Abstract
Purpose:
KEYNOTE-158 (ClinicalTrials.gov identifier: NCT02628067) investigated the efficacy and safety of pembrolizumab across multiple cancers. We present results from patients with previously treated advanced well-differentiated neuroendocrine tumors (NET).
Patients and Methods:
Pembrolizumab 200 mg was administered every 3 weeks for 2 years or until progression, intolerable toxicity, or physician/patient decision. Tumor imaging was performed every 9 weeks for the first year and then every 12 weeks. Endpoints included objective response rate (ORR) per RECIST v1.1 by independent central radiologic review (primary) and duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety (secondary).
Results:
A total of 107 patients with NETs of the lung, appendix, small intestine, colon, rectum, or pancreas were treated. Median age was 59.0 years (range, 29–80), 44.9% had ECOG performance status 1, 40.2% had received ≥3 prior therapies for advanced disease, and 15.9% had PD-L1–positive tumors (combined positive score ≥1). Median follow-up was 24.2 months (range, 0.6–33.4). ORR was 3.7% (95% CI, 1.0–9.3), with zero complete responses and four partial responses (three pancreatic and one rectal) all in patients with PD-L1–negative tumors. Median DOR was not reached, with one of four responses ongoing after ≥21 months follow-up. Median PFS was 4.1 months (95% CI, 3.5–5.4); the 6-month PFS rate was 39.3%. Median OS was 24.2 months (95% CI, 15.8–32.5). Treatment-related adverse events (AE) occurred in 75.7% of patients, 21.5% of whom had grade 3–5 AEs.
Conclusions:
Pembrolizumab monotherapy showed limited antitumor activity and manageable safety in patients with previously treated advanced well-differentiated NETs.
Introduction
Neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms that arise from secretory cells throughout the diffuse neuroendocrine system (1). Well-differentiated NETs are often indolent and may secrete various peptide hormones and biogenic amines. Although NETs are rare, accounting for approximately 0.5% of newly diagnosed malignancies, the incidence has been increasing over recent decades (2, 3). The precise reason for the increase is uncertain; however, improved diagnosis and classification may be contributing factors (3).
Systemic treatment options for advanced NETs include octreotide, lanreotide, 177lutetium-dotatate, everolimus, and sunitinib (4–6). In addition, several novel biologic therapies are being tested for activity in NETs (7). Typically, stable disease (SD) is the most frequent overall response observed for patients with well-differentiated NETs with the use of current therapies (5, 8, 9). Most patients with advanced NETs will eventually experience disease progression (8,10), highlighting the need for novel treatment options.
Programmed death 1 (PD-1) is a T-cell coinhibitory receptor that regulates immune response by interacting with its ligands (PD-L) (11). In cancer, PD-1 promotes tumor escape from host immune responses (12, 13). Several studies suggest that programmed death ligand 1 (PD-L1) expression plays a role in the development, progression, and prognosis of NETs, especially in high-grade tumors. For example, PD-L1 expression was reported in 59% of pulmonary NETs (14) and 54% of insulinoma-like pancreatic NETs (pNET; ref. 15). Across NET sites, PD-L1 expression was detected in 0% of grade 1, 78% of grade 2, and 100% of grade 3 tumors (16). Consistent with these findings, expression of PD-L1 was rare among archival tissue samples from low-grade NETs of the small intestine (n=64) and pancreas (n = 31; ref. 17). Similar associations between PD-L1 expression and tumor grade have been reported in metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NET; ref. 18). Furthermore, associations between higher PD-L1 expression and decreased survival have been reported in metastatic GEP-NETs and pulmonary NETs (14, 18).
Immune checkpoint inhibitors have demonstrated antitumor activity in many tumor types. One such immune checkpoint inhibitor is pembrolizumab, a highly selective, humanized mAb that blocks the interaction of PD-1 with its ligands, PD-L1 and PD-L2 (19). Single-agent pembrolizumab showed antitumor activity in some patients with previously treated, PD-L1–positive carcinoid and pNETs in the phase Ib KEYNOTE-028 study (20). Overall, three patients with carcinoid (12%; 95% CI, 3%–31%) and one patient with pNETs (6%; 95% CI, 0%–30%) had objective responses, and SD rates were 60% (n = 15) and 88% (n = 14), respectively (20). Moreover, durations of response were 6.9, 9.2, and 11.1 months for the carcinoid responders and the pNET responder had an ongoing response of 17.6 months (20). The KEYNOTE-158 phase II basket study investigated the antitumor activity and safety of pembrolizumab monotherapy in multiple cancer types. Here, we present the results from the cohort of biomarker unselected patients with previously treated advanced well-differentiated NETs enrolled in KEYNOTE-158.
