Abstract
Non-convulsive status epilepticus describes the syndrome of unexplained impaired consciousness in critically ill patients. Non-convulsive status epilepticus is very likely to lead to delayed diagnosis and poor outcomes because of the absence of convulsive symptoms. EEG is essential for the diagnosis of non-convulsive status epilepticus to establish the association between periodic discharges and rhythmic delta activity in addition to ictal epileptiform discharges according to the Salzburg criteria. Arterial spin labelling, a type of perfusion MRI, has been applied for rapid and non-invasive evaluation of the ictal state. Ictal cerebral cortical hyperperfusion is the most common finding to demonstrate focal onset seizures. Hyperperfusion of the thalamus on single photon emission computed tomography was found in patients with impaired awareness seizures. We hypothesized that thalamocortical hyperperfusion on arterial spin labelling identifies non-convulsive status epilepticus and such thalamic hyperperfusion specifically associates with periodic/rhythmic discharges producing impaired consciousness without convulsion. We identified 27 patients (17 females; age, 39–91 years) who underwent both arterial spin labelling and EEG within 24 h of suspected non-convulsive status epilepticus. We analysed 28 episodes of suspected non-convulsive status epilepticus and compared hyperperfusion on arterial spin labelling with periodic/rhythmic discharges. We evaluated 21 episodes as a positive diagnosis of non-convulsive status epilepticus according to the Salzburg criteria. We identified periodic discharges in 15 (12 lateralized and 3 bilateral independent) episodes and rhythmic delta activity in 13 (10 lateralized, 1 bilateral independent and 2 generalized) episodes. Arterial spin labelling showed thalamic hyperperfusion in 16 (11 unilateral and 5 bilateral) episodes and cerebral cortical hyperperfusion in 24 (20 unilateral and 4 bilateral) episodes. Thalamic hyperperfusion was significantly associated with non-convulsive status epilepticus (P = 0.0007; sensitivity, 76.2%; specificity, 100%), periodic discharges (P < 0.0001; 93.3%; 84.6%), and rhythmic delta activity (P = 0.0006; 92.3%; 73.3%). Cerebral cortical hyperperfusion was significantly associated with non-convulsive status epilepticus (P = 0.0017; 100%; 57.1%) and periodic discharges (P = 0.0349; 100%; 30.8%), but not with rhythmic delta activity. Thalamocortical hyperperfusion could be a new biomarker of non-convulsive status epilepticus according to the Salzburg criteria in critically ill patients. Specific thalamic hyperexcitability might modulate the periodic discharges and rhythmic delta activity associated with non-convulsive status epilepticus. Impaired consciousness without convulsions could be caused by predominant thalamic hyperperfusion together with cortical hyperperfusion but without ictal epileptiform discharges.
Keywords: periodic discharge, rhythmic delta activity, Salzburg criteria, impaired consciousness, critically ill patients
Thalamocortical hyperperfusion on arterial spin labelling MRI could be a new biomarker of non-convulsive status epilepticus according to the Salzburg criteria in critically ill patients. This thalamic hyperperfusion might contribute to the periodic discharges and rhythmic delta activity associated with non-convulsive status epilepticus.
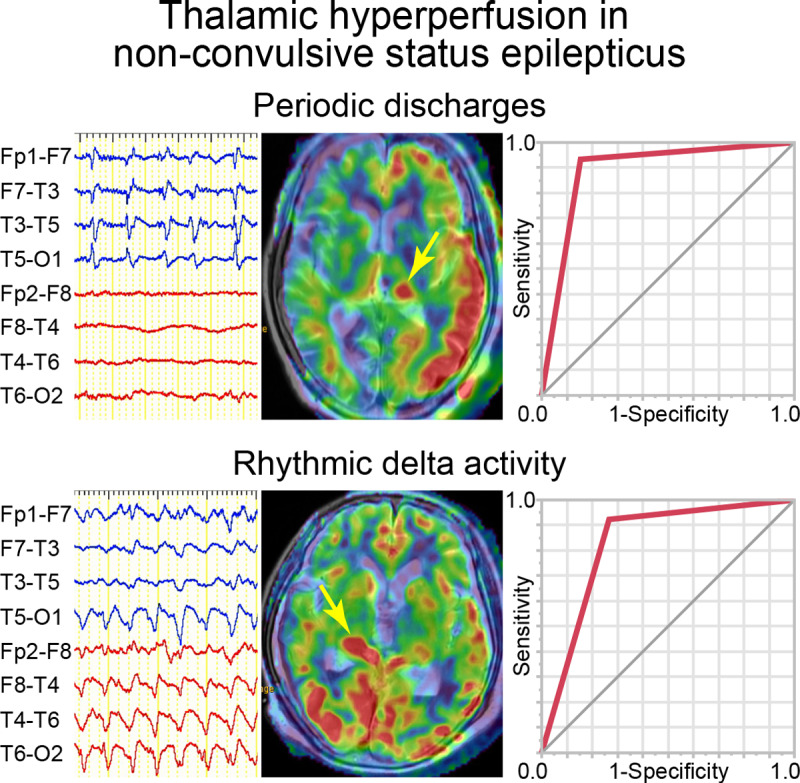
Graphical Abstract
Graphical Abstract.
Introduction
Non-convulsive status epilepticus
Non-convulsive status epilepticus (NCSE) is the term used to describe unexplained impaired consciousness or altered mental status from the baseline in critically ill patients (Sutter et al., 2016). NCSE presents a complex problem in neurocritical care because of the absence of the convulsive symptoms found in convulsive status epilepticus. EEG is essential for the correct diagnosis of NCSE since the clinical signs are very subtle and non-specific (Claassen et al., 2004). Several EEG diagnostic criteria for NCSE have been proposed (Chong and Hirsch, 2005; Kaplan, 2007; Sutter and Kaplan, 2012), and renewed diagnostic criteria for NCSE were proposed by a panel of experts at the fourth London-Innsbruck Colloquium on Status Epilepticus in Salzburg (Beniczky et al., 2013). This Salzburg criteria integrate the standardized critical care EEG terminology of the American Clinical Neurophysiology Society (Hirsch et al., 2013) to reduce the false-positive diagnosis of NCSE (Leitinger et al., 2015b), as well as apply to comatose patients (Trinka and Leitinger, 2015). The Salzburg criteria achieve high diagnostic accuracy and excellent inter-rater agreement for the diagnosis of NCSE in various clinical settings (Leitinger et al., 2016). The interpretation of periodic discharges (PDs) and rhythmic delta activity (RDA) in addition to EEG seizures has established the defining landmark for NCSE in the field of intensive care (Trinka and Leitinger, 2015; Leitinger et al., 2015b, 2016).
Arterial spin labelling
Arterial spin labelling (ASL) is one of the methods used in perfusion MRI studies. ASL can provide non-invasive evaluation of cerebral perfusion without the requirement of exogenous tracer administration (Detre et al., 1992; Williams et al., 1992). ASL has demonstrated cerebral cortical hyperperfusion in acute seizures (Yoo et al., 2017), during the ictal and peri-ictal periods of focal onset seizures (Pizzini et al., 2013; Kim et al., 2016; Schertz et al., 2020), during status epilepticus (Oishi et al., 2012; Matsuura et al., 2015) and in NCSE (Shimogawa et al., 2017). ASL findings of cortical hyperperfusion are more sensitive for status epilepticus seizure than hyperintensity on diffusion-weighted imaging (DWI) (Matsuura et al., 2015; Shimogawa et al., 2017; Schertz et al., 2020). However, any association between thalamic hyperperfusion on ASL and EEG findings, indicating suspected NCSE based on the Salzburg criteria, has not been established.
Thalamus and periodic discharges
The thalamus is the most important component of the arousal system in humans (Steriade et al., 1993). Consciousness is maintained by the ascending reticular activating system consisting of the thalamus, thalamocortical connections and temporolimbic systems. Single photon emission computed tomography (SPECT) study revealed that thalamic hyperperfusion was associated with impaired consciousness during seizures (Lee et al., 2002). Perfusion MRI study demonstrated that thalamic hyperperfusion was associated with prolonged epileptic activities in patients with focal impaired awareness seizure status (Szabo et al., 2005). Therefore, thalamic hyperperfusion could be associated with impaired consciousness in addition to specific EEG findings during NCSE.
Hypothesis
We proposed that the PDs and RDA indispensable to diagnose NCSE are associated with thalamic hyperperfusion on ASL. This study investigated the association of ASL with EEG findings in critically ill patients with suspected NCSE using the Salzburg criteria. We hypothesized that thalamic hyperperfusion on ASL is a surrogate biomarker of NCSE, and represents thalamic hyperexcitability, modulating the PDs and RDA associated with NCSE in critically ill patients.
Materials and methods
Subjects
A total of 1038 adult patients were admitted to our emergency room in the Department of Neurosurgery of South Miyagi Medical Center between November 2013 and December 2018. We retrospectively identified 31 patients with clinically suspected NCSE, manifesting as symptoms consisting of unexplained impaired consciousness or decreasing levels of cognitive performance from the baseline for at least 10 min (Leitinger et al., 2015b). Patients with prior seizure episodes of epileptic disorders were excluded in this study to exclude the long-term effects of anti-epileptic drugs on EEG findings. We excluded four patients in whom ASL and EEG were not performed within 24 h of the onset of suspected NCSE. The study was approved by the local Ethical Committee of South Miyagi Medical Center. The caregivers of all patients provided written informed consent.
