Abstract
Target of rapamycin (TOR) operates as a hub of the signal transduction that integrates nutrient and energy signaling to promote cell proliferation and growth through mediating the transcriptional and post- transcriptional regulator networks in all eukaryotic species. MicroRNAs (miRNAs) are widespread classes of small, single-stranded, non-coding endogenous RNAs and are widely found in eukaryotes, which play a vital role in regulating gene expression by degrading targeted mRNAs or translational repression at post-transcriptional level. Recent studies found that there were necessarily close connections between miRNA and TOR pathways in mammals. However, there is little information about the interplay between the miRNA and TOR in plants. Thus, the aim of this study was to identify potential TOR-miRNA-mRNA regulatory networks in TOR signaling through global mRNA and microRNA expression profiling in potato. Based on the previous high-throughput transcriptome sequencing and filtering, a total of 2,899 genes were significantly differentially expressed in potato under TOR inhibitors treatment. Pathway analysis revealed that these genes were significantly enriched in multiple metabolic processes. Similarly, in the present study, suppression of TOR resulted in 41 miRNAs up-regulated and 45 down-regulated, revealing that TOR plays a crucial role in the regulation of miRNA regulatory network. Furthermore, integrated mRNA and miRNA expression profiling uncovered that these miRNAs participated in large-scale metabolic process in the TOR signal pathway in potato, such as regulation of autophagy and ubiquitination, and biosynthesis of secondary metabolites. Overall, the results shed new insight into TOR related post-transcriptional gene regulatory networks in potato and suggesting TOR-miRNA-targeting genes relevant networks as a potential genetic resource for potato improvement.
Keywords: Target of rapamycin, microRNA, Integrated miRNA-mRNA analysis, Metabolism, Solanum tuberosum L.
Introduction
MicroRNAs (miRNAs) represent an extensive class of small (usually 21–24 nucleotides length), non-coding, single-stranded, endogenous RNAs which are abundant in all eukaryotes. Since the direct cloning of first plant miRNA from Arabidopsis (Arabidopsis thaliana L.) (Park et al., 2002; Llave et al., 2002; Reinhart et al., 2002), more and more miRNAs and their putative targets were identified in different plant species, such as maize (Zea mays L.) (Zhang et al., 2009a), rice (Oryza sativa L.) (Zhang et al., 2017b; Peng et al., 2011), barley (Hordeum vulgare L.) (Curaba et al., 2012), switchgrass (Panicum virgatum L.) (Xie et al., 2014), wheat (Triticum aestivum L.) (Han et al., 2014), sweet potato (Ipomoea babatas L.) (Bian et al., 2016), rapeseed (Brassica napus L.) (Jian et al., 2018), sesame (Sesamum indicu L.) (Marakli, 2018) and radish (Raphanus sativus L.) (Liu et al., 2018), and so on. In plants, the stem-loop secondary structure of RNA Polymerase II transcript (pri-miRNA) was cleaved by Dicer-like 1 (DCL1) protein to produce a hairpin RNA molecule (pre-miRNA), which further cleaved by DCL1 to result in a double stranded intermediate RNA (Jones-Rhoades, Bartel & Bartel, 2006 Zhu et al., 2013). The matured miRNA, which could be incorporated into the effector complex (RISC-RNA Induced Silencing Complex) can target messenger RNA to direct RNA degradation or translational inhibition (Jones-Rhoades, Bartel & Bartel, 2006; Zhu et al., 2013; Iwakawa & Tomari, 2013). MiRNAs were demonstrated to play versatile roles in plant growth, development and responding to stresses, such as temperature, drought, salt, heavy metal stresses and nutrient starvation in plants (Chen, 2009; Sun, 2012,Zhang, 2015). Based on their functions in development, growth, crop yield and stress responses, they are also considered as important genetic resources in crop improvement (Liu & Chen, 2010; Tang & Chu, 2017). As one of the world’s most important staple food, potato (Solanum tuberosum L.) is essential to food security and human health all over the world, especially in solving the poverty (Zhang et al., 2017a). In the last decade, a fast increasing number of potato miRNAs were identified using comprehensive bioinformatic analysis of EST data, comparative genome strategy, computational prediction, and high-throughput sequencing (Zhang et al., 2013; Zhang et al., 2009b; Yang et al., 2016; Yang et al., 2010). Nevertheless, the number of the potato miRNAs (219) deposited in miRBase (http://www.mirbase.org/) was still less than that of rice (1519), Arabidopsis (664), cotton (Gossypium spp L.) (539) and maize (404). The function of some potato miRNAs were also confirmed, for example, miR172 could induce the potato tuberization (Martin et al., 2009), miR396, miR156a, miR157a and four miR169s were drought-induced (Yang et al., 2016; Hwang, Shin & Kwon, 2011), miR156 could modulate potato architecture and tuberization (Bhogale et al., 2014), miR166 and miR159 were responding to salinity (Kitazumia et al., 2015), miR482e could enhance plant sensitivity to Verticillium dahliae infection (Yang et al., 2015), miR397-5P was involved in the PVA infection (Li et al., 2017), miR164 could mediate the lateral root development (Zhang et al., 2018), and miR160 was associated with local defense and systemic acquired resistance against Phytophthora infestans (Natarajan et al., 2018). Herein, a large number of miRNAs have yet to be discovered, and the functions of most miRNAs remain to be investigated in potato.
Target of rapamycin (TOR) was regarded as a central regulator in the signal transduction network through integrating nutrient, energy and stress related cues to coordinate cell proliferation and growth in all eukaryotic species (Xiong & Sheen, 2014; Laplante & Sabatini, 2012,Loewith & Hall, 2011). Many important downstream targets of TOR kinase have been identified in different species, such as AKT, S6K, ATG13, 4EBP1, etc (). TOR can influence gene transcription, protein translation, lipid synthesis, lysosome synthesis, autophagy and energy metabolism through these substrates (Xiong & Sheen, 2012; Laplante & Sabatini, 2012; Van Leene et al., 2019). Recent studies revealed interplays between the TOR signaling pathway and miRNAs during the occurrence and treatments of diseases in mammals (Zhang et al., 2017b). Some miRNAs (miR-7, miR-99 family, miR-101, miR-122, miR-126, etc.) could suppress the upstream signaling pathway of mTOR, while some miRNAs (miR-21, miR-93, miR-96, miR-125b, etc.) could activate the TOR pathway (see review; Zhang et al., 2017c). In addition, global miRNA expression profiling suggested mTOR controls many miRNAs expression in chronic rapamycin (RAP, a specific TOR inhibitor produced by Streptomyces hygroscopicus) treatment of cancer cells and the mouse and human cells with inactivation of TSC complex (an essential repressor of mTOR activation) (Ye et al., 2015; Totary-Jain et al., 2013; Jewell, Flores & Guan, 2015). In mammals, the miRNA biogenesis was regulated by mTORC1-Mdm2-Drosha axis in response to amino acid- and glucose- deprivation (Ye et al., 2015). Moreover, several individual miRNAs (miR-1 (Sun et al., 2010), miR-21 (Bornachea et al., 2012), miR-143 (Fang et al., 2012) and miR-125b (Ge, sun & Chen, 2011)) have been confirmed to be regulated by mTOR signaling, which are known to participate in some physiological functions, including cancer.
Rapamycin (RAP) was the first generation of TOR inhibitor, which could inhibit the activity of TOR only in the presence of 12-kDa FK506 binding protein (FKBP12) through forming a ternary compound RAP-FKBP12-TOR in yeast and animals (Benjamin et al., 2011). Due to the resistance of terrestrial plants to RAP, FKBP12 gene from Homo sapiens L., Saccharomyces cerevisiae L. or Arabidopsis thaliana L. was introduced into plants to generate RAP sensitive plants in the previous study (Deng et al., 2016; Sormani et al., 2007; Ren et al., 2012; Deng et al., 2017; Xiong & Sheen, 2012). In addition to RAP, the second generation of TOR inhibitors (asTORis: AZD8055, Torin1, KU63794 (KU) et al.), which could selectively and efficiently suppress TOR by specifically targeting the ATP-binding pocket of the TOR kinase domain, were also used in revealing the function of TOR in plants (Xiong et al., 2017). In our previous study, a yeast FKBP12 gene was introduced into potato to generate RAP sensitive potato (BP12-OE line), and we found that RAP and asTORis showed synergistic effects on inhibiting potato growth and a great deal of differentially expressed genes (DEGs) were observed in potato under TOR suppression (Deng et al., 2017). MiRNA plays an important role in post-transcriptional gene regulation in plants. Whether TOR is involved in post-transcriptional gene regulation through miRNA remains unclear in potato. Herein, we chose RAP + KU and its control DMSO to construct the potato sRNA libraries for identifying the miRNAs regulated by TOR. The integrative miRNAs and mRNA analysis was done to explicate the biological functions of TOR-miRNA-mRNA regulatory networks in potato metabolism.
Materials and Methods
Plant material and growth condition
In this study, the potato seedling of BP12-OE line was used for the sRNA sequencing (Deng et al., 2017). All plants were grown in Murashige & Skoog (MS) medium under 16 h light/8 h dark in growth chambers at 22 °C. Four-week-old potato seedlings growing on the MS media for 48 h with TOR inhibitors (RAP + KU) and DMSO were collected for the sRNA sequencing.
RNA extraction, library preparation and sequencing
The extraction method and quality control of total RNA were according to Deng et al. (2017). The total amount of 3 ug RNA for each sample was used for small RNA library construction. The NEBNext Multiplex Small RNA Library Prep Set for Illumina (NEB, USA) was used to generate the sequencing libraries and the Agilent Bioanalyzer 2100 system using DNA High Sensitivity Chips was used to assess the library quality. The cBot Cluster Generation System using TruSeq SR Cluster kit v3-cBot-HS (Illumina) was used to cluster the index-coded samples. And then, the libraries were sequenced on an Illumina Hiseq 2500/2000 platform. At last, total of 50 bp single-end reads were generated.