Patients and Methods
Study design and patients
The study design of the KEYNOTE-158 clinical trial (ClinicalTrials.gov identifier: NCT02628067) has been described previously (21). In brief, KEYNOTE-158 is an international, open-label, phase II study of single-agent pembrolizumab across multiple advanced solid tumor types that have progressed on standard-of-care systemic therapy. Key eligibility criteria for the NET cohort included age ≥18 years; well and moderately differentiated NET of the lung, appendix, small intestine, colon, rectum, or pancreas; progression on or intolerance to ≥1 line of standard therapy; measurable disease as assessed by Response Evaluation Criteria in Advanced Solid Tumors version 1.1 (RECIST v1.1) per independent central radiologic review; Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; and adequate organ function. All patients were required to provide tumor tissue from a newly obtained core or excisional biopsy sample (preferred) or archival tumor sample of a nonirradiated lesion for PD-L1 assessment. Patients were enrolled regardless of tumor biomarker expression.
The reasons for exclusion from enrollment included: active central nervous system metastases; active autoimmune disease that required systemic treatment in the previous 2 years; history of noninfectious pneumonitis that required steroids or current pneumonitis; prior therapy with an agent directed against PD-1, PD-L1, PD-L2, or another coinhibitory T-cell receptor; treatment with an antineoplastic monoclonal antibody in the previous 4 weeks; treatment with chemotherapy, targeted small-molecule therapy, or radiotherapy in the previous 2 weeks; or adverse events (AE) from previous therapy that had not resolved to grade ≤1 or baseline.
All patients provided written informed consent. The study protocol was approved by the independent ethics committee or review board at each participating institution. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice.
Study treatment
As described previously (21), pembrolizumab 200 mg was given by intravenous infusion over 30 minutes every 3 weeks for up to 2 years. Reasons for treatment discontinuation included disease progression, intolerable toxicity, physician decision, or patient withdrawal of consent. Clinically stable patients with radiologic disease progression could continue treatment until progression was confirmed at the next imaging assessment (≥4 weeks later) or longer with approval by the study sponsor. Patients who discontinued treatment with SD, partial response (PR), or complete response (CR) and subsequently exhibited disease progression were eligible for an additional 1 year of pembrolizumab.
Assessments
As described previously (21), the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies) at the Neogenomics Laboratories, Inc. testing laboratory was used to analyze tumor PD-L1 expression, determined by using the combined positive score (CPS), the ratio of PD-L1–positive cells (tumor cells, lymphocytes, and macrophages) out of the total number of tumor cells × 100. PD-L1 positivity was defined as CPS ≥1. Tumor imaging by CT (preferred) or MRI was peformed at baseline, at week 9, and every 9 weeks thereafter through 12 months, and then every 12 weeks. Physical examination, vital signs, and laboratory tests were performed at baseline and regularly throughout study treatment. AEs were monitored throughout treatment and for 30 days thereafter (90 days for serious AEs) and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analysis
The statistical analysis methodology has been reported previously (21). The primary endpoint was the objective response rate (ORR; the proportion of patients with a CR or PR). Secondary endpoints included duration of response (the time from first CR or PR to disease progression or death, whichever occurred first); progression-free survival (PFS; the time from first dose to disease progression or death, whichever occurred first); and overall survival (OS; the time from first dose to death). Primary and secondary endpoints were assessed by independent central radiology review based on RECIST v1.1.and were evaluated in the total population and in the PD-L1–positive and PD-L1–negative populations.
Efficacy and safety were assessed in all patients who received ≥1 dose of pembrolizumab. For ORR, point estimates were accompanied by 95% confidence intervals (CIs) using the Clopper–Pearson exact method (22) based on binomial distribution; patients without response data were counted as nonresponders. Duration of response, PFS, and OS were estimated using the Kaplan–Meier method (23). Summary statistics were provided for baseline demographics, disease characteristics, and AEs. This report is based on the data cutoff date of December 6, 2018.