MRI and ASL data collection
MRI was performed with a 3T system (Signa HDxt 3.0T; GE Healthcare, Milwaukee, WI, USA) with a 12-channel phased array head receiving coil for conventional non-contrast sequences and ASL. The conventional sequences included axial echo-planar DWI [b value = 1000 s/mm2; repetition time (TR)/echo time (TE) = 6500/80 ms]; T2 fluid-attenuated inversion recovery (TR/TE/inversion time = 10 000/140/2500 ms); T2-star-weighted imaging (TR/TE = 500/15.0 ms); and three-dimensional time-of-flight magnetic resonance angiography from the carotid bifurcation to all brain arteries (TR/TE = 25/3.4 ms).
ASL was performed using a three-dimensional fast-spin-echo pulsed continuous ASL with background suppression and superimposed T2-weighted imaging. The pulsed continuous ASL sequences consisted of interleaved stack spiral readout with the following parameters: TR, 4600 ms; TE, 9.8 ms; 512 sampling points on 8 spiral arms; field-of-view, 240 mm; section thickness, 4 mm; reconstructed matrix, 512 × 512; reconstructed slice thickness, 5 mm; post-labelling delay, 1525 ms; number of excitations, 2 and total scan time, 3:14 min. T2-weighted imaging used the following parameters: TR, 4700 ms; TE, 100 ms; field-of-view, 240 mm and slice thickness, 4 mm. Quantitative cerebral blood flow (CBF) (ml/100 g/min) was automatically calculated from the pulsed continuous ASL images using the following formula:
where λ is the brain-blood partition coefficient set to 0.9, ST is the saturation time set to 2000 ms, T1t is the grey-matter T1 value set to 1200 ms, T1b is the blood T1 value set to 1600 ms, LT is the labelling duration set to 1500 ms, ε is the labelling efficiency set to 0.80 × 0.75, PW is the difference between labelling and control images, SF is a scaling factor, RPD is the reference proton density images, PLD is the post-labelling delay and NEX is the number of excitations.
Definition of hyperperfusion
We recruited 28 age- and sex-matched subjects (11 males and 17 females; mean age, 75.6 years) as controls to establish the reference values of regional CBF. The control subjects had undergone ASL during the same period for the investigation of primary brain disease. The control subjects were matched with the patients according to the brain disease, without seizures or impaired consciousness episodes. Regions of interest (ROIs) were drawn on the ASL images to measure regional CBF using the workstation of the MRI scanner (AW4.6; GE Healthcare). The area of ROIs was 461 mm2 at the cerebral cortex and 210 mm2 at the thalamus. The total number of ROIs was 48 including three in the thalamus, six in the frontal lobe and five in each of the temporal, parietal and occipital lobes, in the unilateral hemisphere (Supplementary Fig. 1). Based on the ROIs of the control subjects, the mean and standard deviation of regional CBF were calculated as references for both lesion and non-lesion sides in the thalamus and cerebral cortical regions (Supplementary Table 1).
The ROIs were placed by two co-authors (H.A., Y.S.) in the patients to include visually identified hyperperfusion areas in the thalamus (fixed location as in control subjects) and the frontal, temporal, parietal and occipital lobes. These authors independently reviewed ASL data, unaware of the patients’ clinical data including the findings of EEG. The ROIs consisted of three in the thalamus, six in the frontal lobe and five in each of the temporal, parietal and occipital lobes, in the unilateral hemisphere. A total of 48 ROIs were selected in the bilateral hemispheres. The CBF value of each ROI in patients was compared to that of the control subjects in the corresponding region as summarized in Supplementary Table 1. If the CBF value of ROI was 2 standard deviations or more above the mean reference value of CBF in the control subjects, the ROI was considered to indicate hyperperfusion. If one or more hyperperfusion ROIs were identified among the three ROIs in the unilateral thalamus, we defined thalamic hyperperfusion. If two or more hyperperfusion ROIs were identified in each cerebral lobe in the unilateral hemisphere, we defined cortical hyperperfusion in the cerebral lobe.
EEG
Digital scalp EEG (Neurofax; Nihon-Kohden, Tokyo, Japan) was recorded with the international 10–20 system electrode placement and additional T1/T2 electrodes. The EEG recordings were performed for at least 1 h for all patients. Continuous EEG monitoring (more than 6 h) was performed in 15 patients. PDs and RDA were identified using standardized critical care EEG terminology of the American Clinical Neurophysiology Society (Hirsch et al., 2013). The PDs were subcategorized as lateralized, bilateral independent and generalized PDs. The RDA was subcategorized as lateralized, bilateral independent and generalized RDA. The EEG seizures were identified as frequency change and apparent spatiotemporal evolution pattern of epileptiform discharges with a frequency of >25 times per 10 s epoch. Brief rhythmic epileptiform discharges were identified as repetitive epileptiform discharges occurring two or three times per second, lasting only a few seconds. Single spike or sharp waves were not evaluated in this study. Two experienced, board-certified experts of EEG and clinical neurophysiology (S.O., H.O.) independently reviewed the recordings of EEG, unaware of the patients’ clinical data including ASL.
Non-convulsive status epilepticus
NCSE was diagnosed using the Salzburg criteria (Beniczky et al., 2013; Leitinger et al., 2016). The minimal duration of the EEG epoch required to fulfil the Salzburg criteria was defined as 10 s with abnormal findings during the entire EEG recordings (Leitinger et al., 2016). We categorized the EEG findings associated with NCSE using the terminology proposed previously (Leitinger et al., 2015a, 2016) as follows:
Epileptiform discharges with the frequency of >25 times per 10 s epoch (2.5 cycles per second).
-
Epileptiform discharges with the frequency of ≤2.5 cycles per second or continuous (quasi-) RDA with the frequency of >0.5 cycles per second, and at least one of:
(2a) EEG and clinical improvement with intravenous antiepileptic drugs.
(2b) Subtle clinical ictal phenomena during the EEG.
(2c) Typical spatiotemporal evolution.
(2d) Only EEG improvement to intravenous antiepileptic drugs.
(2e) Fluctuation of EEG findings without definitive evolution.
The absence of (1) or (2).
We classified episodes of Salzburg criteria (1), (2a), (2b) and (2c) as NCSE, (2d) and (2e) as possible NCSE, and (3) as no NCSE (Leitinger et al., 2016). Episodes classified as possible NCSE were considered as a positive diagnosis of NCSE in the previous proposal (Leitinger et al., 2016).
Statistical analysis
Fisher’s exact test was used for significance testing between thalamic and cerebral cortical hyperperfusion on ASL, and the presence of NCSE and EEG findings. The sensitivity and specificity of thalamic and cerebral cortical hyperperfusion were calculated using standardized formulas for PDs, RDA, EEG seizures and brief rhythmic epileptiform discharges. Based on the sensitivities and specificities for NCSE, PDs and RDA, receiver operating characteristic curves were calculated, and the differences of the area under the curve (AUC) were compared between thalamic hyperperfusion and cerebral cortical hyperperfusion. For comparison of the AUCs, P-values and 95% confidence intervals (CIs) were calculated using the concordance statistics. The Wilcoxon rank sum test was used to calculate the differences in patients’ age, Glasgow Coma Scale (GCS) at suspected NCSE, times of ASL or EEG starting from suspected NCSE, time interval between ASL and EEG and EEG recording periods. Significance level of α = 0.05 was used for all statistical analyses. The data were analysed using commercially available software (JMP Pro 13.2; SAS Institute Inc.) and the open-source statistical package R (version 3.3.3; The R Project for Statistical Computing).
Gwet’s AC1 (for categorical data) was calculated for the EEG findings to assess the inter-rater agreement (Gwet, 2008). Inter-rater agreement was interpreted according to the following groups: poor (κ < 0), slight (κ = 0.01–0.2), fair (κ = 0.21–0.4), moderate (κ = 0.41–0.6), substantial (κ = 0.61–0.8) and almost perfect agreement (κ> 0.8) (Landis and Koch, 1977).
Data availability
The data used in this study are available upon reasonable request.
Results
Patient profiles
The clinical profiles of the patients are listed in Table 1. ASL and EEG were studied within 24 h for 28 episodes of suspected NCSE in our 27 patients. One patient had two separate episodes (Episodes 16 and 17), both with ASL and EEG. The 27 patients consisted of 17 females and 10 males with a mean age of 77.7 years (range, 39–91 years). The primary diseases of patients were as follows: subarachnoid haemorrhage in nine patients, intracerebral haemorrhage in seven, acute subdural haematoma in seven, chronic subdural haematoma in two and hydrocephalus in one. Three patients had generalized convulsions, including one with acute subdural haematoma and one with hydrocephalus. The laterality of lesions was the left hemisphere in 13 patients, right in 10, bilateral in 2 and non-lesion in 2. Surgical treatments for the primary diseases were performed in 18 patients. GCS at the suspected NCSE ranged from 5 to 11 (median, 8). In addition to impaired consciousness, subtle clinical phenomena were seen in 13 episodes consisting of eye deviation in six, twitching of the mouth in five and eye movement and twitching of the extremities in one each. ASL or EEG started from 1 to 12 h (mean, 6.8) after the suspected NCSE except for three episodes of no recovery of consciousness after generalized convulsion with ASL or EEG 24 or 36 h after the generalized convulsion. The time differences between ASL and EEG ranged from 0.5 to 23 h (mean, 8.0). The recording periods of EEG ranged from 1 to 64 h (mean, 11.9). Continuous EEG monitoring (more than 6 h) was performed in 15 episodes.
Table 1.
