferior frontal gyrus (IFG), i.e., right-hemispheric patients with lesions involving these areas were significantly less likely to show shorter RTs when a warning tone was presented prior to visual target appearance.
Discussion
As a novel and intriguing finding, our results demonstrate that the ability to benefit from a non-spatial, auditory warning tone is critically linked to the right anterior insula and the adjacent IFG. Hence, in neglect patients, an auditory stimulus can enhance the ventral attentional ‘alerting’ system and boost the reaction of the dorsal attentional system up to a level of patients without neglect, but only if these regions are intact.
The anterior insular cortex has been implicated in a wide range of higher cognitive functions (see, for a review, Shura et al., 2014). In particular, the anterior portion of the insula has been conceptualized as part of the ventral attentional network (Uddin, 2015), of which the IFG, which is densely connected with the anterior insula (Flynn et al., 1999), is also a component (Corbetta and Shulman, 2002, 2011). As such, on the one hand, the right anterior insula and the IFG seem to be good candidates as hub between the ventral and dorsal attentional networks. On the other hand, these two cortical regions have been also shown to play an important role in auditory processing; this is of particular relevance for our results, since, in our approach, the alerting stimulus was presented in the auditory modality. The right anterior insula and the IFG show connections to the ipsilateral primary auditory cortex, but also to associative auditory cortices within the planum temporale, via the arcuate fasciculus (Ghaziri et al., 2017; Ibanez et al., 2010). Furthermore, they also receive input from contralateral auditory areas via thalamo-cortical projections (Bamiou et al., 2003). Finally, the anterior insula shows also connections to the frontal and parietal areas of the dorsal attentional network (Menon and Uddin, 2010; Ghaziri et al., 2017; Ptak et al., 2020).
A word of caution should be spent on a potential limitation of the mass univariate approach used in this study for the voxel-based lesion-symptom mapping analyses, i.e., that results can sometimes be spatially biased towards the centre of mass of the middle cerebral artery distribution in patients who suffered thromboembolic strokes (see Mah et al., 2014). However, considering the above-mentioned lines of evidence, highlighting the functional significance of the anterior insula and of the IFG in both attentional and auditory processing, our results appear most likely genuine and not the result of a methodological artefact.
We thus propose that the right anterior insula and IFG are a critical hub through which the ventral attentional network can ‘alert’ and increase the efficiency of the activity of the dorsal attentional network.
This also explains the strong, positive correlation between RTs to visual stimuli and hemispatial neglect severity observed in our patients, and is in line with a previous observation—made by a qualitative analysis in nine hemispatial neglect patients—that damage to the right insula seems to abolish the positive effects of alerting on spatial attention within the left, neglected space (Chica et al., 2012). This is also in line with the potentially important role that has been ascribed to prefrontal cortical areas in hemispatial neglect compensation and recovery (Bartolomeo, 1997; 2000), as well as with the compensatory, intentional leftward orienting in hemispatial neglect that has been shown to be mediated by these areas (Takamura et al., 2016).
The fact that, in hemispatial neglect patients, the detection of visual stimuli can be improved by an alerting tone, can also have important consequences for clinical applications and therapy. Indeed, it has been recognized early on that a punctual enhancement of alerting can trigger a transitory amelioration of hemispatial neglect symptoms (Robertson et al., 1998). Consequently, several therapeutic approaches have been devised, aiming at ameliorating hemispatial neglect symptoms through an alerting enhancement (Chandrakumar et al., 2019; Van Vleet et al., 2020), showing promising results on a group level. However, a great variability between individuals in treatment outcomes is a very common finding for neglect rehabilitation (Bowen et al., 2013). Our findings suggest that this variability in the outcome of alerting approaches can be explained by the integrity of the right anterior insula and the right IFG. Such information seems important to stratify patients according to their lesion patterns and allocate them to the most suitable therapeutic approaches.
Acknowledgements
We would like to thank all participants for taking part in our study. We are grateful to Severin A. Stadler for his help in data collection. We are also grateful to the clinical teams at the Inselspital, Bern University Hospital, Switzerland and the Neurocenter of the Kantonsspital Luzern, Switzerland for their assistance. Furthermore, we would like to acknowledge the research support of the Swiss National Science Foundation (SNSF).
Funding
This work was supported by the Swiss National Science Foundation (SNSF, Switzerland), Grants No. PZ00P3_154714, 320030_169789, and 32003B_196915/1.
Competing interests
The authors report no competing interests.
Glossary
- CoC =
centre of cancellation;
- IFG =
inferior frontal gyrus;
- RT =
reaction time.