Results
Patients
From February 23, 2016, to August 03, 2016, 107 patients were enrolled at 42 sites in 16 countries (Supplementary Table S1). All patients had received ≥1 dose of pembrolizumab. As of the December 6, 2018, data cutoff, the median follow-up duration was 24.2 months (range, 0.6–33.4). Overall, 104 (97.2%) patients discontinued pembrolizumab, most commonly for disease progression (Supplementary Fig. S1). Median duration of pembrolizumab treatment was 4.5 months (range, 0.03–25.3), and the median number of pembrolizumab doses was 7 (range, 1–35). Baseline characteristics are shown in Table 1. The median age was 59.0 years (range, 29–80) and 44.9% had ECOG performance status (PS) 1. The most common sites of disease were the pancreas (37.4%) and small intestine (23.4%). Overall, 1.9% had previously received adjuvant and/or neoadjuvant therapy only, and 40.2% had received ≥3 previous lines of therapy. At baseline, 17 patients (15.9%) had PD-L1–positive tumors, 83 patients (77.6%) had PD-L1–negative tumors; and seven patients (6.5%) had an unknown PD-L1 expression level.
Table 1.
Baseline demographics and disease characteristics.
| Characteristics | N = 107 |
|---|---|
| Age, years, median (range) | 59 (29–80) |
| <65 years, n (%) | 71 (66.4) |
| ECOG performance status 1, n (%) | 48 (44.9) |
| Stage M1 disease, n (%) | 106 (99.1) |
| PD-L1–positive tumor, n (%) | 17 (15.9) |
| Primary site of disease | |
| Pancreas | 40 (37.4) |
| Small intestine | 25 (23.4) |
| Other gastrointestinal | 18 (16.8) |
| Lung | 14 (13.1) |
| Othera | 10 (9.3) |
| Baseline tumor sizeb, mm, median (range) | 139.4 (11.4–370.4) |
| Prior (neo)adjuvant therapy, n (%) | 8 (7.5) |
| No. prior therapies for recurrent/metastatic disease, n (%) | |
| Adjuvant or neoadjuvantc | 2 (1.9) |
| 0d | 4 (3.7) |
| 1 | 25 (23.4) |
| 2 | 33 (30.8) |
| 3 | 12 (11.2) |
| ≥4 | 31 (29.0) |
Note: Data are presented as n (%) unless otherwise noted.
Includes anus (n = 1), liver (n = 1), multiple sites (n = 4), ovary (n = 1), and unknown (n = 3).
Defined as the sum of the longest diameters of target lesions measurable by central radiology review.
Participants received adjuvant/neoadjuvant therapy alone without recurrence.
Participants did not receive systemic chemotherapy.
Antitumor activity
In the total population (n = 107), 0 patients had a CR and four patients had a PR as assessed by RECIST v1.1 per independent central review (three pancreatic and one rectal), resulting in an ORR of 3.7% (95% CI, 1.0–9.3; Table 2). All four responses were in patients with PD-L1–negative tumors. One had a grade 2 pancreatic NET (Ki-67 18%) who had received prior octreotide and capecitabine/temozolomide chemotherapy. Another had a low-grade pancreatic NET that was progressing aggressively after numerous prior treatments, including octreotide, capecitabine/temozolomide, everolimus, sunitinib, and 177Lutetium-dotatate. A third had an intermediate-grade (Ki-67 index 10%–15%) pancreatic NET, and the fourth had an intermediate-grade rectal NET (Ki-67 index 20%), both progressive on octreotide alone. Median time to response was 3.2 months (range, 2.1–6.2) and median duration of response was not reached (range, 2.2+–27.1+ months; Table 2). One of the four responses (one pancreatic) was ongoing after ≥21 months follow-up (Fig. 1A). Sixty patients (56.1%; 95% CI, 46.1–65.7) had SD as best response; 11 (64.7%) in the PD-L1–positive group, and 45 (54.2%) in the PD-L1–negative group (Table 2). The median duration of SD (95% CI) was 7.6 months (5.6–8.3). An analysis of best percentage change from baseline in target lesion size for the 101 patients who had ≥1 evaluable postbaseline imaging assessment is depicted in Fig. 1B.
Table 2.