Data analysis
Quality control and mapping
Raw reads of fastq format were processed through python scripts and custom perl to remove the low quality reads. The GC content, Q20 and Q30 of the raw reads were calculated. All downstream analyses were according to a range of length of clean reads, such as 18–30 nt for plant. The small RNA tags were mapped to the reference potato genome sequence (http://plants.ensembl.org/Solanum_tuberosum/Search/New?db=core) by Bowtie without mismatch to analyze their distribution and expression on the reference (Xu et al., 2011; Langmead et al., 2009).
Bioinformatic identification of known and novel miRNAs, and their differential expression under TOR inhibitors treatment
The mapped small RNA tags were aligned to the miRNA precursor/mature miRNA of plants and animals in the miRBase20.0 database for looking for known miRNA. The potential miRNAs and their secondary structures were obtained using modified software mirdeep2 (Friedlander et al., 2012) and srna-tools-cli (http://srna-tools.cmp.uea.ac.uk/). The Custom scripts were used to analyze the known miRNA counts and their base bias either on each position or on the first position with certain length.
The rRNA, tRNA, snRNA, snoRNA, repeat and protein-coding genes were deleted from the small RNA libraries based on RepeatMasker and Rfam database. And then the remaining sequences were used for predicting the novel miRNAs with the software miREvo (Wen et al., 2012) and mirdeep2 (Friedlander et al., 2012) through exploring the secondary structure, the minimum free energy and the Dicer cleavage site. The analysis of novel miRNA counts and their base bias was the same as that of known miRNA.
All miRNA abundances were evaluated and normalized using the tags per million reads (TPM) method through the following criteria (Zhou et al., 2010): TPM = number of mapped miRNA reads ×106/ number of clean sample reads. Differential expression analysis between the treatment and control was performed using the DESeq R package (1.8.3). The P-values were adjusted using q-value (Storey, 2003). /log2fold-change/ >1 and the corrected P-value (q-value) <0.01 were set as the threshold for significantly differential expression by default.
Prediction and annotation of target genes of miRNAs
The putative target genes of miRNAs were predicted by psRobot-tar in psRobot (Wu et al., 2012) for plants. All the target genes were annotated in the NCBI nr database and potato genome database (http://plants.ensembl.org/Solanum_tuberosum/Search/New?db=core). For further annotating the target gene candidates of differentially expressed miRNAs, Gene Ontology (GO) enrichment and the statistical enrichment of KEGG pathways were implemented using GOseq based Wallenius non-central hyper-geometric distribution and KOBAS, respectively (Mao et al., 2005; Young et al., 2010).
M RNA and miRNA validation by qRT-PCR
Four-week-old potato seedlings growing on the MS media were treated with TOR inhibitors (RAP + KU) and DMSO for 48 h and were collected for qRT-PCR. The mRNA validation was according to Deng et al. (2017). The primers for qRT-PCR designed by Primer premier 5 software were listed in Table S1. To validate the expression of miRNA, 1 µg of total RNA was reverse transcribed using Mir-X™ miRNA qRT-PCR TB Green™ Kit (Takara). The RT-qPCR analysis was performed according to the manufacturer’s protocol (Mir-X™ miRNA qRT-PCR TB Green™ Kit). The miRNA-specific primers were listed in Table S1 and reverse primers was provided by kit. Potato actin gene was used as constitutive references (Nicot et al., 2005; Payyavula, Singh & Navarre, 2013; Liu et al., 2015). All reactions were conducted with 3 biological replicates, and the relative expression of genes was conducted using the 2−ΔΔc(t) method.
Results
sRNA sequencing
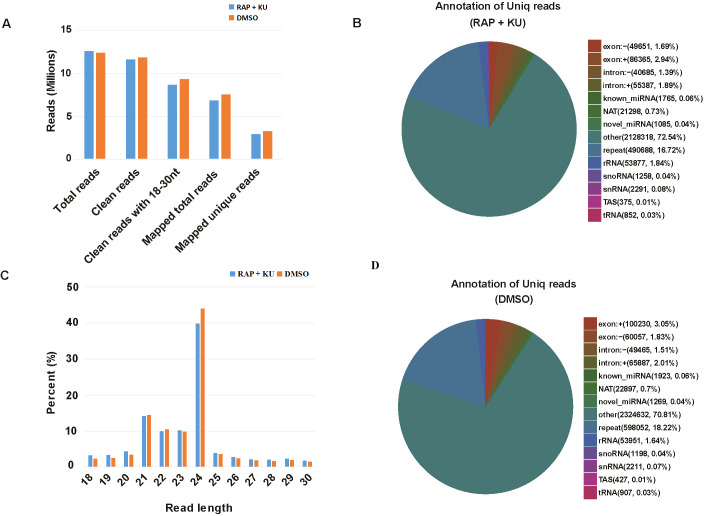
The basic information of sRNA sequencing data from potato (BP12-OE line) with or without TOR inhibitor (RAP + KU) was shown in Fig. 1 and Table S2. The values of Q30 (bases correct recognition rate >99.9%) of the raw data were more than 97%. After removal of adapter contaminants, low quality reads, small reads and polyN, about 92.53% and 95.76% reads in total raw reads were clean reads in two groups, respectively (Fig. 1A). Finally, about 79.18% and 80.61% of the total clean reads with 18–30 nt read length could be mapped and matched to the potato genome sequence with bowtie, respectively (Langmead et al., 2009). For avoiding some sRNAs mapped to more than one annotation, the mapped clean total sRNAs were annotated according to the following priority rule: known miRNA >rRNA >tRNA >snRNA >snoRNA >repeat >NAT-siRNA >gene >novel miRNA >ta-siRNA (Fig. 1B). Most clean sRNA reads ranged from 21 to 24 nt, with 24 nt being the most abundant group of small RNAs, representing the typical length of Dicer-like protein 3 (DCL3)-derived products (Fig. 1C). The dominance of the 24 nt read length sRNA in potato is consistent with previous studies of other species, such as rice (Morin et al., 2008), cucumber (Cucumis sativus L.) (Mao et al., 2012), tomato (Solanum lycopersicum L.) (Cao et al., 2014), tobacco (Nicotiana tabacum L.) (Yin et al., 2015) and radish (Liu et al., 2018). The second abundant group is 21-nt miRNAs, which have the canonical size derivedfrom DCL1 processing. Most of the first nucleotide from the 5′end of the known and novel miRNAs was uridine (U), which was a bias for AGO1 (Mi et al., 2008), suggesting the important character of miRNAs was not changed under the treatment of TOR specific inhibitors (Fig. S1). In addition, in all the libraries, the total rRNA proportion was less than 60% which was used as a marker as the plant samples quality control.
Figure 1. The summary of basic information for the sRNA data.
(A) Statistical analysis of sequencing reads. (B) The number and proportion of different kind of sRNAs, “exon: +”: exon sense strand, “exon”: exon antisense strand, “intron: +”: intron sense strand, “intro: -”: intron antisense strand, NAT: natural antisense transcripts, repeat: repeat sequence, snoRNA: small nucleolar RNA, snRNA: small nuclear RNA, TAS: ta-si RNA. (C) The length distribution of clean sRNA.
Identification of known and potential novel miRNAs in potato
In order for identifying the known miRNAs from sRNA libraries in potato, we compared the sRNA information with the known, up-dated and mature plant miRNAs deposited in the miRBase without any mismatching (Kozomara & Griffiths-Jones, 2014). Based on the blastn searches and further sequence analysis, the total of 193 known miRNA were observed and 174 known miRNA exist both in the RAP + KU and control libraries (Table S3). The read counts of the known miRNAs ranged from 0 to 24,000 in the RAP + KU and control libraries, which showed high diversity. About 17 and 22 known miRNAs were found to have more than 10,000 redundancies in the above groups, respectively (Table 1). Among these miRNAs, the miR166a-3p and miR319a-3p had the most reads.
Table 1. The known miRNA of more than ten thousand redundancies and novel miRNAs of more than 100 redundancies.
| MiRNA | RAP+KU | DMSO | MiRNA | RAP+KU | DMSO |
|---|---|---|---|---|---|
| stu-miR319a-3p | 24000 | 13181 | novel_188 | 6057 | 11099 |
| stu-miR166a-3p | 14823 | 15988 | novel_1 | 5395 | 9175 |
| stu-miR166b | 10331 | 11160 | novel_16 | 2209 | 4329 |
| stu-miR398a-3p | 3832 | 11553 | novel_17 | 2169 | 2028 |
| stu-miR162a-3p | 4850 | 10908 | novel_18 | 839 | 1656 |
| stu-miR396-5p | 5382 | 5157 | novel_40 | 665 | 1026 |
| stu-miR482a-3p | 2292 | 4388 | novel_15 | 487 | 940 |
| stu-miR6022 | 4287 | 4798 | novel_30 | 733 | 764 |
| stu-miR319b | 3656 | 1197 | novel_34 | 604 | 711 |
| stu-miR482c | 1303 | 2880 | novel_56 | 280 | 439 |
| stu-miR4.2e−3p | 1344 | 2470 | novel_36 | 574 | 385 |
| stu-miR1919-5p | 1679 | 1703 | novel_38 | 256 | 322 |
| stu-miR156a | 1446 | 1972 | novel_59 | 162 | 298 |
| stu-miR156d-3p | 1357 | 2244 | novel_67 | 119 | 216 |
| stu-miR6149-5p | 1062 | 1807 | novel_60 | 138 | 195 |
| stu-miR395a | 1109 | 1526 | novel_62 | 128 | 188 |
| stu-miR482d-3p | 1325 | novel_137 | 137 | ||
| stu-miR6027 | 1839 | novel_84 | 118 | ||
| stu-miR482b-3p | 1609 | novel_68 | 106 | ||
| stu-miR384-5p | 1209 | novel_58 | 115 | ||
| stu-miR8036-3p | 1137 | ||||
| stu-miR6024-3p | 1060 |
The available softwares miREvo (Wen et al., 2012) and mirdeep2 (Friedlander et al., 2012) were integrated to predict potential novel miRNAs. Only those with stable hairpin structures were considered, because this was an essential characteristic for identification of novel miRNAs. Additionally, the binding locations of Dicer enzymes and free energies were used to evaluate these candidate miRNAs. Ultimately, 79 novel miRNAs were discovered from two potato libraries (Table S3). The novel miRNAs displayed lower expression levels, ranging from 0 to 11,652, when compared with known miRNAs. In the RAP + KU and control group, only 17 and 19 novel miRNAs were found to have more than 100 redundancies, respectively (Table 1). In general, many novel miRNAs discoveries in potato enriched the plant miRNA dataset.