Clinical profiles
| Episode No. | Age (years) | Sex | Primary disease |
Surgery for primary disease | Subtle clinical phenomena | GCS at the suspected NCSE | Times of ASL/EEG starting from suspected NCSE (h) | Time differences between ASL and EEG (h) | Recording periods of EEG (h) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Brain disease | Laterality of lesion | |||||||||
| 1 | 88 | Male | ASDH | Right | No | 9 | 12 | 3.5 | 1 | |
| 2 | 72 | Male | ASDH | Right | No | Twitching of mouth | 9 | 1.5 | 1 | 1 |
| 3 | 87 | Female | ASDH, GC | Right | No | Eye deviation | 5 | 24a | 1 | 1 |
| 4 | 87 | Female | ASDH | Left | No | 8 | 4.5 | 21 | 1 | |
| 5 | 58 | Male | ICH | Right | Yes | Eye deviation | 5 | 4.5 | 22 | 1 |
| 6 | 81 | Female | ASDH | Left | No | 9 | 3.5 | 0.5 | 12 | |
| 7 | 70 | Female | SAH | Left | Yes | Twitching of mouth | 8 | 5 | 7 | 1 |
| 8 | 83 | Female | ASDH | Bilateral | No | 8 | 6 | 5 | 1 | |
| 9 | 75 | Female | ICH, ASDH | Right | Yes | 10 | 12 | 18 | 1 | |
| 10 | 84 | Female | ICH | Right | Yes | Eye deviation | 7 | 12 | 4.5 | 19 |
| 11 | 80 | Male | CSH | Bilateral | Yes | Eye movement | 9 | 2.5 | 2 | 16 |
| 12 | 75 | Female | SAH | Right | Yes | 8 | 8.5 | 16 | 1 | |
| 13 | 78 | Female | SAH | Left | Yes | Twitching of extremities | 9 | 8.5 | 15 | 1 |
| 14 | 86 | Female | SAH | Left | Yes | Eye deviation | 8 | 4.5 | 7.5 | 16 |
| 15 | 91 | Male | CSH | Left | Yes | Twitching of mouth | 9 | 12 | 2 | 15 |
| 16b | 80 | Female | SAH | Left | Yes | Eye deviation | 5 | 5 | 6 | 17 |
| 17b | 80 | Female | SAH | Left | Yes | Eye deviation | 7 | 4 | 4 | 18 |
| 18 | 83 | Male | ICH | Left | Yes | Twitching of mouth | 8 | 4 | 8 | 17 |
| 19 | 72 | Male | ICH | Right | Yes | 9 | 2.5 | 3.5 | 64 | |
| 20 | 80 | Female | GC | – | No | Twitching of mouth | 5 | 36a | 22 | 48 |
| 21 | 86 | Female | SAH | Left | Yes | 7 | 12 | 3 | 6 | |
| 22 | 71 | Female | Hydrocephalus, GC | – | Yes | 11 | 24a | 5 | 24 | |
| 23 | 85 | Male | ASDH | Left | No | 10 | 12 | 23 | 1.5 | |
| 24 | 39 | Male | ICH | Left | Yes | 6 | 6 | 4 | 1 | |
| 25 | 91 | Female | SAH | Left | No | 5 | 1 | 7 | 43 | |
| 26 | 62 | Male | ICH | Right | Yes | 6 | 5.5 | 4.5 | 2 | |
| 27 | 75 | Female | SAH | Right | Yes | 10 | 12 | 1.5 | 14 | |
| 28 | 78 | Female | SAH | Left | Yes | 9 | 9 | 6 | 17 | |
No recovery of consciousness after generalized convulsion.
Episodes 16 and 17 were separate episodes in the same patient.
Abbreviations: ASDH, acute subdural haematoma; ASL, arterial spin labelling; CSH, chronic subdural haematoma; GC, generalized convulsion; GCS, Glasgow Coma Scale; ICH, intracerebral haemorrhage; NCSE, non-convulsive status epilepticus; SAH, subarachnoid haemorrhage.
EEG
The EEG findings are described in Table 2. PDs were identified in 15 episodes including lateralized PDs in 12 and bilateral independent PDs in 3. Generalized PDs were not found in any episodes. The laterality of PDs was bilateral in six episodes, left in five and right in four. The locations of PDs consisted of multifocal in six episodes, hemispheric in five, regional in two and focal in two. The frequency of PDs ranged from 1 to 2.5 Hz.
Table 2.
Diagnosis of NCSE by Salzburg criteria, EEG findings and hyperperfusion on ASL
| Episode No. | Diagnosis of NCSE by Salzburg criteria | PDs |
RDA |
EEG seizures | Brief rhythmic epileptiform discharges |
Hyperperfusion on ASL |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thalamus | Cerebral cortex |
||||||||||||||
| Patterns | Laterality | Locations | Frequency (Hz) | Patterns | Laterality | Locations | Frequency (Hz) | Locations | Laterality | Locations | Laterality | Laterality | Locations | ||
| 1 | 1 | Lateralized | Right | T, P, O | 1 | Lateralized | Right | T, P | 3–4 | Right T → Right F, T | Right | T | Right | Right | T, P, O |
| 2 | 2c | Lateralized | Right | F, C, T (STE) | 1–2 | Generalized | Bilateral | F, C, T | 1.5 | Right | Right | F | |||
| 3 | 2b | Lateralized | Right > Left | P, O (Left); F, P, O (Right) | 1.5–2 | Lateralized | Right > Left | P, O (Left); F, P, O (Right) | 1.5–2 | Right | Bilateral | O | |||
| 4 | 1 | Lateralized | Left | P, O | 1.5 | Lateralized | Left | P, O | 2 | Left T, O | Left | P, O | Left | Left | F |
| 5 | 1 | Lateralized | Right | T | 1.5 | Lateralized | Right | T | 2 | Right T → Right T, O | Right | T, P | Left | Bilateral | P, O (Left); T (Right) |
| 6 | 1 | Lateralized | Left | F, T, P | 1 | Left P → Left T, P | Left | T, P | Left | Left | F, T, P, O | ||||
| 7 | 2b | Lateralized | Left | F, T | 1.5 | Left | F, T | Left | Bilateral | F, T, O (Left); T (Right) | |||||
| 8 | 1 | Right F, C → Generalized | Right | F | Right | F | |||||||||
| 9 | 1 | Right C, P | Right | F, C, P | Right | F, P | |||||||||
| 10 | 2b | Bilateral independent | Left > Right | F, T, P (Left); F, T (Right) | 1–2 | Lateralized | Left | F, T | 1.5–2 | Bilateral | Right | F, T, P, O | |||
| 11 | 2c | Lateralized | Right | F, P, O (STE) | 0.5–1.5 | Bilateral | Right | P, O | |||||||
| 12 | 2e | Bilateral independent | Right > Left | F, T (Left); F, C, T (Right) (Fluct) | 1–1.5 | Lateralized | Right | F, C, T | 0.5–1 | Bilateral | C | Left | Bilateral | O (Left); T (Right) | |
| 13 | 2b | Bilateral independent | Right > Left | F, T (Left); F, T (Right) | 1.5 | Right | F | Bilateral | Left | F | |||||
| 14 | 2b | Lateralized | Left > Right | T, P (Left); T, O (Right) | 1–1.5 | Right | Right | T, O | |||||||
| 15 | 2b | Lateralized | Left | T, P | 1 | Lateralized | Right | F, C | 2 | Bilateral | O | Left | Left | T | |
| 16a | 1 | Left F → Left F, T, P | Left | F, P, O | Left | F, T, P, O | |||||||||
| 17a | 2b | Lateralized | Right > Left | F, T (Left); F, T, P, O (Right) | 1 | Lateralized | Right > Left | F, T (Left); F, T, P, O (Right) | 1–2 | Right | P | Bilateral | Left | F, T | |
| 18 | 1 | Lateralized | Left | F, C, P, O | 1 | Lateralized | Left | F, C, P, O | 1.5–2 | Left O → Left F, C | Left | F, P | Bilateral | Left | F |
| 19 | 1 | Right C, P → Right T | Right | C, P | Right | F, T | |||||||||
| 20 | 2b | Lateralized | Left | F | 2–2.5 | Left | F | Left | Left | F | |||||
| 21 | 2e | Bilateral independent | Right > Left | F, T (Left); C, T, P (Right) (Fluct) | 1–1.5 | Left | F | Left | T, P | ||||||
| 22 | 3 | Generalized | Bilateral | Generalized | 1–1.5 | ||||||||||
| 23 | 3 | Left | F, T | ||||||||||||
| 24 | 3 | Left | F, T, O | ||||||||||||
| 25 | 3 | ||||||||||||||
| 26 | 3 | Left | F | ||||||||||||
| 27 | 3 | Right | F, T | ||||||||||||
| 28 | 3 | ||||||||||||||
Episodes 16 and 17 were separate episodes in the same patient.
Abbreviations: C, central; F, frontal; Fluct, fluctuation; O, occipital; P, parietal; PDs, periodic discharges; RDA, rhythmic delta activity; SCP, subtle clinical phenomena; STE, spatiotemporal evolution; T, temporal.
RDA was identified in 13 episodes including lateralized RDA in 10, bilateral independent RDA in one and generalized RDA in two. The laterality of RDA was bilateral in five episodes, left in four and right in four. The locations of RDA consisted of regional in five episodes, multifocal in three, hemispheric in three and focal and generalized in one each. The frequency of RDA ranged from 1 to 4 Hz.