Summary of response assessed per RECIST v1.1 by independent central review.
| Total population | Overalla N = 107 |
PD-L1+ N = 17 |
PD-L1− N = 83 |
|---|---|---|---|
| ORR,b % (95% CI) | 3.7 (1.0–9.3) | 0 (0.0–19.5) | 4.8 (1.3–11.9) |
| Best overall response, n (%) | |||
| Complete response | 0 | 0 | 0 |
| Partial responsec | 4 (3.7) | 0 | 4 (4.8) |
| Stable disease | 60 (56.1) | 11 (64.7) | 45 (54.2) |
| Progressive disease | 34 (31.8) | 6 (35.3) | 25 (30.1) |
| Nonevaluabled | 5 (4.7) | 0 | 5 (6.0) |
| No assessmente | 4 (3.7) | 0 | 4 (4.8) |
| Patients with response | N = 4 | N = 0 | N = 4 |
| Time to response, months, median (range) | 3.2 (2.1–6.2) | — | 3.2 (2.1–6.2) |
| Responders without subsequent disease progression, n (%) | 1 (25.0) | — | 1 (25.0) |
| Duration of response, months, median (range) | NR (2.2+-27.1+) | — | NR (2.2+-27.1+) |
Abbreviations: CI, confidence interval; NR, not reached; PR, partial response.
Includes seven patients with unknown PD-L1 expression level.
At the time of analysis, all responses were confirmed.
Responses were three pancreatic and one rectal.
Patients for whom not all target lesions were captured on ≥1 postbaseline imaging assessment.
Patients for whom no postbaseline tumor assessment was performed.
Figure 1.
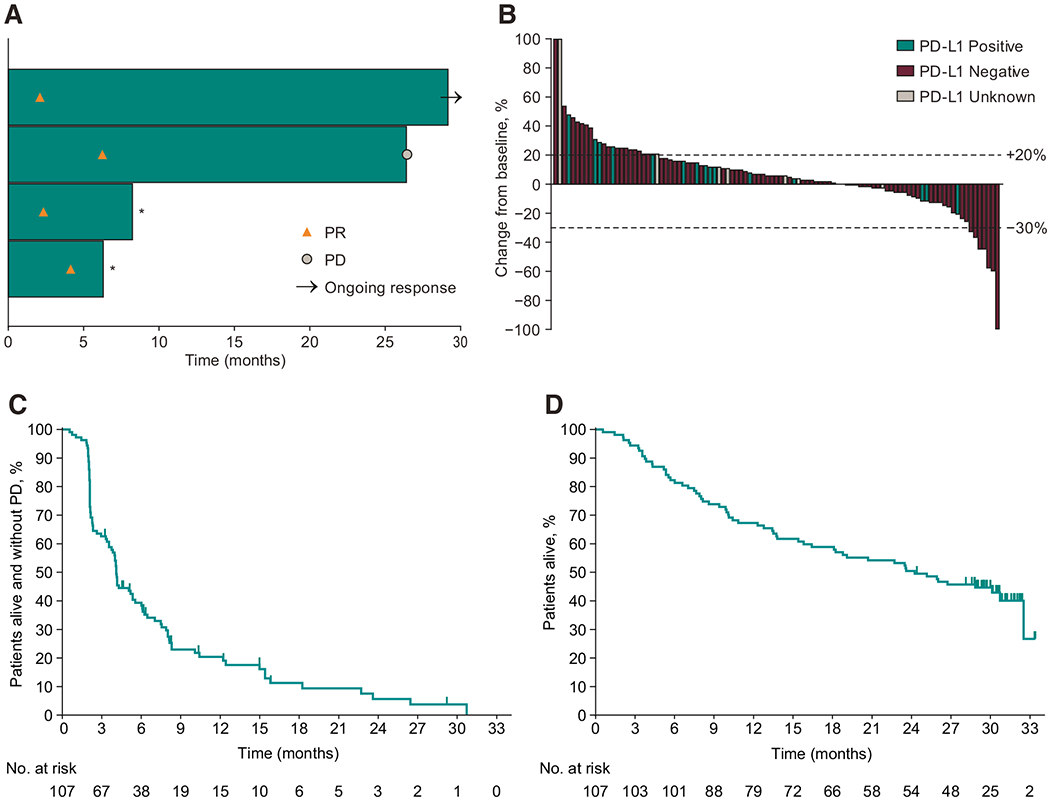
Antitumor activity of pembrolizumab in the total population. A, Time to and duration of response assessed by RECIST v1.1 per independent central review in patients whose best overall response was partial response (n = 4). The length of the bars represents the time to the last imaging assessment. *Started new anticancer therapy without progressive disease. PR, partial response. B, Best change from baseline in target lesion size assessed by RECIST v1.1 per independent central review in patients with ≥1 evaluable postbaseline imaging assessment (n = 101). C, Kaplan-Meier estimates of progression-free survival assessed by RECIST v1.1 per independent central review in the efficacy population (N = 107); D, Kaplan-Meier estimates of overall survival in the efficacy population (N = 107). PR, partial response.