TOR regulates potato miRNA expression
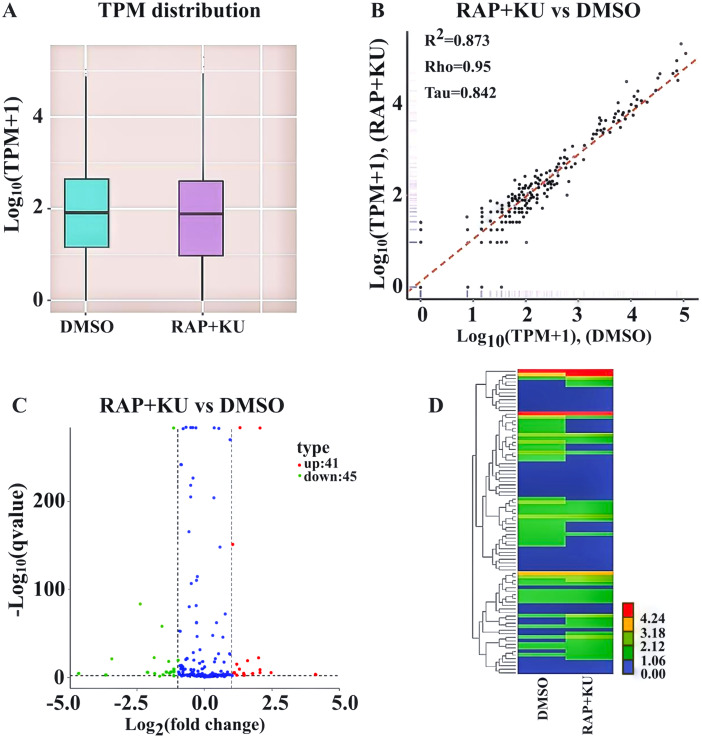
After obtaining the readcounts of all the miRNAs, the quantification and normalization of them were conducted by TPM (transcript per million) (Zhou et al., 2010) (Table S4). The two treatments have the similar distributions of the expression levels of all the miRNAs, and the high correlation (R2 = 0.873) of miRNA expression level showed the experimental reliability and reasonable sample selection (Figs. 2A–2B). MiRNAs with TPMs over 60 were regarded as expressing at a very high level and miRNAs with TPMs in the interval 0–1 were deemed not to be expressed at very low levels. About 86% of the total miRNAs (272) were expressed (TPM ≥ 1) and more than 146 miRNAs were highly expressed (TPM > 60) in the two treatments. Among them, one novel and 19 known miRNA were only observed in the sRNA libraries from TOR inhibition (RAP + KU), while 2 novel and 19 known miRNA were only detected in the control DMSO library, indicating TOR may completely inhibit or induce these miRNAs expression in potato.
Figure 2. The differentially expressed miRNAs between the treatment group (RAP + KU) and control (DMSO).
(A) Boxplot of the log transformed TPM expression values. (B) The correlation of miRNAs expression level between the treatment group (RAP + KU) and control (DMSO). (C) The number of differentially expressed miRNAs. (D) Hierarchical clustering of the differentially expressed miRNAs.
In order to identify the differentially expressed miRNAs, we compared the expression of the known and novel miRNAs between the TOR inhibitors and control treatment samples using the DEGseq (2010) R package. Total of 86 differentially expressed miRNAs were detected in the RAP + KU vs DMSO group, including 41 up-regulated and 45 down-regulated miRNAs (Fig. 2C). To obtain the miRNA expression patterns, we performed the hierarchical clustering of all the differentially expressed miRNAs based on the log10 (TPMs + 1) for the two sRNA libraries (Fig. 2D). The expression levels of all the differentially expressed miRNAs were listed in Table 2 and these miRNAs (stu-miR5303c, stu-miR8010, stu-miR8024a-3p and stu-miR8028-5p) had remarkable up-regulated differences, while these miRNAs (stu-miR3627-5p, stu-miR7985, stu-miR156f-3p and stu-miR8013) had remarkable down-regulated differences. The differential expression of about 1/3 of total miRNAs suggested that TOR may play a crucial role in the regulation of miRNAs expression in potato.
Table 2. The differentially expressed miRNAs in potato between the treatment of RAP + KU and DMSO.
| MiRNA | Log2 (RAP + KU)/ DMSO | Q valve | MiRNA | Log2 (RAP + KU)/ DMSO | Q valve |
|---|---|---|---|---|---|
| stu-miR3627-5p | −6.01 | 9.80E−10 | novel_36 | 1.03 | 1.08E−15 |
| stu-miR7985 | −4.69 | 6.26E−05 | novel_99 | 1.04 | 5.16E−05 |
| stu-miR156f-3p | −4.69 | 6.26E−05 | stu-miR8031 | 1.08 | 1.09E−06 |
| stu-miR8013 | −4.69 | 6.26E−05 | novel_115 | 1.11 | 4.12E−05 |
| novel_111 | −3.69 | 2.74E−03 | stu-miR4376-3p | 1.19 | 1.56E−15 |
| stu-miR169a-3p | −3.69 | 2.74E−03 | novel_53 | 1.19 | 1.34E−03 |
| stu-miR1886 h | −3.69 | 2.74E−03 | stu-miR172c-3p | 1.19 | 1.34E−03 |
| stu-miR1886i-3p | −3.69 | 2.74E−03 | stu-miR8032a-5p | 1.32 | 5.10E−10 |
| stu-miR477a-3p | −3.69 | 2.74E−03 | stu-miR319a-3p | 1.32 | 0.00E+00 |
| stu-miR7986 | −3.69 | 2.74E−03 | stu-miR8033-3p | 1.45 | 7.30E−03 |
| stu-miR7998 | −3.69 | 2.74E−03 | novel_117 | 1.45 | 1.01E−05 |
| stu-miR8015-3p | −3.69 | 2.74E−03 | stu-miR8020 | 1.45 | 8.78E−04 |
| stu-miR8034 | −3.69 | 2.74E−03 | stu-miR399a-3p | 1.60 | 1.48E−19 |
| stu-miR8041a-5p | −3.69 | 2.74E−03 | stu-miR8025-5p | 1.78 | 3.42E−05 |
| novel_154 | −3.69 | 2.74E−03 | stu-miR399j-3p | 1.98 | 1.74E−22 |
| stu-miR7987 | −3.69 | 2.74E−03 | stu-miR8030-5p | 2.04 | 8.87E−07 |
| stu-miR8023 | −3.69 | 2.74E−03 | novel_161 | 2.04 | 2.93E−09 |
| stu-miR8050-5p | −3.69 | 2.74E−03 | stu-miR319b | 2.06 | 0.00E+00 |
| stu-miR7979 | −3.69 | 2.74E−03 | stu-miR5304-5p | 2.45 | 4.44E−06 |
| stu-miR8004 | −3.69 | 2.74E−03 | stu-miR8008b | 2.45 | 4.44E−06 |
| stu-miR8045 | −3.69 | 2.74E−03 | stu-miR7995 | 2.45 | 4.44E−06 |
| stu-miR7122-5p | −3.45 | 1.86E−21 | stu-miR399g-3p | 4.14 | 1.21E−03 |
| novel_103 | −2.40 | 8.42E−84 | stu-miR8001b-3p | 4.14 | 1.21E−03 |
| stu-miR5303j | −2.13 | 9.01E−07 | stu-miR8014-3p | 4.14 | 1.21E−03 |
| stu-miR7984c-3p | −2.13 | 9.01E−07 | stu-miR8024a-5p | 4.14 | 1.21E−03 |
| stu-miR171d-5p | −1.87 | 2.36E−05 | stu-miR7990b | 4.14 | 1.21E−03 |
| novel_120 | −1.87 | 5.85E−23 | stu-miR167a-3p | 4.14 | 1.21E−03 |
| stu-miR169f-3p | −1.87 | 2.36E−05 | stu-miR7983-5p | 4.14 | 1.21E−03 |
| stu-miR8032b-3p | −1.87 | 2.36E−05 | stu-miR166c-5p | 4.14 | 1.21E−03 |
| stu-miR397-3p | −1.59 | 6.32E−59 | stu-miR8048-3p | 4.14 | 1.21E−03 |
| stu-miR164-5p | −1.55 | 4.66E−04 | stu-miR8012 | 4.14 | 1.21E−03 |
| stu-miR7991a | −1.55 | 4.66E−04 | stu-miR5304-3p | 4.14 | 1.21E−03 |
| stu-miR8007a-3p | −1.55 | 4.66E−04 | stu-miR8018 | 4.14 | 1.21E−03 |
| stu-miR8038a-5p | −1.42 | 2.91E−07 | stu-miR3627-3p | 4.14 | 1.21E−03 |
| novel_93 | −1.33 | 3.38E−19 | stu-miR6023 | 4.14 | 1.21E−03 |
| novel_162 | −1.28 | 3.82E−06 | novel_182 | 4.14 | 1.21E−03 |
| stu-miR7997a | −1.28 | 3.82E−06 | stu-miR8011a-3p | 4.14 | 1.21E−03 |
| stu-miR172d-5p | −1.25 | 3.20E−07 | stu-miR5303c | 5.14 | 1.01E−05 |
| stu-miR398a-3p | −1.14 | 0.00E+00 | stu-miR8028-5p | 5.14 | 1.01E−05 |
| novel_193 | −1.13 | 2.95E−07 | stu-miR8024a-3p | 5.73 | 9.15E−08 |
| stu-miR399a-5p | −1.13 | 5.49E−03 | stu-miR8010 | 5.73 | 9.15E−08 |
| novel_116 | −1.13 | 1.58E−10 | |||
| stu-miR5303 h | −1.13 | 2.39E−08 | |||
| stu-miR7980b-5p | −1.13 | 5.49E−03 | |||
| novel_108 | −1.01 | 7.44E−20 |
The prediction of miRNA target genes
In order to investigate the function of all the previously identified miRNAs in different biological processes, their potential target genes were predicted using psRobot_tar in psRobot for plants (Wu et al., 2012). Overall, 192 miRNAs got total 4,127 predicted target genes in 272 identified miRNAs (Table S5). Except the numbered EPlSTUG genes, all the rest (4081) numbered PGSC0003DMG genes were annotated in the website (http://plants.ensembl.org/Solanum_tuberosum/Search/New?db=core) and listed in Table S6. The number of potential targets in the potato is variable for each miRNA from 1 to 772, and most of them have multiple target genes, which are consistent with other reports (Curaba et al., 2012; Xie, Frazier & Zhang, 2011). The top three miRNAs are stu-miR7797a (772), stu-miR5303h (751) and stu-miR5303g (743). The results indicated the single miRNA might possess wide-ranging functions and involve in different kinds of signal pathways in potato. The products of the target genes include functional protein, transcriptional factors and enzyme, etc.