References
- Asplund C, Todd J, Snyder A, Marois R. A central role for the lateral prefrontal cortex in goal directed and stimulus-driven attention. Nat Neurosci 2010; 13: 507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamiou D, Musiek F, Luxon L. The insula (Island of Reil) and its role in auditory processing: literature review. Brain Res Rev 2003; 42: 143–54. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P. The novelty effect in recovered hemineglect. Cortex 1997; 33: 323–32. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P. Inhibitory processes and compensation for spatial bias after right hemisphere damage. Neuropsychol Rehabil 2000; 10: 511–26. [Google Scholar]
- Bowen A, Hazelton C, Pollock A, Lincoln NB. Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database Syst Rev 2013; CD003586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrakumar D, Keage H, Gutteridge D, Dorrian J, Banks S, Loetscher T. Interactions between spatial attention and alertness in healthy adults: a meta-analysis. Cortex 2019; 119: 61–73. [DOI] [PubMed] [Google Scholar]
- Chica A, Thiebaut de Schotten M, Toba M, Malhotra P, Lupiáñez J, Bartolomeo P. Attention networks and their interactions after right-hemisphere damage. Cortex 2012; 48: 654–63. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman G. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002; 3: 201–5. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman G. Spatial neglect and attention networks. Annu Rev Neurosci 2011; 34: 569–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn F, Benson D, Ardila A. Anatomy of the insula - functional and clinical correlates. Aphasiology 1999; 13: 55–78. [Google Scholar]
- Gauthier L, Dehaut F, Joanette Y. The Bells test: a quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol 1989; 11: 49–54. [Google Scholar]
- Ghaziri J, Tucholka A, Girard G, Houde J, Boucher O, Gilbert G, et al. The corticocortical structural connectivity of the human insula. Cerebral Cortex 2017; 27: 1216–28. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 2007; 53: 905–18. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E, 1993. Neglect and related disorders In: Heilman KM, Valenstein E, editors, Clinical neuropsychology. New York: Oxford University Press; p. 279–336. [Google Scholar]
- Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect. Nat Rev Neurosci 2003; 4: 26–36. [DOI] [PubMed] [Google Scholar]
- Ibanez A, Gleichgerrcht E, Manes F. Clinical effects of insular damage in humans. Brain Struct Funct 2010; 214: 397–410. [DOI] [PubMed] [Google Scholar]
- Japee S, Holiday K, Satyshur M, Mukai I, Ungerleider L. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci 2015; 9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunven M, Bartolomeo P. Attention and spatial cognition: neural and anatomical substrates of visual neglect. Ann Phys Rehabil Med 2017; 60: 124–9. [DOI] [PubMed] [Google Scholar]
- Mah YH, Husain M, Rees G, Nachev P. Human brain lesion-deficit inference remapped. Brain 2014; 137: 2522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin L. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010; 214: 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyffeler T, Vanbellingen T, Kaufmann B, Pflugshaupt T, Bauer D, Frey J, et al. Theta burst stimulation in neglect after stroke: functional outcome and response variability origins. Brain 2019; 142: 992–1008. [DOI] [PubMed] [Google Scholar]
- Ptak R, Bourgeois A, Cavelti S, Naz Doganci N, Schnider A, Rita Iannotti G. Discrete patterns of cross-hemispheric functional connectivity underlie impairments of spatial cognition after stroke. J Neurosci 2020; 40: 6638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart S, Kerkhoff LE. G. SNT-single und SNT-dual: Zwei neue sensitive Neglecttests für den leichten und chronischen neglect. Neurol Rehabil 2016; 22: 98–104. [Google Scholar]
- Robertson I, Mattingley J, Rorden C, Driver J. Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature 1998; 395: 169–72. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H, Bonilha L. Improving lesion–symptom mapping. J Cogn Neurosci 2007; 19: 1081–8. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H. A simple measure of neglect severity. Neuropsychologia 2010; 48: 2758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shura R, Hurley R, Taber K. Insular cortex: structural and functional neuroanatomy. J Neuropsychiatry Clin Neurosci 2014; 26: 276–82. [DOI] [PubMed] [Google Scholar]
- Takamura Y, Imanishi M, Osaka M, Ohmatsu S, Tominaga T, Yamanaka K, et al. Intentional gaze shift to neglected space: a compensatory strategy during recovery after unilateral spatial neglect. Brain 2016; 139: 2970–82. [DOI] [PubMed] [Google Scholar]
- Uddin L. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 2015; 16: 55–61. [DOI] [PubMed] [Google Scholar]
- Van Vleet T, Bonato P, Fabara E, Dabit S, Kim S, Chiu C, et al. Alertness training improves spatial bias and functional ability in spatial neglect. Ann Neurol 2020; 00: 1–12. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Mesulam M. Mental state assessment of young and elderly adults in behavioral neurology Philadelphia: F. A. Davis; 1985. [Google Scholar]
- Wilson B, Cockburn J, Halligan PW. The behavioural inattention test manual. England: Thames Valley Test Company; 1987. [Google Scholar]
- Zimmermann P, Fimm B. Testbatterie zur Aufmerksamkeitsprüfung (TAP): Psytest. Würselen, Germany; 1993.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