At the time of data cutoff, 92 (86.0%) patients in the total population experienced disease progression or death. Median PFS was 4.1 months (95% CI, 3.5–5.4), and the estimated PFS rate at 12 months was 20.4% (Fig. 1C). Median time-to-progression (95% CI) was 4.3 months (4.0–8.0). A total of 62 (57.9%) patients in the total population had died. Median OS was 24.2 months (95% CI, 15.8–32.5) in the total population (Fig. 1D); the 12-month and 18-month estimates of OS were 67.3% and 58.9%, respectively. None of the four responders had died as of the data cutoff date.
Safety
Overall, 81 (75.7%) patients experienced ≥1 treatment-related AE, including 23 (21.5%) with ≥1 grade 3–5 event (Table 3). There was one (0.9%) treatment-related AE (autoimmune hepatitis) that led to death. Ten (9.3%) patients discontinued pembrolizumab because of treatment-related AEs. The most common treatment-related AEs were fatigue (22.4%) and diarrhea (13.1%). The only treatment-related AEs of grade 3–5 severity that occurred in ≥2 patients were colitis [(n = 2 (1.9%)], ulcerative colitis [n = 2 (1.9%)], autoimmune hepatitis [n = 2 (1.9%)], and hypotension [n = 2 (1.9%)].
Table 3.
Treatment-related AEs of any grade that occurred in ≥5 patients or of ≥grade 3 that occurred in ≥2 patients.
|
N = 107 |
||
|---|---|---|
| Any grade | Grade ≥3 | |
| Any,a n (%) | 81 (75.7) | 23 (21.5) |
| Led to death, n (%) | 1 (0.9) | 1 (0.9) |
| Specific events, n (%) | ||
| Fatigue | 24 (22.4) | 1 (0.9) |
| Diarrhea | 14 (13.1) | 1 (0.9) |
| Asthenia | 12 (11.2) | 1 (0.9) |
| Pruritus | 12 (11.2) | 1 (0.9) |
| Hypothyroidism | 11 (10.3) | 0 |
| Rash | 11 (10.3) | 1 (0.9) |
| Decreased appetite | 11 (10.3) | 0 |
| Nausea | 8 (7.5) | 0 |
| Arthralgia | 7 (6.5) | 0 |
| Headache | 6 (5.6) | 0 |
| Vomiting | 5 (4.7) | 0 |
| Maculopapular rash | 5 (4.7) | 1 (0.9) |
| Colitis | 2 (1.9) | 2 (1.9) |
| Ulcerative colitis | 2 (1.9) | 2 (1.9) |
| Hypotension | 2 (1.9) | 2 (1.9) |
| Autoimmune hepatitis | 2 (1.9) | 2 (1.9) |
| Immune-mediated AEs and infusion reactions that occurred in ≥1 patient | ||
| Any, n (%)b | 24 (22.4) | 9 (8.4) |
| Led to death, n (%) | 1 (0.9) | 1 (0.9) |
| Specific events, n (%) | ||
| Hypothyroidism | 11 (10.3) | 0 |
| Hyperthyroidism | 4 (3.7) | 0 |
| Pneumonitis | 3 (2.8) | 0 |
| Hepatitis | 3 (2.8) | 3 (2.8) |
| Severe skin reactions | 3 (2.8) | 3 (2.8) |
| Colitis | 2 (1.9) | 2 (1.9) |
| Adrenal insufficiency | 2 (1.9) | 1 (0.9) |
| Infusion reaction | 1 (0.9) | 0 |
Note: Data are presented as n (%), where n is the number of patients who experienced ≥1 episode of a given event. Relatedness to treatment was determined by the investigator. Immune-mediated events were based on a list of terms specified by the sponsor and considered regardless of attribution to treatment or immune relatedness by the investigator; related terms were included.
Abbreviation: AE, adverse event.
Immune-mediated AEs and infusion reactions, which were based on a list of terms specified by the sponsor and considered regardless of attribution to study treatment or immune relatedness by the investigator, occurred in 24 (22.4%) patients, including nine (8.4%) who experienced ≥1 grade 3–5 event. The only immune-mediated AE that led to death was the aforementioned autoimmune hepatitis. The most common immune-mediated AE was hypothyroidism (10.3%%; Table 3). The immune-mediated AEs of grade 3–5 severity that occurred in ≥2 patients were hepatitis [n = 3 (2.8%)], severe skin reactions [n = 3 (2.8%)], and colitis [n = 2 (1.9%)].