The putative function of differentially expressed miRNAs
Some differential expression miRNAs had been reported in other species, such as miR156 and miR169 etc., (Xiong et al., 2013, Jiao et al., 2010; Meng et al., 2011; Wu et al., 2009). In addition to these miRNAs, there are a large number of miRNA functions that have not been studied. In order to understand the regulatory functions of differentially expressed miRNAs, we carried out the miRNA target prediction analysis and annotated the target genes according to the website (http://plants.ensembl.org/Solanum_tuberosum/Search/New?db=core) (Table S7). The putative miRNA target genes participated in many biological processes such as cell wall modification, the cell cycle, photosynthesis, nutrient transport, carbon and nitrogen utilization, autophagy, ubiquitination, senescence, protein and lipid metabolism, chromatin structure, hormone metabolism, signaling and stress-related processes. This result was also consistent with differentially expressed genes related to the TOR-regulated pathways reported by previous transcriptome studies (Park et al., 2002; Payyavula, Singh & Navarre, 2013; Xiong & Sheen, 2014; Caldana et al., 2013). And then Numerous GO functional and KEGG pathway enrichment of differentially expressed miRNAs were detected, suggesting TOR regulated miRNAs participated in many biological metabolic processes in potato (Tables S8A–S8B). These results suggest that miRNA may play a vital role in the regulation of metabolic processes in TOR signaling.
Integrative miRNA-mRNA expression and function analysis
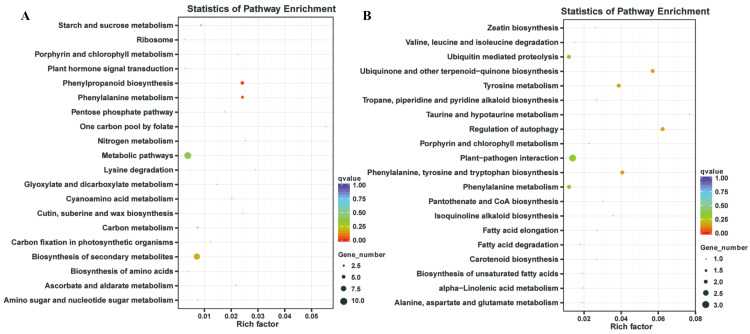
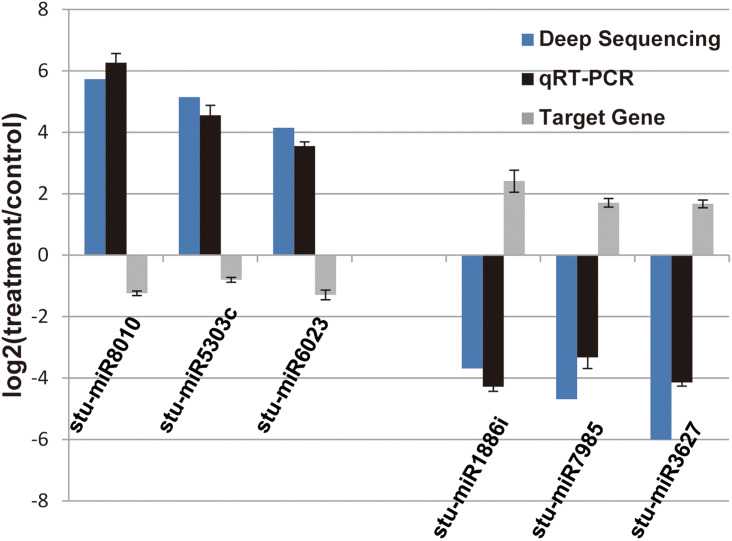
In our previous study, our focus is on the relationship between auxin, TOR and adventitious root formation (Deng et al., 2017). Our transcriptome data showed that a large number of genes associated with root development were differentially expressed in potato seedling under TOR inhibitor treatment. Loss of auxin signaling after TOR suppression results in significant down-regulation of these genes (Morin et al., 2008; Deng et al., 2017). In addition, a large number of genes related to biosynthesis and metabolism are differentially expressed. How TOR affects the expression of these genes is still unclear. To further determine the regulatory function of TOR at post-transcriptional level, we performed miRNA-mRNA reverse correlation analysis to identify reliable miRNA and their targets. In present study, thirteen up-regulated miRNAs and 14 down-regulated miRNAs were corresponding to 47 down-regulated 82 up-regulated mRNAs, respectively (Tables S9A–S9D). GO functional (Table S10A–S10B) and KEGG pathway (Fig. 3) analysis showed that the negatively correlated miRNA/mRNA interaction pairs participate in some key metabolic processes such as lipid synthesis, amino acid metabolism, nitrogen metabolism, flavonoid biosynthesis and so on. To validate the results of high-throughput sRNA sequencing, the expression patterns of 8 miRNAs were analyzed by qRT-PCR. Meanwhile, to detect the expression patterns of the targets identified by transcriptome analysis, 6 target genes were selected for qRT-PCR analysis. The results showed that all the selected miRNAs and targets shared similar expression tendency with the original results (Fig. 4).
Figure 3. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis.
(A) KEGG enrichment based on down-regulated DEGs under the TOR inhibition. (B) KEGG enrichment based on up-regulated DEGs under the TOR inhibition.
Figure 4. Verification of the selected miRNAs and their potential target genes by qRT-PCR.
Relative expression levels of the selected six miRNAs (stu-miR8010, stu-miR5303c, stu-miR6023, stu-miR1886i, stu-miR7985 and stu-miR3627) and their potential target genes (PGSC0003DMG400026112, PGSC0003DMG400028214, PGSC0003DMG400008584, PGSC0003DMG400023703, PGSC0003DMG402018257 and PGSC0003DMG401025397) were measured by RT-qPCR. U6 and actin were used as internal control for miRNAs and mRNA RT-qPCR, respectively.
Differentially expressed miRNAs were involved in some anabolic and biosynthetic pathways
TOR, as a central regulator of anabolism, participates in many biosynthetic processes, such as ribosome biosynthesis, and protein translation and synthesis. In the integrative miRNA-mRNA data, DE miRNAs target genes also participate in many anabolism processes. As shown in Fig. 3 and Table 3, differentially expressed miRNAs were involved in phenylpropanoid biosynthesis, cutin, suberine and wax biosynthesis. On the other hand, TOR also negatively regulates the synthesis of some metabolites, such as glucosinolates and flavonoids. Suppression of TOR could up-regulated genes responsible glucosinolates and flavonoids synthesis (Caldana et al., 2013). It was found that the differentially expressed miRNAs target genes were also involved in the biosynthesis of flavonoids, suggesting that TOR may regulate the biosynthesis of flavonoids relying on miRNAs. Interestingly, TOR, as the core regulatory element of ribosomal biosynthesis, large number genes of ribosome was differentially expressed in transcriptomics. But only few differentially expressed miRNAs target genes were involved in ribosomal biosynthesis. These results indicated that miRNAs are only part of TOR regulation networks in transcription and post-transcriptional level.
Table 3. Differentially expressed miRNAs under the TOR inhibition in potato involved in catabolic process, anabolic process and biosynthetic pathways.