EEG seizures were detected in nine episodes. Seven episodes showed spatiotemporal evolutions. The other two episodes showed no spatial evolution. Brief rhythmic epileptiform discharges were detected in 17 episodes. The laterality of brief rhythmic epileptiform discharges was right in eight episodes, left in seven and bilateral in two. The locations of brief rhythmic epileptiform discharges were focal in eight episodes, regional in seven and hemispheric in two. The inter-rater agreement was 1.0 for PDs, 0.93 for RDA, 1.0 for EEG seizures and 0.93 for brief rhythmic epileptiform discharges, indicating almost perfect agreement in all categories.
Non-convulsive status epilepticus
The diagnoses of NCSE are described in Table 2. Based on the EEG findings and subtle clinical phenomena under Salzburg criteria, we diagnosed NCSE in 19 episodes, possible NCSE in 2 and no NCSE in 7. Nine episodes showed epileptiform discharges with the frequency of >25 times per 10 s epoch (Salzburg criteria 1). Eight episodes showed subtle clinical ictal phenomena during the EEG (Salzburg criteria 2b). Two episodes showed typical spatiotemporal evolution of the epileptiform discharges with the frequency of ≤2.5 cycles per second (Salzburg criteria 2c). Two episodes showed fluctuation of PDs or RDA without definitive evolution (Salzburg criteria 2e). These 21 episodes were considered as a positive diagnosis of NCSE. The other seven episodes did not fulfil the criteria of NCSE (Salzburg criteria 3). No significant differences were found between the NCSE and the possible NCSE groups and no NCSE group in patients’ age, GCS at suspected NCSE, times of ASL or EEG starting from suspected NCSE, time differences between ASL and EEG and EEG recording periods (Supplementary Table 2).
Arterial spin labelling
The ASL findings are described in Table 2. Magnetic resonance angiography confirmed the absence of main artery stenosis or occlusion affecting CBF in all 27 patients in this study.
Thalamic hyperperfusion was identified in 16 (57%) of 28 episodes. The laterality of thalamic hyperperfusion was left in seven episodes, right in four and bilateral in five. Mean CBF of thalamic hyperperfusion was 70.1 ml/100 g/min in the lesion side and 70.5 ml/100 g/min in the non-lesion side (Supplementary Table 3).
Cerebral cortical hyperperfusion was identified in 24 (86%) of 28 episodes. The laterality of cerebral cortical hyperperfusion was left in 12 episodes, right in 8 and bilateral in 4. The locations of cerebral cortical hyperperfusion were focal in nine episodes, regional in seven, hemispheric in five and multifocal in three. Mean CBF of cerebral cortical hyperperfusion ranged from 81.9 to 85.9 ml/100 g/min in the lesion side and from 68.8 to 82.2 ml/100 g/min in the non-lesion side (Supplementary Table 3).
Comparison between ASL, and NCSE and EEG findings
The results of statistical analyses between thalamic hyperperfusion and NCSE and EEG findings are described in Table 3. Thalamic hyperperfusion was detected in 16 (76%) of 21 episodes with NCSE including two episodes with possible NCSE. Thalamic hyperperfusion was significantly associated with NCSE (P = 0.0007). The sensitivity of thalamic hyperperfusion for NCSE was 76.2% and the specificity was 100.0%. Thalamic hyperperfusion was detected in 14 (93%) of 15 episodes with PDs. Thalamic hyperperfusion was significantly associated with PDs consisting of lateralized and bilateral independent PDs (P < 0.0001; odds ratio, 77.0; 95% CI, 6.2–963.7). The sensitivity of thalamic hyperperfusion for PDs was 93.3% and the specificity was 84.6%. Thalamic hyperperfusion was detected in 12 (92%) of 13 episodes with RDA. Thalamic hyperperfusion was significantly associated with RDA (P = 0.0006; odds ratio, 33.0; 95% CI, 3.2–342.3). The sensitivity of thalamic hyperperfusion for RDA was 92.3% and the specificity was 73.3%. In the subcategory of RDA, thalamic hyperperfusion was significantly associated with lateralized and bilateral independent RDA (P = 0.0002). The sensitivity of thalamic hyperperfusion for lateralized and bilateral independent RDA was 100.0% and the specificity was 70.6%. There was no significant association between thalamic hyperperfusion, and generalized RDA, EEG seizures and brief rhythmic epileptiform discharges (Table 3).
Table 3.
Association between thalamic hyperperfusion and NCSE and EEG findings
| N | Thalamic hyperperfusion, N (%) |
P value | Odds ratio (95% CI) | Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| NCSE | 21 | 16 (76) | 5 (24) | 0.0007 | N/A | 76.2 | 100.0 |
| EEG findings | |||||||
| PDs (lateralized and bilateral independent)a | 15 | 14 (93) | 1 (7) | <0.0001 | 77.0 (6.2–963.7) | 93.3 | 84.6 |
| RDA | 13 | 12 (92) | 1 (8) | 0.0006 | 33.0 (3.2–342.3) | 92.3 | 73.3 |
| Lateralized and bilateral independent | 11 | 11 (100) | 0 (0) | 0.0002 | N/A | 100.0 | 70.6 |
| Generalized | 2 | 1 (50) | 1 (50) | 0.8254 | 0.7 (0.0–13.0) | 50.0 | 42.3 |
| EEG seizures | 9 | 5 (56) | 4 (44) | 0.7014 | 0.9 (0.2–4.5) | 55.6 | 42.1 |
| Brief rhythmic epileptiform discharges | 17 | 11 (65) | 6 (35) | 0.2693 | 2.2 (0.5–10.4) | 64.7 | 54.5 |
Generalized PDs were not found in any episodes.
Abbreviations: CI, confidence interval; N/A, not applicable.
The results of statistical analyses between cerebral cortical hyperperfusion and NCSE and EEG findings are described in Table 4. Cerebral cortical hyperperfusion was detected in all 21 episodes with NCSE including two episodes with possible NCSE. Cerebral cortical hyperperfusion was significantly associated with NCSE (P = 0.0017). The sensitivity of cerebral cortical hyperperfusion for NCSE was 100.0% and the specificity was 57.1%. Cerebral cortical hyperperfusion was detected in all 15 episodes with PDs. Cerebral cortical hyperperfusion was significantly associated with PDs consisting of lateralized and bilateral independent PDs (P = 0.0349). The sensitivity of cerebral cortical hyperperfusion for PDs was 100.0% and the specificity was 30.8%. There was no significant association between cerebral cortical hyperperfusion, and RDA, EEG seizures and brief rhythmic epileptiform discharges (Table 4).
Table 4.
Association between cerebral cortical hyperperfusion and NCSE and EEG findings
| N | Cerebral cortical hyperperfusion, N (%) |
P value | Odds ratio (95% CI) | Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| NCSE | 21 | 21 (100) | 0 (0) | 0.0017 | N/A | 100.0 | 57.1 |
| EEG findings | |||||||
| PDs (lateralized and bilateral independent)a | 15 | 15 (100) | 0 (0) | 0.0349 | N/A | 100.0 | 30.8 |
| RDA | 13 | 12 (92) | 1 (8) | 0.3556 | 3.0 (0.3–33.1) | 92.3 | 20.0 |
| Lateralized and bilateral independent | 11 | 11 (100) | 0 (0) | 0.1162 | N/A | 100.0 | 23.5 |
| Generalized | 2 | 1 (50) | 1 (50) | 0.9841 | 0.1 (0.0–2.7) | 50.0 | 11.5 |
| EEG seizures | 9 | 9 (100) | 0 (0) | 0.1893 | N/A | 100.0 | 21.1 |
| Brief rhythmic epileptiform discharges | 17 | 16 (94) | 1 (6) | 0.1531 | 6.0 (0.5–67.3) | 94.1 | 27.3 |
Generalized PDs were not found in any episodes.
Abbreviations: CI, confidence interval; N/A, not applicable.
Comparisons of the receiver operating characteristic curves between thalamic and cerebral cortical hyperperfusion for NCSE, PDs and RDA are shown in Fig. 1. For NCSE, the AUC of thalamic hyperperfusion was 0.8810 and AUC of cerebral cortical hyperperfusion was 0.7857, with no difference between thalamic and cerebral cortical hyperperfusion (0.0952, P = 0.3938) (Fig. 1A). For PDs, the AUC of thalamic hyperperfusion was 0.8897 and AUC of cerebral cortical hyperperfusion was 0.6538, with a significant difference between thalamic and cerebral cortical hyperperfusion (0.2359, P = 0.0029; 95% CI, 0.08–0.39). Thalamic hyperperfusion showed significantly higher association with PDs than cerebral cortical hyperperfusion (Fig. 1B). For RDA, the AUC of thalamic hyperperfusion was 0.8282 and AUC of cerebral cortical hyperperfusion was 0.5615, with a significant difference between thalamic and cerebral cortical hyperperfusion (0.2667, P < 0.0001; 95% CI, 0.14–0.40). Thalamic hyperperfusion showed significantly higher association with RDA than cerebral cortical hyperperfusion (Fig. 1C).
Figure 1.