Discussion
In the KEYNOTE-158 clinical trial, pembrolizumab showed limited antitumor activity in patients with previously treated advanced well-differentiated NETs. The ORR was 3.7%, with no patients achieving a CR and four patients achieving a PR (three pancreatic and one rectal). Three of the four patients had histologically aggressive grade 2 NETs (Ki-67 10%–20%) and the fourth had a history of low-grade pancreatic NET on remote biopsy, but aggressively progressive disease at the time of study enrollment. Given the emergence of advanced pancreatic NETs as a distinct subtype compared with other well-differentiated NETs, it is noteworthy that the response rate in the pancreatic NETs subgroup was 7.5% (three of 40). A recently presented study of the PD-1 inhibitor spartalizumab in NETs reported a response rate of 3% (1/33) in pancreatic NETs (24), suggesting that responses are uncommon in this population. The response rate we observed in gastrointestinal NETs of 2% (1/43) was also similar to the response rate observed with spartalizumab of 3% (1/32). In patients with a PR, the median time to response was 3.2 months. In addition, the responses were durable, with a median duration of response that had not been reached after a 24.2-month median follow-up, and two of the four responses ongoing after 18 months. In NETs, PRs are encouraging because available treatment options typically result in disease stabilization rather than objective responses (4–7). Median OS was 24.2 months (range, 15.8–32.5 months) and OS rates at 12 and 18 months were 67.3% and 58.9%, respectively; however, given the indolent nature of NETs, survival is typically quite long, even in patients with advanced disease.
The current findings are generally consistent with previous results from the phase Ib KEYNOTE-028 clinical trial of pembrolizumab in previously treated patients with PD-L1–positive advanced NETs (20). In that study, pembrolizumab was associated with three PRs in patients with carcinoid tumors (n = 25) and 1 PR in patients with pNETs (n = 16), which resulted in ORRs of 12.0% (95% CI, 2.5–31.2) and 6.3% (95% CI, 0.2–30.2), respectively (20). The median duration of response was 9.2 (range 6.9–11.1) months in the carcinoid cohort, and not reached in the pNET cohort after approximately 24 months of treatment (20).
Tumor PD-L1 expression has been associated with pembrolizumab efficacy across several tumor types (25–28). In contrast to KEYNOTE-028, patients in this study were enrolled regardless of tumor PD-L1 expression. Here, the majority of patients (77.6%) had tumors that did not express PD-L1. There were no objective responses in patients with PD-L1–positive tumors; however, the small number of enrolled patients with PD-L1–positive tumors hampers the reliable comparison of response rates by tumor PD-L1 expression. In addition, recent data signal a potential role for other immune biomarkers and combinations thereof for identifying patients with NETs who would derive optimal benefit from immunotherapy (15). Research to identify which biomarkers may help predict response to immunotherapy is needed.
Pembrolizumab was generally well tolerated in patients with NETs. The incidence of treatment-related AEs was similar to that observed in a former study of pembrolizumab in NETs (20) and in other solid tumor types, with fatigue and diarrhea being the most common events. Overall, 10 patients (9.3%) discontinued pembrolizumab because of treatment-related AEs and 1 treatment-related death occurred. Treatment-related grade 3–5 AEs occurred in 23 patients (21.5%); no single treatment-related grade 3–5 event occurred in ≥3 patients. Immune-mediated AEs and infusion reactions occurred in 24 patients (22.4%) with hypothyroidism being the most common event.
This study had several limitations. One of the main limitations is the lack of a comparator arm. In addition, because this study included patients with previously treated, well-, and moderately differentiated NETs, the results should not be generalized to patients with more aggressive, poorly differentiated neuroendocrine carcinomas. Finally, tumor-specific information regarding tumor grade, mitotic count, and Ki-67 proliferative index, which could influence response to pembrolizumab, was not collected as part of this basket trial.
In conclusion, pembrolizumab monotherapy showed durable antitumor activity in a small subset of patients with previously treated, advanced well-differentiated NETs. All four partial responders had clinical or pathologic indicators of relatively aggressive disease of non-midgut primary. The safety profile was consistent with that previously observed for pembrolizumab in patients with advanced cancer, and no new safety signals were noted. Additional research to inform molecular or immunologic features of responders may help identify a patient population with NETs who would derive clinical benefit from treatment with immune checkpoint inhibitors. Several clinical trials investigating the antitumor efficacy of immune checkpoint inhibitors in NETs are currently underway, including monotherapy with pembrolizumab (NCT02939651, NCT03136055, NCT03190213), avelumab (NCT03278379, NCT03278405, NCT03147404), and other PD-1 receptor antibodies (NCT02955069, NCT03167853), and combination therapy with pembrolizumab and lanreotide depot (NCT03043664), ipilimumab and nivolumab (NCT02923934, NCT02834013), and durvalumab and tremelimumab (NCT03095274).