| MiRNA | Target gene_id | Log2FoldChange | Pval | Gene description |
|---|---|---|---|---|
| Ribosome | ||||
| stu-miR8020 | PGSC0003DMG400030153 | −0.31601 | 1.55E−04 | Ribosomal protein L18a |
| Biosynthesis of amino acids | ||||
| stu-miR7983-5p | PGSC0003DMG400022088 | −0.40026 | 1.28E−07 | Transketolase, C-terminal |
| Cutin, suberine and wax biosynthesis | ||||
| stu-miR8011a-3p | PGSC0003DMG400004844 | −0.77456 | 4.95E−07 | Glucose-methanol-choline oxidoreductase, N-terminal |
| Phenylpropanoid biosynthesis | ||||
| stu-miR4376-3p | PGSC0003DMG400020795 | −2.9897 | 1.54E−06 | Haem peroxidase, plant/fungal/bacterial |
| stu-miR5303c | PGSC0003DMG400003013 | −0.60889 | 3.06E−04 | Glycoside hydrolase family 3 |
| stu-miR7983-5p | PGSC0003DMG400005872 | −2.6826 | 2.42E−19 | Plant peroxidase |
| stu-miR4376-3p | PGSC0003DMG400022541 | −0.73675 | 1.21E−03 | Haem peroxidase, plant/fungal/bacterial |
| stu-miR4376-3p | PGSC0003DMG400020801 | −3.3592 | 4.72E−10 | Peroxidases heam-ligand binding site |
| Ubiquinone and other terpenoid-quinone biosynthesis | ||||
| stu-miR7997a | PGSC0003DMG400021276 | 2.1196 | 3.17E−19 | Pyridoxal phosphate-dependent transferase, major region, subdomain 1 |
| stu-miR8045 | PGSC0003DMG400017707 | 0.52448 | 3.78E−08 | 4-hydroxyphenylpyruvate dioxygenase |
| Phenylalanine, tyrosine and tryptophan biosynthesis | ||||
| stu-miR5303 h,stu-miR5303j | PGSC0003DMG400011282 | 1.4353 | 4.63E−03 | Tryptophan synthase, beta chain, conserved site |
| stu-miR7997a | PGSC0003DMG400021276 | 2.1196 | 3.17E−19 | Pyridoxal phosphate-dependent transferase, major region, subdomain 1 |
| Isoquinoline alkaloid biosynthesis | ||||
| stu-miR7997a | PGSC0003DMG400021276 | 2.1196 | 3.17E−19 | Pyridoxal phosphate-dependent transferase, major region, subdomain 1 |
| Pantothenate and CoA biosynthesis | ||||
| stu-miR5303 h,stu-miR5303j,stu-miR7997a | PGSC0003DMG400013511 | 0.37163 | 1.54E−04 | ATCOAA—Type II pantothenate kinase |
| Tropane, piperidine and pyridine alkaloid biosynthesis | ||||
| stu-miR7997a | PGSC0003DMG400021276 | 2.1196 | 3.17E−19 | Pyridoxal phosphate-dependent transferase, major region, subdomain 1 |
| Fatty acid elongation | ||||
| stu-miR8004 | PGSC0003DMG400014549 | 0.73372 | 1.65E−04 | Thiolase-like |
| Carotenoid biosynthesis | ||||
| stu-miR5303 h,stu-miR5303j | PGSC0003DMG402018475 | 1.1371 | 1.49E−03 | Cytochrome P450 |
| Zeatin biosynthesis | ||||
| stu-miR5303 h,stu-miR5303j | PGSC0003DMG400023732 | 1.0493 | 3.57E−08 | UDP-glucuronosyl/UDP-glucosyltransferase |
| Biosynthesis of unsaturated fatty acids | ||||
| stu-miR8050-5p | PGSC0003DMG400020620 | 0.40349 | 8.59E−06 | ACX2—Acyl-CoA dehydrogenase/oxidase C-terminal |
| Lysine degradation | ||||
| stu-miR5303c | PGSC0003DMG400001557 | −0.33784 | 2.01E−03 | Histone H3-K9 methyltransferase, plant |
| Regulation of autophagy | ||||
| stu-miR5303 h,stu-miR5303j,stu-miR7997a | PGSC0003DMG402022314 | 0.28852 | 2.09E−03 | Ubiquitin-related domain |
| stu-miR7997a | PGSC0003DMG402012477 | 0.33886 | 3.04E−04 | Autophagy protein Atg8 ubiquitin-like |
| Ubiquitin mediated proteolysis | ||||
| stu-miR5303 h,stu-miR5303j,stu-miR7997a | PGSC0003DMG400003897 | 0.29401 | 1.31E−03 | Zinc finger, RING-type |
| stu-miR5303 h,stu-miR5303j,stu-miR7997a | PGSC0003DMG400019395 | 0.43331 | 9.66E−07 | Ubiquitin-conjugating enzyme/RWD-like |
| Fatty acid degradation | ||||
| stu-miR8050-5p | PGSC0003DMG400020620 | 0.40349 | 8.59E−06 | ACX2—Acyl-CoA dehydrogenase/oxidase C-terminal |
| Valine, leucine and isoleucine degradation | ||||
| stu-miR5303 h,stu-miR5303j | PGSC0003DMG400011330 | 0.7956 | 4.46E−15 | BCE2—Chloramphenicol acetyltransferase-like domain |
Differentially expressed miRNAs related to some catabolic processes
Catabolic pathways is another set of metabolism that breaks down large molecules (such as lipids, polysaccharides, proteins and nucleic acids) into smaller unites (such as fatty acids, monosaccharides, amino acids and nucleotides, respectively) which are either oxidized to release energy or used in other anabolic reactions. Autophagy is a natural regulated project of the cell that disassembles unnecessary or dysfunctional components for turnover and recycling of intracellular macro molecules and whole organelles. In the mammals, many miRNAs (such as miR-145, miR-181, miR-93-5p, miR-33 and miR-21) (Zhang et al., 2017b) as the upstream regulator of TOR signaling pathway were shown to modulate disease biogenesis through inhibiting autophagy. In this study, the KEGG pathway “regulation of autophagy” was affected under the TOR suppression, and a total of three down-regulated miRNAs as the downstream of the TOR signaling pathway were targeting the autophagy-related genes (Table 3). Ubiquitination is a key posttranslational modification carried out by a set of three enzymes: ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin-protein ligase (E3). The “ubiquitin mediated proteolysis” KEGG pathway was one of the most enriched pathways in the miRNA data. A total of four differentially expressed ubiquitination-related miRNAs were observed and three were significantly down-regulated (Table 3). These observations strongly support the previous results in which TOR negatively regulated the catabolic process (autophagy and ubiquitination) in yeast, mammals and plants (Xiong & Sheen, 2014; Dong et al., 2015). In previously studies, it has showed that suppression of TOR could lead to accumulation of sugars and lipids in Arabidopsis (Caldana et al., 2013; Dobrenel et al., 2013). In this study, we found that many miRNA targeted genes were involved in these metabolic processes. TOR may influence the metabolic processes of sugars and lipids by regulating corresponding miRNAs. Thus, our results showed that miRNAs may act as a bridge between TOR and catabolism.
Discussion
MiRNAs play an important role in plant growth, development and stress response. Through high-throughput sequencing technologies, many of the evolutionary conserved and novel miRNAs were detected in different species. A large number of miRNAs are still unidentified due to its low expression levels, tissue specificity or spatio-temporal expression specificity. In this study, total of 193 known miRNAs and 79 novel miRNAs were identified, largely increasing the number of miRNAs in potato. Furthermore, we found that the expression of a large number of miRNAs was affected by TOR in potato. About 86 miRNAs were differentially expressed between the libraries of TOR inhibition and the control, suggesting TOR play a crucial role in regulation the expression of miRNA. The expression levels of stu-miR5303c, stu-miR8010, stu-miR8024a-3p and stu-miR166c-5p had remarkable up-regulated differences, while that of stu-miR3627-5p, novel_111, stu-miR169a-3p and stu-miR1886h, etc., had remarkable down-regulated differences. These data suggested TOR plays a crucial role in regulation the expression of miRNA. The functions of these miRNAs have been reported in other species, such as miR156, miR164 and miR319 et al. The role of miR156 in controlling flower development is highly conserved in rice, tomato and maize (Hong & Jackson, 2015). MiR164 can target to NAC transcription factors to modulate root development and drought resistance in Z. mays and rice, respectively (Li et al., 2012; Fang, Xie & Xiong, 2014). Zhao et al. (2015) showed that miR319/TCP4 module played a crucial role in systemic defensive response in tomato. In addition, the role of these differentially expressed miRNAs was partially revealed in potato as well, such as miR156, miR172 and miR169. MiR156 and miR172 is involved in regulating developmental timing in Arabidopsis (Wu et al., 2009). Consistently, miR156 and miR172 affect not only flowering time, but also the timing of tuberization in potato. Similarly, miR169 is conserved related with salt stress in potato and other species (Yang et al., 2016; Zhao et al., 2009; Luan et al., 2015). Indeed, it has been reported that both TOR and miRNAs are involved in many different signaling pathways to regulate plant growth and development in previous studies (Jones-Rhoades, Bartel & Bartel, 2006; Shi, Wu & Sheen, 2018). Whether TOR can regulate plant growth and development through these known miRNAs identified in this study may be a field worthy of research. In addition, many differentially expressed miRNAs identified in this study are unknown function miRNAs. Integrated mRNA and miRNA expression profiling will be helpful to predict the function of these unknown miRNAs. In this study, through the combined analysis of miRNAomics and transcriptomics, the obtained RNA-miRNA regulatory networks partially reveal the function of TOR in post-transcriptional regulation. Our data revealed more than 120 miRNA-mRNA regulatory networks involved in TOR signaling and thus provided circumstantial evidence for miRNAs involving in the role of TOR in modulating gene expression at post-transcriptional level. These data will provide valuable information for further investigation of the molecular mechanisms of miRNA involved in the TOR signaling pathway.
TOR as a core regulator of cell growth can regulate the many biological process of the organism at different levels such as: gene transcription, protein translation and synthesis and metabolic processes. A number of transcriptome analyses have showed that TOR was involved in the regulation of genes expression at transcriptional level in plants (Dong et al., 2015; Caldana et al., 2013; Song et al., 2017; Xiong et al., 2013). It had found that some transcription factors are involved in TOR signaling pathway in mammals, such as SREBP1, HIF1α and YY1 (Laplante & Sabatini, 2012; Zoncu, Efeyan & Sabatini, 2011; Li et al., 2011; Porstmann et al., 2008; Cunningham et al., 2007). In addition, Xiong & Sheen (2014) and Xiong et al. (2013) had firstly found the transcription factors E2Fa and E2Fb which could directly activate by TOR kinase in Arabidopsis. However, compared with the thousands differential expression genes after TOR inhibition, transcription factors directly regulated by TOR signaling pathway are very limited. In previous studies, it has shown that miRNAs can target to signaling proteins, enzymes, transcription factors and other genes, and Cui et al. declare that miRNAs preferentially target the downstream components of the adaptors, which have potential to recruit more downstream components, such as TFs based on bioinformatics analysis (Cui et al., 2006). In this study, we found that many differentially expressed miRNAs can target to TFs, such as miR164, miR156, miR169, miR172 and so on (Jiao et al., 2010; Wu et al., 2009; Zhao et al., 2009; Guo et al., 2005). In addition, we also found that some function unknown miRNAs can target to TFs based on integrated mRNA and miRNA expression profiling, such as miR5303j- PGSC0003DMG400028381 (a WRKY transcription factor) module and miR8050- PGSC0003DMT400021748 (a GRAS transcription factor) module. Furthermore, Cui et al. showed that variation of expression of miRNA targets is significantly lower than that of other genes in different species (Cui et al., 2007), implying that TOR-miRNA-mRNA(TF) module may be an important complement to TOR- substrate and TOR-TFs pathway in TOR-related conserved functions in eukaryotes, such as ribosome biosynthesis and lipid synthesis (Shi, Wu & Sheen, 2018). On the other hand, our data showed that differentially expressed miRNAs only participates in part of the function of TOR, suggesting that the regulation of gene transcripts by TOR signaling may rely on transcription factors at transcriptional level and other long non-coding RNA at transcriptional or post-transcriptional level. As an important cash crop, potato tubers contain abundant sugar, protein and other nutrients. TOR is a core regulator of metabolism in eukaryotes. MiRNAs also play key roles in many biological processes in animals and plants (Vaucheret, 2006; Shabalina & Koonin, 2008). How to modify these signals to regulate potato metabolism and optimize the nutritional value of potato tuber (secondary metabolites, lipid content and fatty acid composition, starch content or amino acid content) is a future course. In our current study, we predicted multiple TOR-miRNA-RNA regulatory networks for its relevance in biological metabolism. However, their roles in potato growth and development and application values in crop improvement needs further study.