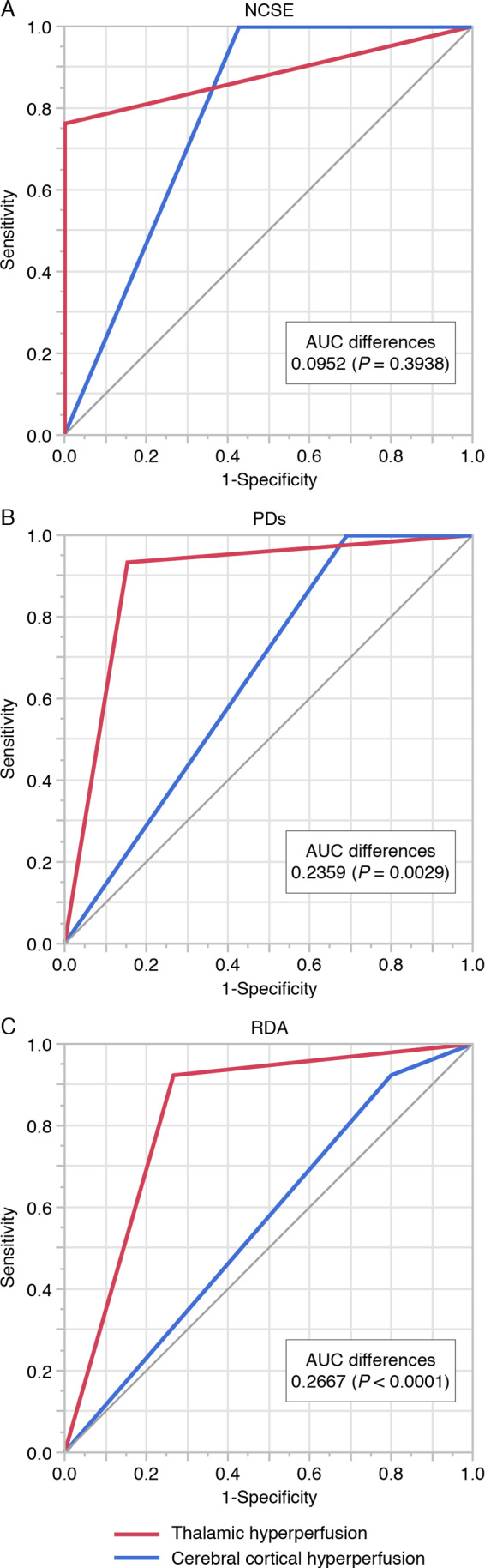
Comparisons of receiver operating characteristic curves between thalamic and cerebral cortical hyperperfusion for NCSE, PDs and RDA. (A) Receiver operating characteristic curves of thalamic (red line) and cerebral cortical hyperperfusion (blue line) for NCSE. Area under the curve (AUC) of thalamic hyperperfusion was 0.8810 and AUC of cerebral cortical hyperperfusion was 0.7857, with no difference for NCSE between thalamic and cerebral cortical hyperperfusion (0.0952, P = 0.3938). (B) Receiver operating characteristic curves of thalamic (red line) and cerebral cortical hyperperfusion (blue line) for PDs. AUC of thalamic hyperperfusion was 0.8897 and AUC of cerebral cortical hyperperfusion was 0.6538, with a significant difference between thalamic and cerebral cortical hyperperfusion (0.2359, P = 0.0029). (C) Receiver operating characteristic curves of thalamic (red line) and cerebral cortical hyperperfusion (blue line) for RDA. AUC of thalamic hyperperfusion was 0.8282 and AUC of cerebral cortical hyperperfusion was 0.5615, with a significant difference between thalamic and cerebral cortical hyperperfusion (0.2667, P < 0.0001).
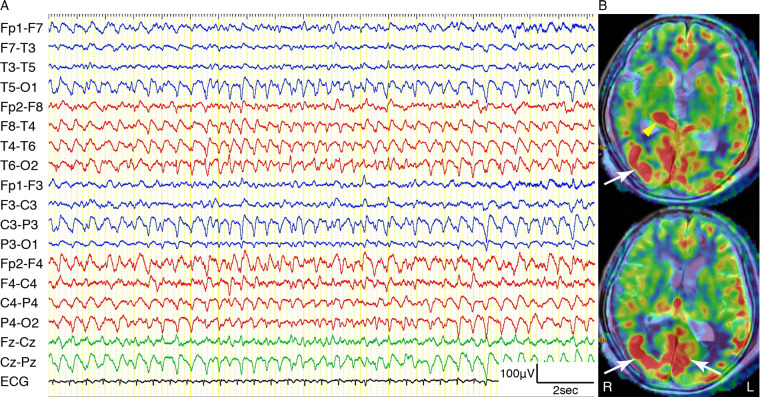
Illustrative case: Episode 3
An 87-year-old female with a traumatic brain injury was diagnosed with NCSE. She was hit by an automobile at an intersection. Initial brain CT showed thin right temporo-occipital acute subdural haematoma. She presented with generalized convulsion 9 days later. The convulsive seizures were controlled by fosphenytoin sodium and levetiracetam, but her consciousness with GCS 5 had not recovered 24 h later. EEG showed bilateral 1.5–2 Hz PDs and RDA with right hemispheric predominance (Fig. 2A). Her eyes deviated to the left during the periods of PDs and RDA. ASL showed right thalamic and bilateral occipital cortical hyperperfusion (Fig. 2B). After additional administration of propofol, her consciousness recovered to GCS 14.
Figure 2.
Example of thalamic and cerebral cortical hyperperfusion on ASL in an 87-year-old female with NCSE (Episode 3). (A) EEG recording on a longitudinal bipolar montage showing bilateral 1.5–2 Hz continuous RDA with right hemispheric predominance. (B) ASL superimposed onto the T2-weighted MRI showing right thalamic hyperperfusion (yellow arrowhead) and bilateral occipital cortical hyperperfusion (white arrows).
Illustrative case: Episode 6
An 81-year-old female with subdural haematoma was diagnosed with NCSE. She was admitted with complaints of right paresis and motor aphasia without impaired consciousness with GCS 12. Initial brain CT showed left hemispheric idiopathic acute subdural haematoma due to blood coagulation disorder. Her consciousness deteriorated to GCS 9 2 days later. Continuous EEG showed 1 Hz lateralized PDs over the left hemisphere (Fig. 3A) as well as EEG seizure pattern over the left temporo-parietal regions. ASL showed left thalamic and left hemispheric cortical hyperperfusion (Fig. 3B). After administration of levetiracetam and midazolam, her consciousness recovered to GCS 14 with resolving right paresis and motor aphasia.
Figure 3.
Example of thalamic and cerebral cortical hyperperfusion on ASL in an 81-year-old female with NCSE (Episode 6). (A) EEG recording on a longitudinal bipolar montage showing 1 Hz lateralized PDs over the left hemisphere. (B) ASL superimposed onto the T2-weighted MRI showing left thalamic hyperperfusion (yellow arrowhead) and left hemispheric cortical hyperperfusion (white arrows).
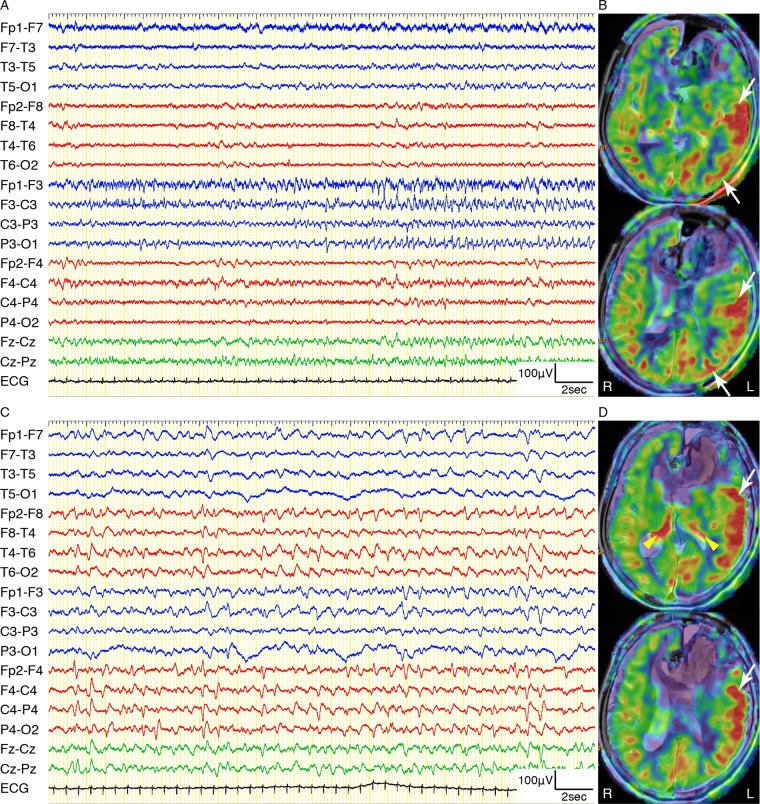
Illustrative case: Episodes 16 and 17
An 80-year-old female with subarachnoid haemorrhage was twice diagnosed with NCSE during separate periods. She was admitted with complaints of headache, right paresis, aphasia and moderately impaired consciousness with GCS 10. Initial brain CT showed subarachnoid haemorrhage with left frontal subcortical haematoma due to a ruptured aneurysm of the left anterior cerebral artery. We immediately performed clipping of the aneurysm and removal of the haematoma. Her consciousness deteriorated to GCS 5 with eye deviation to the right over the next 3 days. Continuous EEG showed several EEG seizure patterns from the left frontal to left temporo-parietal regions (Fig. 4A). ASL showed left hemispheric cortical hyperperfusion (Fig. 4B). After administration of levetiracetam and midazolam, her consciousness improved to GCS 9. However, her consciousness deteriorated to GCS 7 with eye deviation to the right again after 13 days. Subsequent continuous EEG showed bilateral 1 Hz PDs and 1–2 Hz RDA with right hemispheric predominance (Fig. 4C). Repeat ASL showed bilateral thalamic hyperperfusion and left fronto-temporal cortical hyperperfusion (Fig. 4D). After additional administration of lacosamide, her consciousness recovered to GCS 10.