Supplementary Material
Translational Relevance.
Pembrolizumab monotherapy showed durable antitumor activity in a small subset of patients with previously treated advanced well-differentiated neuroendocrine tumors. The safety profile was consistent with that previously observed for pembrolizumab in patients with advanced cancer and no new safety signals were noted. Additional research to inform molecular or immunologic features of responders may help identify a patient population with neuroendocrine tumors who would derive clinical benefit from treatment with immune checkpoint inhibitors.
Acknowledgments
We thank the patients and their families and caregivers for participating in this study. We also thank all site personnel, the Merck & Co., Inc. study team, and NeoGenomics Laboratories, Inc. for performing tumor PD-L1 assessment. Medical writing and editorial assistance were provided by Christine McCrary Sisk and Michele McColgan, both of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. Also from Merck & Co., Inc., Scott Pruitt contributed to acquisition of funding, collection of data, supervision of research, administrative or logistical support, and study design; Lei Xu contributed statistical expertise; and Susan Zeigenfuss contributed to writing, collection of data, supervision of research, provision of study materials or patients, administrative or logistical support, and clinical science. This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
Disclosure of Potential Conflicts of Interest
J.R. Strosberg reports receiving speakers bureau honoraria from Ipsen and Lexicon. N. Mizuno reports receiving commercial research grants from Taiho Pharmaceutical, AstraZeneca, NanoCarrier, Eisai, MSD, Novartis, Dainippon Sumitomo Pharma, ASLAN Pharmaceuticals, Pharma Valley Center, Incyte Inc., and Yakult Honsha, speakers bureau honoraria from Taiho Pharmaceutical, Novartis, Ono Pharmaceutical, Yakult Honsha, and Teijin Pharma, and is an advisory board member/unpaid consultant for Teijin Pharma. E. Grande reports receiving commercial research grants from Roche, Pfizer, Astra Zeneca, Lexicon, and MSD and speakers bureau honoraria from Pfizer, MSD, Roche, Eusa Pharma, Bristol-Myers Squibb, IPSEN, and ADACAP. R. Shapira-Frommer is an employee/paid consultant for Merck, Clovis Oncology, VBL therapeutics, and Novartis and reports receiving speakers bureau honoraria from Merck, Bristol-Myers Squibb, Astra Zeneca, Novartis, Roche, Medison, and Neopharm. E.K. Bergsland reports receiving research grants from Merck and Novartis (to institution). M. Fakih is an employee/paid consultant for Array, Amgen, and Bayer, and reports receiving speakers bureau honoraria from Amgen. S. Takahashi reports receiving commercial research grants and speakers bureau honoraria from MSD, Daiichi Sankyo, Sanofi, Eisai, Bayer, and Taiho. S.A. Piha-Paul reports receiving other commercial research support from AbbVie, Inc, Alkermes, Aminex Therapeutics, Inc., Amphivena Therapeutics, Inc., BioMarin Pharmaceutical, Inc., Boehringer Ingelheim, Bristol Myers Squibb, Cerulean Pharma Inc., Chugai Pharmaceutical Co., Ltd, Curis, Inc., Daichi Sanko, Eli Lilly, Five Prime Therapeutics, Genmab A/S, GlaxoSmithKline, Helix BioPharma Corp, Incyte Corp., Jacobio Pharmaceuticals Co., Ltd, Medimmune, LLC., Medivation, Inc., Merck Sharp and Dohme Corp., NewLink Genetics Corp./Blue LinkPharmaceuticals, Novartis Pharmaceuticals, Pieris Pharmaceuticals, Inc., Pfizer, Principia Biopharma, Inc., Puma Biotechhology, Inc., Rapt Therapeutics, Inc., Seattle Genetics, Syneos Health, Taiho Oncology, Tesaro, Inc., TransThera Bio, and XuanZhu Biopharma. B.H. O’Neil is an employee/paid consultant for Lilly. S. Thomas reports receiving speakers bureau honoraria from Thomas & Chander Medical Consulting. M.P. Lolkema is an employee/paid consultant for MSD, Amgen, Johnson & Johnson, Roche, Bayer, Sanofi, and Pfizer and reports receiving commercial research grants from MSD, Johnson & Johnson, Astellas, and Sanofi. N. Ibrahim holds ownership interest (including patents) in GlaxoSmithKline and Merck. K.G. Norwood is an employee/paid consultant for Merck. J. Hadoux is an advisory board member/unpaid consultant for IPSEN, and reports receiving other remuneration from Pfizer. No potential conflicts of interest were disclosed by the other authors.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Previous presentation: Presented at the Gastrointestinal Cancers Symposium; January 17–19, 2019; San Francisco, CA; abstract 190.