Conclusion
In conclusion, we identified 86 miRNAs that are regulated by TOR signaling in potato. Combined miRNAomics and transcriptomics analysis, more than 120 miRNA-mRNA regulatory networks were identified. These miRNAs may participate in TOR-related pathways including ribosome biosynthesis and lipid synthesis. Our results indicated a need for further experimental studies to reveal the role of TOR-related miRNAs in potato improvement.
Supplemental Information
(A) GO functional enrichments of differentially expressed miRNAs. (B) KEGG pathway enrichments of differentially expressed miRNAs.
(A) Up-regulated miRNAs. (B) Down-regulated miRNAs. (C) Up-regulated mRNAs. (D) Down-regulated mRNAs.
(A) The GO function analysis based on the down expressed mRNA/up expressed miRNA under the TOR inhibition with RAP + KU. (B) The GO function analysis based on the up expressed mRNA/down expressed miRNA under the TOR inhibition with RAP + KU.
Funding Statement
This work was funded by the National Key R&D Program of China (grant number 2018YFD0200808), the National Natural Science Foundation of China (grant number 63004401, 31672206, 31801271 and 31801913), and the China Postdoctoral Science Foundation (grant number 2018M633320). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Pan Dong, Email: dongpan@cqu.edu.cn.
Maozhi Ren, Email: renmaozhi01@caas.cn.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Kexuan Deng and Pan Dong conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Huan Yin performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Fangjie Xiong performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Li Feng performed the experiments, prepared figures and/or tables, and approved the final draft.
Maozhi Ren conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Data is available at GenBank: GSE155877.
References
- Benjamin et al. (2011).Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nature Reviews. Drug Discovery. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- Bhogale et al. (2014).Bhogale S, Mahajan AS, Natarajan B, Rajabhoj M, Thulasiram HV, Banerjee AK. MicroRNA156: a potential graft-transmissible microRNA that modulates plant architecture and tuberization in Solanum tuberosum ssp. andigena. Plant Physiology. 2014;164:1011–1027. doi: 10.1104/pp.113.230714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian et al. (2016).Bian XZE, Ma P, Jia Z, Guo X, Xie Y. Identification of miRNAs in sweet potato by Solexa sequencing. Russian Journal of Plant Physiology. 2016;63:283–292. doi: 10.1134/S1021443716020060. [DOI] [Google Scholar]
- Bornachea et al. (2012).Bornachea O, Santos M, Martinez-Cruz AB, Garcia-Escudero R, Duenas M, Costa C, Segrelles C, Lorz C, Buitrago A, Saiz-Ladera C, Agirre X, Grande T, Paradela B, Maraver A, Ariza JM, Prosper F, Serrano M, Sanchez-Cespedes M, Paramio JM. EMT and induction of miR-21 mediate metastasis development in Trp53-deficient tumours. Scientific Reports. 2012;2:434. doi: 10.1038/srep00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldana et al. (2013).Caldana C, Li Y, Leisse A, Zhang Y, Bartholomaeus L, Fernie AR, Willmitzer L, Giavalisco P. Systemic analysis of inducible target of rapamycin mutants reveal a general metabolic switch controlling growth in Arabidopsis thaliana. The Plant Journal: for Cell and Molecular Biology. 2013;73:897–909. doi: 10.1111/tpj.12080. [DOI] [PubMed] [Google Scholar]
- Cao et al. (2014).Cao X, Wu Z, Jiang F, Zhou R, Yang Z. Identification of chilling stress-responsive tomato microRNAs and their target genes by high-throughput sequencing and degradome analysis. BMC Genomics. 2014;15:1130. doi: 10.1186/1471-2164-15-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen (2009).Chen X. Small RNAs and their roles in plant development. Annual Review of Cell and Developmental Biology. 2009;25:21–44. doi: 10.1146/annurev.cellbio.042308.113417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui et al. (2006).Cui Q, Yu Z, Purisima EO, Wang E. Principles of microRNA regulation of a human cellular signaling network. Molecular Systems Biology. 2006;2 doi: 10.1038/msb4100089. Article 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui et al. (2007).Cui Q, Yu Z, Purisima EO, Wang E. MicroRNA regulation and interspecific variation of gene expression. Trends in Genetics. 2007;23:372–375. doi: 10.1016/j.tig.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Cunningham et al. (2007).Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- Curaba et al. (2012).Curaba J, Spriggs A, Taylor J, Li Z, Helliwell C. miRNA regulation in the early development of barley seed. BMC Plant Biology. 2012;12:120. doi: 10.1186/1471-2229-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng et al. (2017).Deng K, Dong P, Wang W, Feng L, Xiong F, Wang K, Zhang S, Feng S, Wang B, Zhang J, Ren M. The TOR pathway is involved in adventitious root formation in arabidopsis and potato. Frontiers in Plant. 2017;8 doi: 10.3389/fpls.2017.00784. Article 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng et al. (2016).Deng K, Yu L, Zheng X, Zhang K, Wang W, Dong P, Zhang J, Ren M. Target of rapamycin is a key player for auxin signaling transduction in arabidopsis. Frontiers in Plant Science. 2016;7 doi: 10.3389/fpls.2016.00291. Article 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrenel et al. (2013).Dobrenel T, Marchive C, Azzopardi M, Clement G, Moreau M, Sormani R, Robaglia C, Meyer C. Sugar metabolism and the plant target of rapamycin kinase: a sweet operaTOR? Frontiers in Plant Science. 2013;4:93. doi: 10.3389/fpls.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al. (2015).Dong P, Xiong F, Que Y, Wang K, Yu L, Li Z, Ren M. Expression profiling and functional analysis reveals that TOR is a key player in regulating photosynthesis and phytohormone signaling pathways in Arabidopsis. Frontiers in Plant Science. 2015;6 doi: 10.3389/fpls.2015.00677. Article 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang et al. (2012).Fang R, Xiao T, Fang Z, Sun Y, Li F, Gao Y, Feng Y, Li L, Wang Y, Liu X, Chen H, Liu XY, Ji H. MicroRNA-143 (miR-143) regulates cancer glycolysis via targeting hexokinase 2 gene. The Journal of Biological Chemistry. 2012;287:23227–23235. doi: 10.1074/jbc.M112.373084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, Xie & Xiong (2014).Fang Y, Xie K, Xiong L. Conserved miR164-targeted NAC genes negatively regulate drought resistance in rice. Journal of Experimental Botany. 2014;65:2119–2135. doi: 10.1093/jxb/eru072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander et al. (2012).Friedlander MR, Mackowiak SD, Li N, Chen W, Rajewsky N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Research. 2012;40:37–52. doi: 10.1093/nar/gkr688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, sun & Chen (2011).Ge Y, sun Y, Chen J. IGF-II is regulated by microRNA-125b in skeletal myogenesis. The Journal of Cell Biology. 2011;192:69–81. doi: 10.1083/jcb.201007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo et al. (2005).Guo HS, Xie Q, Fei JF, Chua NH. MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for arabidopsis lateral root development. Plant Cell. 2005;17:1376–1386. doi: 10.1105/tpc.105.030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han et al. (2014).Han R, Jian C, Lv J, Yan Y, Chi Q, Li Z, Wang Q, Zhang J, Liu X, Zhao H. Identification and characterization of microRNAs in the flag leaf and developing seed of wheat (Triticum aestivum L.) BMC Genomics. 2014;15:289. doi: 10.1186/1471-2164-15-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong & Jackson (2015).Hong Y, Jackson S. Floral induction and flower formation–the role and potential applications of miRNAs. Plant Biotechnology Journal. 2015;13:282–292. doi: 10.1111/pbi.12340. [DOI] [PubMed] [Google Scholar]
- Hwang, Shin & Kwon (2011).Hwang EW, Shin SJ, Kwon HB. Identification of MicroRNAs and their putative targets that respond to drought stress in Solanum tuberosum. Journal of the Korean Society for Applied Biological Chemistry. 2011;54:317–324. doi: 10.3839/jksabc.2011.051. [DOI] [Google Scholar]
- Iwakawa & Tomari (2013).Iwakawa HO, Tomari Y. Molecular insights into microRNA-mediated translational repression in plants. Molecular Cell. 2013;52:591–601. doi: 10.1016/j.molcel.2013.10.033. [DOI] [PubMed] [Google Scholar]
- Jewell, Flores & Guan (2015).Jewell JL, Flores F, Guan K-L. Micro(RNA) Managing by mTORC1. Molecular Cell. 2015;57:575–576. doi: 10.1016/j.molcel.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian et al. (2018).Jian H, Yang B, Zhang A, Ma J. Genome-Wide Identification of MicroRNAs in response to cadmium stress in oilseed rape (Brassica napus L.) using high-throughput sequencing. International Journal of Molecular Sciences. 2018;19(5) doi: 10.3390/ijms19051431. Article 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao et al. (2010).Jiao Y, Wang YD, Wang J, Yan M, Liu G, Dong G, Zeng D, Lu Z, Zhu X, Qian Q. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nature Genetics. 2010;42:541–544. doi: 10.1038/ng.591. [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades, Bartel & Bartel (2006).Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAS and their regulatory roles in plants. Annual Review of Plant Biology. 2006;57:19–53. doi: 10.1146/annurev.arplant.57.032905.105218. [DOI] [PubMed] [Google Scholar]
- Kitazumia et al. (2015).Kitazumia A, Kawahara Y, Onda TS, De Koeyer D, De los Reyes BG. Implications of miR166 and miR159 induction to the basal response mechanisms of an andigena potato (Solanum tuberosum subsp, andigena) to salinity stress, predicted from network models in Arabidopsis. Genome. 2015;58:13–24. doi: 10.1139/gen-2015-0011. [DOI] [PubMed] [Google Scholar]