Figure 4.
Example of thalamic and cerebral cortical hyperperfusion on ASL in an 80-year-old female with separate two NCSE episodes (Episodes 16 and 17). (A, B) Episode 16; (C, D) Episode 17. (A) EEG recording on a longitudinal bipolar montage showing EEG seizure patterns with apparent spatiotemporal evolution from the left frontal to left temporo-parietal regions. (B) ASL superimposed onto the T2-weighted MRI showing left hemispheric cortical hyperperfusion (white arrows) without thalamic hyperperfusion. (C) EEG recording on a longitudinal bipolar montage showing bilateral 1 Hz PDs with right hemispheric predominance. (D) ASL superimposed onto the T2-weighted MRI showing bilateral thalamic hyperperfusion (yellow arrowheads) and left fronto-temporal cortical hyperperfusion (white arrows).
Discussion
Summary of findings
The thalamic hyperperfusion demonstrated by ASL was significantly associated with NCSE, PDs and RDA on scalp EEG with high sensitivity and specificity in critically ill patients who presented with impaired awareness without convulsions. The cerebral cortical hyperperfusion demonstrated by ASL was significantly associated with NCSE and PDs with high sensitivity but lower specificity than the thalamic hyperperfusion. The cerebral cortical hyperperfusion demonstrated by ASL showed no association with RDA. ASL hyperperfusion over both the thalamus and the cerebral cortex was associated with NCSE without significant difference. The PDs and RDA on EEG were significantly more associated with thalamic hyperperfusion than cerebral cortical hyperperfusion.
Thalamic hyperperfusion in NCSE involved with impaired consciousness
We hypothesized that thalamic hyperperfusion in NCSE indicated hyperexcitability of the thalamocortical network and was associated with impaired consciousness. The degree of thalamocortical synchrony by stereotactic EEG is correlated with loss of consciousness (Guye et al., 2006). Secondary involvement of the thalamus during focal impaired awareness seizures may play a major role in impaired consciousness in temporal lobe epilepsy (Blumenfeld et al., 2004). Excessive synchronization of the thalamocortical networks by stereotactic EEG is correlated with the degree of impaired consciousness (Arthuis et al., 2009). Thalamic hyperperfusion was demonstrated during focal impaired awareness seizures by SPECT, suggesting that thalamic hyperactivity was associated with impaired consciousness during seizures (Lee et al., 2002; Blumenfeld et al., 2004).
Altered level of consciousness during focal seizures requires hypersynchrony between the thalamus and the association cortices (Bartolomei et al., 2014). Among the thalamic nuclei, the medial pulvinar nucleus functionally connects with the association cortex of the temporoparietal junction, frontoparietal opercular cortex, insula and both lateral and mesial temporal regions (Rosenberg et al., 2009). Perfusion MRI (Szabo et al., 2005) and SPECT studies (Lee et al., 2002) in patients with focal impaired awareness seizures disclosed hyperperfusion of the pulvinar nucleus during the sequelae of prolonged ictal brain activity. Regions of thalamic hyperperfusion were observed to some extent and contained multiple nuclei including the pulvinar of the thalamus. Hyperexcitability of the reciprocal connectivity between the thalamus and the cerebral cortex might elicit the impaired consciousness of NCSE in critically ill patients.
Anatomical studies in monkeys demonstrated that the reciprocal connections of the medial pulvinar nucleus reached all cerebral lobes (Romanski et al., 1997). Thalamic connectivity in the human brain corresponds to the findings from monkeys (Rosenberg et al., 2009). DWI hyperintensities were found in both the cerebral cortex and the thalamus in patients with status epilepticus (Lansberg et al., 1999; Szabo et al., 2005; Toledo et al., 2008; Huang et al., 2009; Katramados et al., 2009; Chatzikonstantinou et al., 2011; Ohe et al., 2014; Nakae et al., 2016; Rennebaum et al., 2016; Jabeen et al., 2017; Giovannini et al., 2018). Among 225 patients with seizures, 17 patients exhibited DWI abnormalities of the pulvinar corresponding to status epilepticus (Ohe et al., 2014). Such DWI abnormalities of pulvinar can be expected to cause hyperexcitability of the thalamus connecting various areas of cerebral cortex. The medial pulvinar nucleus is mainly connected with the association cortices of the temporal and parietal lobes and to a lesser degree with those of the frontal and occipital lobes (Rosenberg et al., 2009).
Thalamic hyperperfusion associated with PDs and RDA
Thalamic hyperperfusion on ASL was significantly associated with PDs and RDA, especially lateralized PDs and lateralized RDA during NCSE. Thalamic hyperexcitability could modulate PDs and/or RDA associated with NCSE. The PDs and RDA often occurred without apparent EEG seizures during NCSE, and were often indistinguishable from EEG seizures as well. The Salzburg criteria are the breakthrough for the diagnosis of NCSE by combining the frequency of epileptiform discharges and clinical symptoms to differentiate NCSE, possible NCSE and no NCSE (Trinka and Leitinger, 2015; Leitinger et al., 2016). The PDs and RDA consist of epileptiform discharges with the frequency of ≤2.5 cycles per second without evolution. The diagnosis of NCSE using the Salzburg criteria has been confirmed in 220 cases (Leitinger et al., 2016). This study has now demonstrated the ASL findings associated with NCSE, PDs and RDA under the Salzburg criteria.
Lateralized PDs were formerly called periodic lateralized epileptiform discharges (PLEDs). However, lateralized PDs are not consistently associated with epileptiform discharges. Therefore, the American Clinical Neurophysiology Society standardized EEG terminology discarded the ‘E’ of epileptiform discharges (Hirsch et al., 2013). PLEDs are associated with both cortical lesions, and subcortical grey- and white-matter lesions in diffuse encephalopathies (Gloor et al., 1968). Acute lesions may be the most common structural abnormalities seen in PLEDs, whereas chronic lesions, subcortical lesions and non-lesions are not uncommon (Kalamangalam et al., 2007). Thalamic hyperintensities on DWI were associated with PLEDs in various cases of status epilepticus (Huang et al., 2009; Rennebaum et al., 2016). Other studies of DWI showed thalamic hyperintensities associating with seizure propagations other than PLEDs (Katramados et al., 2009; Giovannini et al., 2018). The thalamus is involved in the propagation and synchronization of epileptic discharges on EEG (Szabo et al., 2005; Guye et al., 2006; Rosenberg et al., 2006). PLEDs represent an abnormal response of both cortical and thalamocortical neurons to the rhythmic burst firing generated by the reticular nucleus of the thalamus (Gross et al., 1998). Consequently, the thalamus could modulate lateralized PDs with ictal nature.
Our results indicated that thalamic hyperperfusion is associated with lateralized RDA in addition to lateralized PDs. Lateralized RDA in critically ill patients is indicated to have similar clinical significance to lateralized PDs (Gaspard et al., 2013). Lateralized RDA reflects the presence of a focal lesion and is associated with a high risk of acute non-convulsive seizures (Gaspard et al., 2013). Lateralized RDA in critically ill patients is associated with seizures if the frequency is 1.5 Hz or higher (Rodriguez Ruiz et al., 2017). The study of cat models demonstrated that unilateral diffuse polymorphic delta activity appears on the side of thalamic or hypothalamic lesions (Gloor et al., 1977), whereas the reticular thalamic nucleus can generate synchronous delta oscillations (Steriade et al., 1993). Our results indicated that lateralized RDA with 1.5 Hz or higher frequency mainly dominated in episodes with thalamic hyperperfusion. We speculated that thalamus is involved in modulating the rhythmicity of delta activity in addition to PDs in critically ill patients with NCSE.
Cerebral cortical hyperperfusion associated with NCSE
The cerebral cortical hyperperfusion on ASL was associated with all NCSE episodes in our series, and was also associated with PDs during NCSE. However, cortical hyperperfusion was not significantly associated with RDA. Regional cortical hyperperfusion on SPECT was associated with PLEDs representing a form of focal status epilepticus (Assal et al., 2001). Cerebral cortical hyperintensities on DWI were associated with PLEDs in patients with focal seizures (Narayanan, 2016).
The sensitivities of both thalamic and cerebral cortical hyperperfusion for NCSE were high, but the specificity of cerebral cortical hyperperfusion for NCSE was low. Cortical hyperperfusion was demonstrated in all 15 patients with NCSE (Shimogawa et al., 2017), whereas cortical hyperperfusion has been widely detected on ASL during seizure-related events including ictal or peri-ictal periods other than NCSE (Pizzini et al., 2013; Matsuura et al., 2015; Yoo et al., 2017). The sensitivity and specificity of cortical hyperperfusion on ASL to identify the seizure focus were 74 and 0%, respectively (Kim et al., 2016). In our results, cerebral cortical hyperperfusion was not associated with RDA, whereas thalamic hyperperfusion was associated with lateralized PDs and lateralized RDA. We speculated that cerebral cortical hyperperfusion in critically ill patients reflects cortical functional abnormality. Thalamic hyperperfusion might indicate the hyperexcitability of thalamocortical networks due to specifically recognized lateralized PDs and lateralized RDA during NCSE. Such cortical dysfunction might be modulated by the thalamus.