Supplementary Material Access the most recent supplemental material at: http://clincancerres.aacrjournals.org/content/suppl/2020/01/24/1078-0432.CCR-19-3014.DC1
References
- 1.Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9: 61–72. [DOI] [PubMed] [Google Scholar]
- 2.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huguet I, Grossman AB, O’Toole D. Changes in the epidemiology of neuroendocrine tumours. Neuroendocrinology 2017;104:105–11. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Neuroendocrinetumors Version 3; 2017. https://oncolife.com.ua/doc/nccn/Neuroendocrine_Tumors.pdf.
- 5.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501–13. [DOI] [PubMed] [Google Scholar]
- 6.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of (177)lu-dotatate for midgut neuroendocrine tumors. N Engl J Med 2017; 376:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavel ME, Sers C. WOMEN IN CANCER THEMATIC REVIEW: Systemic therapies in neuroendocrine tumors and novel approaches toward personalized medicine. Endocr Relat Cancer 2016;23:T135–T54. [DOI] [PubMed] [Google Scholar]
- 8.Liu IH, Kunz PL. Biologics in gastrointestinal and pancreatic neuroendocrine tumors. J Gastrointest Oncol 2017;8:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunz PL. Carcinoid and neuroendocrine tumors: building on success. J Clin Oncol 2015;33:1855–63. [DOI] [PubMed] [Google Scholar]
- 11.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 2013;14:1212–8. [DOI] [PubMed] [Google Scholar]
- 12.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99: 12293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- 14.Fan Y, Ma K, Wang C,Ning J, Hu Y,Dong D,et al. Prognostic value of PD-L1 and PD-1 expression in pulmonary neuroendocrine tumors. Onco Targets Ther 2016;9:6075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young K, Ragulan C, Nyamundanda G, Patil Y, Lawlor RT, Cunningham D, et al. Immune landscape of pancreatic neuroendocrine tumours (PanNETs). Ann Oncol 2017;28 (suppl_5:abstr 4280). [Google Scholar]
- 16.Cavalcanti E, Armentano R, Valentini AM, Chieppa M, Caruso ML. Role of PD-L1 expression as a biomarker for GEP neuroendocrine neoplasm grading. Cell Death Dis 2017;8:e3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Silva A, Bowden M, Zhang S, Masugi Y, Thorner AR, Herbert ZT, et al. Characterization of the neuroendocrine tumor immune microenvironment. Pancreas 2018;47:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim ST, Ha SY, Lee S, Ahn S, Lee J, Park SH, et al. The impact of PD-L1 expression in patients with metastatic GEP-NETs. J Cancer 2016;7:484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoja L, Butler MO, Kang SP, Ebbinghaus S, Joshua AM. Pembrolizumab. J Immunother Cancer 2015;3:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehnert JM, Rugo HS, O’Neil BH, Santoro A, Schellens JHM, Cohen RB, et al. Pembrolizumab for patients with PD-L1-positive advanced carcinoid or pancreatic neuroendocrine tumors: results from the KEYNOTE-028 study. Ann Oncol 2017;28. [DOI] [PubMed] [Google Scholar]
- 21.Chung HC, Ros W, Delord J-P, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2019;37: 1470–8. [DOI] [PubMed] [Google Scholar]
- 22.Clopper C, Pearson S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404–13. [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 24.Yao CJ, Strosberg J, Fazio N, Pavel ME, Ruszniewski P, Bergsland E, et al. Activity & safety of spartalizumab (PDR001) in patients (pts) with advanced neuroendocrine tumors (NET) of pancreatic (Pan), gastrointestinal (GI), or thoracic (T) origin, & gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progressed on prior treatment (Tx). Ann Oncol 29:2018. (suppl_8:abstr 13080). [Google Scholar]
- 25.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372: 2521–32. [DOI] [PubMed] [Google Scholar]
- 26.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 27.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- 28.Hui R, Garon EB, Goldman JW, Leighl NB, Hellmann MD, Patnaik A, et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: a phase 1 trial. Ann Oncol 2017;28: 874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