- Kozomara & Griffiths-Jones (2014).Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Research. 2014;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead et al. (2009).Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Bology. 2009;10 doi: 10.1186/gb-2009-10-3-r25. Article R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante & Sabatini (2012).Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2012).Li J, Guo G, Guo W, Guo G, Tong D, Ni Z, Sun Q, Yao Y. miRNA164-directed cleavage of ZmNAC1 confers lateral root development in maize (Zea mays L.) BMC Plant Biology. 2012;12:220. doi: 10.1186/1471-2229-12-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2017).Li Y, Hu X, Chen J, Wang W, Xiong X, He C. Integrated mRNA and microRNA transcriptome analysis reveals miRNA regulation in response to PVA in potato. Scientific Reports. 2017;7:16925. doi: 10.1038/s41598-017-17059-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2011).Li S, Ogawa W, Emi A, Hayashi K, Senga Y, Nomura K, Hara K, Yu D, Kasuga M. Role of S6K1 in regulation of SREBP1c expression in the liver. Biochemical and Biophysical Research Communications. 2011;412:197–202. doi: 10.1016/j.bbrc.2011.07.038. [DOI] [PubMed] [Google Scholar]
- Liu & Chen (2010).Liu Q, Chen YQ. A new mechanism in plant engineering: the potential roles of microRNAs in molecular breeding for crop improvement. Biotechnology Advances. 2010;28:301–307. doi: 10.1016/j.biotechadv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2015).Liu Y, Lin-Wang K, Deng C, Warran B, Wang L, Yu B, Yang H, Wang J, Espley RV, Zhang J, Wang D, Allan AC. Comparative Transcriptome Analysis of White and Purple Potato to Identify Genes Involved in Anthocyanin Biosynthesis. PLOS ONE. 2015;10:e0129148. doi: 10.1371/journal.pone.0129148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2018).Liu C, Liu X, Xu W, Fu W, Wang F, Gao J, Li Q, Zhang Z, Li J, Wang S. Identification of miRNAs and their targets in regulating tuberous root development in radish using small RNA and degradome analyses. 3 Biotech. 2018;8 doi: 10.1007/s13205-018-1330-z. Article 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llave et al. (2002).Llave C, Xie Z, Kasschau KD, Carrington JC. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science. 2002;297:2053–2056. doi: 10.1126/science.107631. [DOI] [PubMed] [Google Scholar]
- Loewith & Hall (2011).Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan et al. (2015).Luan M, Xu M, Lu Y, Zhang L, Fan Y, Wang L. Expression of zma-miR169 miRNAs and their target ZmNF-YA genes in response to abiotic stress in maize leaves. Gene. 2015;555:178–185. doi: 10.1016/j.gene.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Mao et al. (2005).Mao X, Cai T, Olyarchuk JG, Wei L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics (Oxford, England) 2005;21:3787–3793. doi: 10.1093/bioinformatics/bti430. [DOI] [PubMed] [Google Scholar]
- Mao et al. (2012).Mao W, Li Z, Xia X, Li Y, Yu J. A combined approach of high-throughput sequencing and degradome analysis reveals tissue specific expression of microRNAs and their targets in cucumber. PLOS ONE. 2012;7:e33040. doi: 10.1371/journal.pone.0033040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marakli (2018).Marakli S. Identification and functional analyses of new sesame miRNAs (Sesamum indicum L.) and their targets. Molecular Biology Reports. 2018;45:2145–2155. doi: 10.1007/s11033-018-4373-7. [DOI] [PubMed] [Google Scholar]
- Martin et al. (2009).Martin A, Adam H, Diaz-Mendoza M, Zurczak M, Gonzalez-Schain ND, Suarez-Lopez P. Graft-transmissible induction of potato tuberization by the microRNA miR172. Development. 2009;136:2873–2881. doi: 10.1242/dev.031658. [DOI] [PubMed] [Google Scholar]
- Meng et al. (2011).Meng Z, Hong D, Jian-Kang Z, Fusuo Z, Wen-Xue L. Involvement of miR169 in the nitrogen-starvation responses in Arabidopsis. New Phytologist. 2011;190:906–915. doi: 10.1111/j.1469-8137.2011.03647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi et al. (2008).Mi S, Cai T, Hu Y, Chen Y, Hodges E, Ni F, Wu L, Li S, Zhou H, Long C, Chen S, Hannon GJ, Qi Y. Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′terminal nucleotide. Cell. 2008;133:116–127. doi: 10.1016/j.cell.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin et al. (2008).Morin RD, Aksay G, Dolgosheina E, Ebhardt HA, Magrini V, Mardis ER, Sahinalp SC, Unrau PJ. Comparative analysis of the small RNA transcriptomes of Pinus contorta and Oryza sativa. Genome Research. 2008;18:571–584. doi: 10.1101/gr.6897308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan et al. (2018).Natarajan B, Kalsi HS, Godbole P, Malankar N, Thiagarayaselvam A Siddappa, S, Thulasiram HV, Chakrabarti SK, Banerjee AK. MiRNA160 is associated with local defense and systemic acquired resistance against Phytophthora infestans infection in potato. Journal of Experimental Botany. 2018;69:2023–2036. doi: 10.1093/jxb/ery025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot et al. (2005).Nicot N, Hausman JF, Hoffmann L, Evers D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. Journal of Experimental Botany. 2005;56:2907–2914. doi: 10.1093/jxb/eri285. [DOI] [PubMed] [Google Scholar]
- Park et al. (2002).Park W, Li J, Song R, Messing J, Chen X. CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Current Biology. 2002;12:1484–1495. doi: 10.1016/S0960-9822(02)01017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payyavula, Singh & Navarre (2013).Payyavula RS, Singh RK, Navarre DA. Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism. Journal of Experimental Botany. 2013;64:5115–5131. doi: 10.1093/jxb/ert303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng et al. (2011).Peng T, Lv Q, Zhang J, Li J, Du Y, Zhao Q. Differential expression of the microRNAs in superior and inferior spikelets in rice (Oryza sativa) Journal of Experimental Botany. 2011;62:4943–4954. doi: 10.1093/jxb/err205. [DOI] [PubMed] [Google Scholar]
- Porstmann et al. (2008).Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metabolism. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart et al. (2002).Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel D. MicroRNAs in plants. Genes & Development. 2002;16:1616–1626. doi: 10.1101/gad.1004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren et al. (2012).Ren M, Venglat P, Qiu S, Feng L, Cao Y, Wang E, Xiang D, Wang J, Alexander D, Chalivendra S, Logan D, Mattoo A, Selvaraj G, Datla R. Target of rapamycin signaling regulates metabolism, growth, and life span in Arabidopsis. Plant Cell. 2012;24:4850–4874. doi: 10.1105/tpc.112.107144.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina & Koonin (2008).Shabalina SA, Koonin EV. Origins and evolution of eukaryotic RNA interference. Trends in Ecology & Evolution. 2008;23:578–587. doi: 10.1016/j.tree.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Wu & Sheen (2018).Shi L, Wu Y, Sheen J. TOR signaling in plants: conservation and innovation. Development. 2018;145(13) doi: 10.1242/dev.160887. Article dev160887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al. (2017).Song Y, Zhao G, Zhang X, Li L, Xiong F, Zhuo F, Zhang C, Yang Z, Datla R, Ren M, Li F. The crosstalk between Target of Rapamycin (TOR) and Jasmonic Acid (JA) signaling existing in Arabidopsis and cotton. Scientific Reports. 2017;7:45830. doi: 10.1038/srep45830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani et al. (2007).Sormani R, Yao L, Menand B, Ennar N, Lecampion C, Meyer C, Robaglia C. Saccharomyces cerevisiae FKBP12 binds Arabidopsis thaliana TOR and its expression in plants leads to rapamycin susceptibility. BMC Plant Biology. 2007;7:26. doi: 10.1186/1471-2229-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey (2003).Storey JD. The positive false discovery rate: a Bayesian interpretation and the q -value. Annals of Statistics. 2003;31:2013–2035. doi: 10.1214/aos/1074290335. [DOI] [Google Scholar]
- Sun (2012).Sun G. MicroRNAs and their diverse functions in plants. Plant Molecular Biology. 2012;80:17–36. doi: 10.1007/s11103-011-9817-6. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2010).Sun Y, Ge Y, Drnevich J, Zhao Y, Band M, Chen J. Mammalian target of rapamycin regulates miRNA-1 and follistatin in skeletal myogenesis. The Journal of Cell Biology. 2010;189:1157–1169. doi: 10.1083/jcb.200912093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang & Chu (2017).Tang J, Chu C. MicroRNAs in crop improvement: fine-tuners for complex traits. Nature Plants. 2017;3 doi: 10.1038/nplants.2017.77. Article 17077. [DOI] [PubMed] [Google Scholar]
- Totary-Jain et al. (2013).Totary-Jain H, Sanoudou D, Ben-Dov IZ, Dautriche CN, Guarnieri P, Marx SO, Tuschl T, Marks AR. Reprogramming of the microRNA transcriptome mediates resistance to rapamycin. The Journal of Biological Chemistry. 2013;288:6034–6044. doi: 10.1074/jbc.M112.416446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leene et al. (2019).Van Leene J, Han C, Gadeyne A, Eeckhout D, Matthijs C, Cannoot B, De Winne N, Persiau G, Van De Slijke E, Van de Cotte B, Stes E, Van Bel M, Storme V, Impens F, Gevaert K, Vandepoele K, De Smet I, De Jaeger G. Capturing the phosphorylation and protein interaction landscape of the plant TOR kinase. Nature Plants. 2019;5:316–327. doi: 10.1038/s41477-019-0378-z. [DOI] [PubMed] [Google Scholar]