Thalamocortical hyperperfusion as a new biomarker of NCSE
PLEDs represent an EEG signature of a dynamic pathophysiological state involving unstable neurobiological processes described as the ‘ictal–interictal continuum’ applied for uncertain EEG falling between ictal EEG features with evolution and interictal periodic/rhythmic activities without evolution (Pohlmann-Eden et al., 1996). Since continuous EEG monitoring has been established in the intensive care unit for patients with suspected NCSE, an indistinguishable pattern is often observed between EEG seizures, and PDs and RDA. Lateralized PDs in critically ill patients are highly associated with clinical seizures regardless of the frequency of epileptiform discharges (Rodriguez Ruiz et al., 2017). The concept of ictal–interictal continuum was naturally accepted for such ambiguous EEG findings (Pohlmann-Eden et al., 1996). The spectrum of PDs together with ictal–interictal continuum is well known to associate with impaired awareness seizures and altered mental status with or without seizures (Chong and Hirsch, 2005).
Delayed diagnosis and treatment of NCSE lead to poor outcomes (Young et al., 1996). Prolonged video EEG monitoring is essential for the diagnosis of NCSE since the clinical signs are very subtle (Claassen et al., 2004). The cohort study of 2111 participants with continuous EEG showed that any highly epileptiform discharges during the first hour of EEG (i.e. a 2HELPS2B score of ≥2) indicated that at least 24 h of recording was recommended to detect seizures (Struck et al., 2020). In 121 critically ill children, the first non-convulsive seizures were all captured within 24 h in 28 non-convulsive seizures (McCoy et al., 2011). In contrast, the acquisition time of ASL in MRI requires only about 3 min. Initial use of ASL could promote the early diagnosis of NCSE (Shimogawa et al., 2017). The prompt application of ASL for critically ill patients may be valuable to establish a diagnosis of NCSE.
No evidence-based guidelines have been established for the treatment of ictal–interictal continuum patterns in critically ill patients. If the spectrum of ictal–interictal continuum together with PDs and RDA is one of the diagnoses of NCSE, aggressive treatment would be required. Lateralized PDs regardless of frequencies and lateralized RDA with high frequencies are associated with seizures (Rodriguez Ruiz et al., 2017 ). Brief potentially ictal rhythmic discharges may indicate ictal–interictal continuum for consideration of the treatment of seizures (Yoo et al., 2014; Struck et al., 2020). One of the ictal EEG criteria must persist for longer than 10 s under the Salzburg criteria (Leitinger et al., 2016). We can often identify brief periodic or rhythmic epileptiform discharges shorter than 10 s with and without evolution in critically ill patients. Thalamic hyperperfusion on ASL was most sensitive to PDs and RDA. Single brief potentially ictal rhythmic discharges might be shorter for ASL. If ASL demonstrates cerebral cortical hyperperfusion during the period of frequent brief potentially ictal rhythmic discharges, impaired awareness may associate with micro-seizures in NCSE.
The mean regional CBF of the hyperperfusion area was about 70 ml/100 g/min in the thalamus and 80 ml/100 g/min in the cerebral cortex. Magnetic resonance angiography confirmed the absence of main artery stenosis or occlusion in all patients in this study. The regional CBF of the deep brain structures on ASL is not influenced by the anatomic variant types of the circle of Willis (Hendrikse et al., 2010). Previous ASL study has indicated resting CBF in normal aging subjects (>60 years) is 45.1 ± 9.4 ml/100 g/min in the thalamus and 42.7 ± 8.8 ml/100 g/min in the cortex on average (Chen et al., 2011). Our reference data in the thalamus (non-lesion side) were 48.5 ± 6.4 ml/100 g/min, which was in the same range as the normal perfusion values in a similar age group (Supplementary Table 1). Our reference data in the cerebral cortex (non-lesion side) were ranged from 47.1 ± 7.2 to 51.7 ± 7.9 ml/100 g/min (Supplementary Table 1). Because the control subjects were matched with the patients according to brain disease, the values of CBF in the cerebral cortex may have slightly increased compared to the normal perfusion values shown in the previous study (Chen et al., 2011).
Limitations
This retrospective study showed no consistent time course of ASL and EEG for patients with impaired consciousness. We used the shortest interval possible between ASL and EEG. Early or delayed studies may impact the associations between thalamic hyperperfusion, and PDs and RDA. However, medications and treatments were not started before both ASL and EEG.
The inconsistent duration of EEG monitoring might impact some of the EEG findings. Continuous EEG monitoring was not routinely performed at the beginning of the study. For consistency of EEG findings, we selected suspected NCSE cases with more than 1 h EEG recording.
The various characteristics examined in this study, such as hyperperfusion, NCSE and EEG findings, were evaluated at discrete times. Evaluation of these characteristics as continuous variables may identify correlations between thalamic and cerebral cortical hyperperfusion and NCSE, PDs and RDA.
Conclusion
ASL findings of hyperperfusion in both the thalamus and the cerebral cortex may be a new biomarker of NCSE according to the Salzburg criteria in critically ill patients with impaired consciousness level without convulsions. Thalamic hyperexcitability might modulate the PDs and RDA associated with NCSE. The prompt application of ASL and evidence of thalamocortical abnormalities in critically ill patients may be valuable to identify and treat NCSE.
Supplementary Material
Acknowledgements
Hiroshi Otsubo is a member of Japanese Science and Technology, CREST (JPMJCR 1784). The authors thank Dr Jia Wenting, Dr Takashi Sasaki and Dr Youhei Takeuchi as well as the EEG technologists, the radiological technologists and nursing staff of South Miyagi Medical Center for their assistance in this work.
Supplementary material
Supplementary material is available at Brain Communications online.
Competing interests
The authors report no competing interests.
Glossary
- ASL =
arterial spin labelling
- AUC =
area under the curve
- CBF =
cerebral blood flow
- CI =
confidence interval
- DWI =
diffusion-weighted imaging
- GCS =
Glasgow Coma Scale
- NCSE =
non-convulsive status epilepticus
- PD =
periodic discharge
- PLED =
periodic lateralized epileptiform discharge
- RDA =
rhythmic delta activity
- ROI =
region of interest
- SPECT =
single photon emission computed tomography
- TE =
echo time
- TR =
repetition time
Contributor Information
Satoru Ohtomo, Department of Neurosurgery, South Miyagi Medical Center, Shibata-gun, Miyagi, Japan; Department of Neurosurgery, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan; Department of Epileptology, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan.
Hiroshi Otsubo, Division of Neurology, The Hospital for Sick Children, Toronto, Ontario, Canada; Department of Pediatrics, University of Toronto, Toronto, Ontario, Canada.
Hiroaki Arai, Department of Neurosurgery, South Miyagi Medical Center, Shibata-gun, Miyagi, Japan.
Yoshiteru Shimoda, Department of Neurosurgery, South Miyagi Medical Center, Shibata-gun, Miyagi, Japan; Department of Neurosurgery, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan.
Yoichiro Homma, Department of General Internal Medicine, Seirei-Hamamatsu General Hospital, Hamamatsu, Shizuoka, Japan.
Teiji Tominaga, Department of Neurosurgery, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan.