- Wen et al. (2012).Wen M, Shen Y, Shi S, Tang T. miREvo: an integrative microRNA evolutionary analysis platform for next-generation sequencing experiments. BMC Bioinformatics. 2012;13:140. doi: 10.1186/1471-2105-13-140.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucheret (2006).Vaucheret H. Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes & Development. 2006;20:759–771. doi: 10.1101/gad.1410506. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2009).Wu G, Park MY, Conway SR, Wang JW, Weigel D, Poethig RS. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell. 2009;138:750–759. doi: 10.1016/j.cell.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2012).Wu HJ, Ma YK, Chen T, Wang M, Wang XJ. PsRobot: a web-based plant small RNA meta-analysis toolbox. Nucleic Acids Research. 2012;40:W22–W28. doi: 10.1093/nar/gks554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Frazier & Zhang (2011).Xie F, Frazier TP, Zhang B. Identification, characterization and expression analysis of MicroRNAs and their targets in the potato (Solanum tuberosum) Gene. 2011;473:8–22. doi: 10.1016/j.gene.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Xie et al. (2014).Xie F, Stewart Jr CN, Taki FA, He Q, Liu H, Zhang B. High-throughput deep sequencing shows that microRNAs play important roles in switchgrass responses to drought and salinity stress. Plant Biotechnology Journal. 2014;12:354–366. doi: 10.1111/pbi.12142. [DOI] [PubMed] [Google Scholar]
- Xiong et al. (2013).Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J. Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature. 2013;496:181–186. doi: 10.1038/nature12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong & Sheen (2012).Xiong Y, Sheen J. Rapamycin and glucose-target of rapamycin (TOR) protein signaling in plants. The Journal of Biological Chemistry. 2012;287:2836–2842. doi: 10.1074/jbc.M111.300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong & Sheen (2014).Xiong Y, Sheen J. The role of target of rapamycin signaling networks in plant growth and metabolism. Plant Physiology. 2014;164:499–512. doi: 10.1104/pp.113.229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong et al. (2017).Xiong F, Zhang R, Meng Z, Deng K, Que Y, Zhuo F, Feng L, Guo S, Datla R, Ren M. Brassinosteriod Insensitive 2 (BIN2) acts as a downstream effector of the Target of Rapamycin (TOR) signaling pathway to regulate photoautotrophic growth in Arabidopsis. The New Phytologist. 2017;213:233–249. doi: 10.1111/nph.14118. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2011).Xu X, Pan S, Cheng S, Zhang B, Mu D, Ni P, Zhang G, Yang S, Li R, Wang J, Orjeda G, Guzman F, Torres M, Lozano R, Ponce O, Martinez D, De la Cruz G, Chakrabarti SK, Patil VU, Skryabin KG, Kuznetsov BB, Ravin NV, Kolganova TV, Beletsky AV, Mardanov AV, Di Genova A, Bolser DM, Martin DM, Li G, Yang Y, Kuang H, Hu Q, Xiong X, Bishop GJ, Sagredo B, Mejia N, Zagorski W, Gromadka R, Gawor J, Szczesny P, Huang S, Zhang Z, Liang C, He J, Li Y, He Y, Xu J, Zhang Y, Xie B, Du Y, Qu D, Bonierbale M, Ghislain M, MR Del Herrera, Giuliano G, Pietrella M, Perrotta G, Facella P, O’Brien K, Feingold SE, Barreiro LE, Massa GA, Diambra L, Whitty BR, Vaillancourt B, Lin H, Massa AN, Geoffroy M, Lundback S, DellaPenna D, Buell CR, Sharma SK, Marshall DF, Waugh R, Bryan GJ, Destefanis M, Nagy I, Milbourne D, Thomson SJ, Fiers M, Jacobs JM, Nielsen KL, Sonderkaer M, Iovene M, Torres GA, Jiang J, Veilleux RE, Bachem CW, De Boer J, Borm T, Kloosterman B, Van Eck H, Datema E, Hekkert B, Goverse A, Van Ham RC, Visser RG. Genome sequence and analysis of the tuber crop potato. Nature. 2011;475:189–195. doi: 10.1038/nature10158. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2010).Yang W, Liu X, Zhang J, Feng J, Li C, Chen J. Prediction and validation of conservative microRNAs of Solanum tuberosum L. Molecular Biology Reports. 2010;37:3081–3087. doi: 10.1007/s11033-009-9881-z. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2015).Yang L, Mu X, Liu C, Cai J, Shi K, Zhu W, Yang Q. Overexpression of potato miR482e enhanced plant sensitivity to Verticillium dahliae infection. Journal of integrative plant biology. 2015;57:1078–1088. doi: 10.1111/jipb.12348. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2016).Yang J, Ning Z, Zhou X, Si H, Di W. Identification of four novel stu-miR169s and their target genes in Solanum tuberosum and expression profiles response to drought stress: = Entwicklungsgeschichte und Systematik der Pflanzen. Plant Systematics & Evolution. 2016;302:55–66. doi: 10.1007/s00606-015-1242-x. [DOI] [Google Scholar]
- Ye et al. (2015).Ye P, Liu Y, Chen C, Tang F, Wu Q, Wang X, Liu C-G, Liu X, Liu R, Liu Y, Zheng P. An mTORC1-Mdm2-Drosha axis for miRNA biogenesis in response to glucose- and amino acid-deprivation. Molecular Cell. 2015;57:708–720. doi: 10.1016/j.molcel.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin et al. (2015).Yin F, Qin C, Gao J, Liu M, Luo X, Zhang W, Liu H, Liao X, Shen Y, Mao L, Zhang Z, Lin H, Lubberstedt T, Pan G. Genome-wide identification and analysis of drought-responsive genes and microRNAs in tobacco. International Journal of Molecular Sciences. 2015;16:5714–5740. doi: 10.3390/ijms16035714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young et al. (2010).Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biology. 2010;11 doi: 10.1186/gb-2010-11-2-r14. Article R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang (2015).Zhang B. MicroRNA: a new target for improving plant tolerance to abiotic stress. Journal of Experimental Botany. 2015;66:1749–1761. doi: 10.1093/jxb/erv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2009a).Zhang L, Chia JM, Kumari S, Stein JC, Liu Z, Narechania A, Maher CA, Guill K, McMullen MD, Ware D. A genome-wide characterization of microRNA genes in maize. PLOS Genetics. 2009a;5:e1000716. doi: 10.1371/journal.pgen.1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2017c).Zhang Y, Huang B, Wang HY, Chang A, Zheng XFS. Emerging Role of MicroRNAs in mTOR Signaling. Cellular and Molecular Life Sciences. 2017c;74:2613–2625. doi: 10.1007/s00018-017-2485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2009b).Zhang W, Luo Y, Gong X, Zeng W, Li S. Computational identification of 48 potato microRNAs and their targets. Computational Biology and Chemistry. 2009b;33:84–93. doi: 10.1016/j.compbiolchem.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2013).Zhang R, Marshall D, Bryan GJ, Hornyik C. Identification and characterization of miRNA transcriptome in potato by high-throughput sequencing. PLOS ONE. 2013;8:e57233. doi: 10.1371/journal.pone.0057233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2017a).Zhang H, Xu F, Wu Y, Hu H-h, Dai X-f. Progress of potato staple food research and industry development in China. Journal of Integrative Agriculture. 2017a;16:2924–2932. doi: 10.1016/S2095-3119(17)61736-2. [DOI] [Google Scholar]
- Zhang et al. (2018).Zhang L, Yao L, Zhang N, Yang J, Zhu X, Tang X, Calderon-Urrea A, Si H. Lateral root development in potato is mediated by stu-mi164 regulation of NAC transcription factor. Frontiers in Plant Science. 2018;9 doi: 10.3389/fpls.2018.00383. Article 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2017b).Zhang H, Zhang J, Yan J, Gou F, Mao Y, Tang G, Botella JR. Short tandem target mimic rice lines uncover functions of miRNAs in regulating important agronomic traits. Proceedings of the National Academy of Sciences of the United States of America. 2017b;114:5277–5282. doi: 10.1073/pnas.1703752114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. (2009).Zhao B, Ge L, Liang R, Li W, Ruan K, Lin H, Jin Y. Members of miR-169 family are induced by high salinity and transiently inhibit the NF-YA transcription factor. BMC Molecular Biology. 2009;10:29. doi: 10.1186/1471-2199-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. (2015).Zhao W, Li Z, Fan J, Hu C, Yang Rand Qi, X, Chen H, Zhao F, Wang S. Identification of jasmonic acid-associated microRNAs and characterization of the regulatory roles of the miR319/TCP4 module under root-knot nematode stress in tomato. Journal of Experimental Botany. 2015;66:4653–4667. doi: 10.1093/jxb/erv238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2010).Zhou L, Chen J, Li Z, Li X, Hu X, Huang Y, Zhao X, Liang C, Wang Y, Sun L, Shi M, Xu X, Shen F, Chen M, Han Z, Peng Z, Zhai Q, Chen J, Zhang Z, Yang R, Ye J, Guan Z, Yang H, Gui Y, Wang J, Cai Z, Zhang X. Integrated profiling of microRNAs and mRNAs: microRNAs located on Xq27.3 associate with clear cell renal cell carcinoma. PLOS ONE. 2010;5:e15224. doi: 10.1371/journal.pone.0015224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2013).Zhu H, Zhou Y, Castillo-Gonzalez C, Lu A, Ge C, Zhao YT, Duan L, Li Z, Axtell MJ, Wang XJ, Zhang X. Bidirectional processing of pri-miRNAs with branched terminal loops by Arabidopsis Dicer-like1. Nature structural & Molecular Biology. 2013;20:1106–1115. doi: 10.1038/nsmb.2646.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu, Efeyan & Sabatini (2011).Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature reviews. Molecular Cell Biology. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) GO functional enrichments of differentially expressed miRNAs. (B) KEGG pathway enrichments of differentially expressed miRNAs.
(A) Up-regulated miRNAs. (B) Down-regulated miRNAs. (C) Up-regulated mRNAs. (D) Down-regulated mRNAs.
(A) The GO function analysis based on the down expressed mRNA/up expressed miRNA under the TOR inhibition with RAP + KU. (B) The GO function analysis based on the up expressed mRNA/down expressed miRNA under the TOR inhibition with RAP + KU.
Data Availability Statement
The following information was supplied regarding data availability:
Data is available at GenBank: GSE155877.