References
- Arthuis M, Valton L, Régis J, Chauvel P, Wendling F, Naccache L, et al. Impaired consciousness during temporal lobe seizures is related to increased long-distance cortical-subcortical synchronization. Brain 2009; 132: 2091–101. [DOI] [PubMed] [Google Scholar]
- Assal F, Papazyan JP, Slosman DO, Jallon P, Goerres GW. SPECT in periodic lateralized epileptiform discharges (PLEDs): a form of partial status epilepticus? Seizure 2001; 10: 260–5. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, McGonigal A, Naccache L. Alteration of consciousness in focal epilepsy: the global workspace alteration theory. Epilepsy Behav 2014; 30: 17–23. [DOI] [PubMed] [Google Scholar]
- Beniczky S, Hirsch LJ, Kaplan PW, Pressler R, Bauer G, Aurlien H, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia 2013; 54 (Suppl 6): 28–9. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex 2004; 14: 892–902. [DOI] [PubMed] [Google Scholar]
- Chatzikonstantinou A, Gass A, Förster A, Hennerici MG, Szabo K. Features of acute DWI abnormalities related to status epilepticus. Epilepsy Res 2011; 97: 45–51. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage 2011; 55: 468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol 2005; 22: 79–91. [DOI] [PubMed] [Google Scholar]
- Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004; 62: 1743–8. [DOI] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med 1992; 23: 37–45. [DOI] [PubMed] [Google Scholar]
- Gaspard N, Manganas L, Rampal N, Petroff OA, Hirsch LJ. Similarity of lateralized rhythmic delta activity to periodic lateralized epileptiform discharges in critically ill patients. JAMA Neurol 2013; 70: 1288–95. [DOI] [PubMed] [Google Scholar]
- Giovannini G, Kuchukhidze G, McCoy MR, Meletti S, Trinka E. Neuroimaging alterations related to status epilepticus in an adult population: definition of MRI findings and clinical-EEG correlation. Epilepsia 2018; 59 (Suppl 2): 120–7. [DOI] [PubMed] [Google Scholar]
- Gloor P, Ball G, Schaul N. Brain lesions that produce delta waves in the EEG. Neurology 1977; 27: 326–33. [DOI] [PubMed] [Google Scholar]
- Gloor P, Kalabay O, Giard N. The electroencephalogram in diffuse encephalopathies: electroencephalographic correlates of grey and white matter lesions. Brain 1968; 91: 779–802. [Google Scholar]
- Gross DW, Quesney LF, Sadikot AF. Chronic periodic lateralized epileptiform discharges during sleep in a patient with caudate nucleus atrophy: insights into the anatomical circuitry of PLEDs. Electroencephalogr Clin Neurophysiol 1998; 107: 434–8. [DOI] [PubMed] [Google Scholar]
- Guye M, Régis J, Tamura M, Wendling F, McGonigal A, Chauvel P, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain 2006; 129: 1917–28. [DOI] [PubMed] [Google Scholar]
- Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol 2008; 61: 29–48. [DOI] [PubMed] [Google Scholar]
- Hendrikse J, Petersen ET, Chng SM, Venketasubramanian N, Golay X. Distribution of cerebral blood flow in the nucleus caudatus, nucleus lentiformis, and thalamus: a study of territorial arterial spin-labeling MR imaging. Radiology 2010; 254: 867–75. [DOI] [PubMed] [Google Scholar]
- Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 2013; 30: 1–27. [DOI] [PubMed] [Google Scholar]
- Huang YC, Weng HH, Tsai YT, Huang YC, Hsiao MC, Wu CY, et al. Periictal magnetic resonance imaging in status epilepticus. Epilepsy Res 2009; 86: 72–81. [DOI] [PubMed] [Google Scholar]
- Jabeen SA, Cherukuri P, Mridula R, Harshavardhana KR, Gaddamanugu P, Sarva S, et al. A prospective study of diffusion weighted magnetic resonance imaging abnormalities in patients with cluster of seizures and status epilepticus. Clin Neurol Neurosurg 2017; 155: 70–4. [DOI] [PubMed] [Google Scholar]
- Kalamangalam GP, Diehl B, Burgess RC. Neuroimaging and neurophysiology of periodic lateralized epileptiform discharges: observations and hypotheses. Epilepsia 2007; 48: 1396–405. [DOI] [PubMed] [Google Scholar]
- Kaplan PW. EEG criteria for nonconvulsive status epilepticus. Epilepsia 2007; 48 (Suppl 8): 39–41. [DOI] [PubMed] [Google Scholar]
- Katramados AM, Burdette D, Patel SC, Schultz LR, Gaddam S, Mitsias PD. Periictal diffusion abnormalities of the thalamus in partial status epilepticus. Epilepsia 2009; 50: 265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Lee ST, Yun TJ, Lee SK, Paeng JC, Jun J, et al. Capability of arterial spin labeling MR imaging in localizing seizure focus in clinical seizure activity. Eur J Radiol 2016; 85: 1295–303. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. [PubMed] [Google Scholar]
- Lansberg MG, O’Brien MW, Norbash AM, Moseley ME, Morrell M, Albers GW. MRI abnormalities associated with partial status epilepticus. Neurology 1999; 52: 1021–7. [DOI] [PubMed] [Google Scholar]
- Lee KH, Meador KJ, Park YD, King DW, Murro AM, Pillai JJ, et al. Pathophysiology of altered consciousness during seizures: subtraction SPECT study. Neurology 2002; 59: 841–6. [DOI] [PubMed] [Google Scholar]
- Leitinger M, Beniczky S, Rohracher A, Gardella E, Kalss G, Qerama E, et al. Salzburg consensus criteria for non-convulsive status epilepticus—approach to clinical application. Epilepsy Behav 2015b; 49: 158–63. [DOI] [PubMed] [Google Scholar]
- Leitinger M, Kalss G, Rohracher A, Pilz G, Novak H, Höfler J, et al. Predicting outcome of status epilepticus. Epilepsy Behav 2015a; 49: 126–30. [DOI] [PubMed] [Google Scholar]
- Leitinger M, Trinka E, Gardella E, Rohracher A, Kalss G, Qerama E, et al. Diagnostic accuracy of the Salzburg EEG criteria for non-convulsive status epilepticus: a retrospective study. Lancet Neurol 2016; 15: 1054–62. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Maeda M, Okamoto K, Araki T, Miura Y, Hamada K, et al. Usefulness of arterial spin-labeling images in periictal state diagnosis of epilepsy. J Neurol Sci 2015; 359: 424–9. [DOI] [PubMed] [Google Scholar]
- McCoy B, Sharma R, Ochi A, Go C, Otsubo H, Hutchison JS, et al. Predictors of nonconvulsive seizures among critically ill children. Epilepsia 2011; 52: 1973–8. [DOI] [PubMed] [Google Scholar]
- Nakae Y, Kudo Y, Yamamoto R, Dobashi Y, Kawabata Y, Ikeda S, et al. Relationship between cortex and pulvinar abnormalities on diffusion-weighted imaging in status epilepticus. J Neurol 2016; 263: 127–32. [DOI] [PubMed] [Google Scholar]
- Narayanan J. Can diffusion-weighted imaging be used as a tool to predict seizures in patients with PLEDS? Epileptic Disord 2016; 18: 440–6. [DOI] [PubMed] [Google Scholar]
- Ohe Y, Hayashi T, Deguchi I, Fukuoka T, Horiuchi Y, Maruyama H, et al. MRI abnormality of the pulvinar in patients with status epilepticus. J Neuroradiol 2014; 41: 220–6. [DOI] [PubMed] [Google Scholar]
- Oishi M, Ishida G, Morii K, Hasegawa K, Sato M, Fujii Y. Ictal focal hyperperfusion demonstrated by arterial spin-labeling perfusion MRI in partial epilepsy status. Neuroradiology 2012; 54: 653–6. [DOI] [PubMed] [Google Scholar]
- Pizzini FB, Farace P, Manganotti P, Zoccatelli G, Bongiovanni LG, Golay X, et al. Cerebral perfusion alterations in epileptic patients during peri-ictal and post-ictal phase: PASL vs DSC-MRI. Magn Reson Imaging 2013; 31: 1001–5. [DOI] [PubMed] [Google Scholar]
- Pohlmann-Eden B, Hoch DB, Cochius JI, Chiappa KH. Periodic lateralized epileptiform discharges—a critical review. J Clin Neurophysiol 1996; 13: 519–30. [DOI] [PubMed] [Google Scholar]
- Rennebaum F, Kassubek J, Pinkhardt E, Hübers A, Ludolph AC, Schocke M, et al. Status epilepticus: clinical characteristics and EEG patterns associated with and without MRI diffusion restriction in 69 patients. Epilepsy Res 2016; 120: 55–64. [DOI] [PubMed] [Google Scholar]
- Rodriguez Ruiz A, Vlachy J, Lee JW, Gilmore EJ, Ayer T, Haider HA, et al. for the Critical Care EEG Monitoring Research Consortium. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol 2017; 74: 181–8. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol 1997; 379: 313–32. [PubMed] [Google Scholar]
- Rosenberg DS, Mauguière F, Catenoix H, Faillenot I, Magnin M. Reciprocal thalamocortical connectivity of the medial pulvinar: a depth stimulation and evoked potential study in human brain. Cereb Cortex 2009; 19: 1462–73. [DOI] [PubMed] [Google Scholar]
- Rosenberg DS, Mauguière F, Demarquay G, Ryvlin P, Isnard J, Fischer C, et al. Involvement of medial pulvinar thalamic nucleus in human temporal lobe seizures. Epilepsia 2006; 47: 98–107. [DOI] [PubMed] [Google Scholar]
- Schertz J, Benzakoun M, Pyatigorskaya N, Belkacem S, Sahli-Amor M, Navarro V, et al. Specificities of arterial spin labeling (ASL) abnormalities in acute seizure. J Neuroradiol 2020; 47: 20–6. [DOI] [PubMed] [Google Scholar]
- Shimogawa T, Morioka T, Sayama T, Haga S, Kanazawa Y, Murao K, et al. The initial use of arterial spin labeling perfusion and diffusion-weighted magnetic resonance images in the diagnosis of nonconvulsive partial status epileptics. Epilepsy Res 2017; 129: 162–73. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993; 262: 679–85. [DOI] [PubMed] [Google Scholar]
- Struck AF, Tabaeizadeh M, Schmitt SE, Ruiz AR, Swisher CB, Subramaniam T, et al. Assessment of the validity of the 2HELPS2B score for inpatient seizure risk prediction. JAMA Neurol 2020; 77: 500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter R, Kaplan PW. Electroencephalographic criteria for nonconvulsive status epilepticus: synopsis and comprehensive survey. Epilepsia 2012; 53 (Suppl 3): 1–51. [DOI] [PubMed] [Google Scholar]
- Sutter R, Semmlack S, Kaplan PW. Nonconvulsive status epilepticus in adults–insights into the invisible. Nat Rev Neurol 2016; 12: 281–93. [DOI] [PubMed] [Google Scholar]
- Szabo K, Poepel A, Pohlmann-Eden B, Hirsch J, Back T, Sedlaczek O, et al. Diffusion-weighted and perfusion MRI demonstrates parenchymal changes in complex partial status epilepticus. Brain 2005; 128: 1369–76. [DOI] [PubMed] [Google Scholar]
- Toledo M, Munuera J, Sueiras M, Rovira R, Álvarez-Sabín J, Rovira A. MRI findings in aphasic status epilepticus. Epilepsia 2008; 49: 1465–9. [DOI] [PubMed] [Google Scholar]
- Trinka E, Leitinger M. Which EEG patterns in coma are nonconvulsive status epilepticus? Epilepsy Behav 2015; 49: 203–22. [DOI] [PubMed] [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA 1992; 89: 212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JY, Rampal N, Petroff OA, Hirsch LJ, Gaspard N. Brief potentially ictal rhythmic discharges in critically ill adults. JAMA Neurol 2014; 71: 454–62. [DOI] [PubMed] [Google Scholar]
- Yoo RE, Yun TJ, Yoon BW, Lee SK, Lee ST, Kang KM, et al. Identification of cerebral perfusion using arterial spin labeling in patients with seizures in acute settings. PLoS One 2017; 12: e0173538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology 1996; 47: 83–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are available upon reasonable request.