Abstract
T lymphoblastic lymphoma/leukemia (T-LBL/ALL) is a highly malignant hematological tumor common in young males. Most T-LBL/ALL patients usually initially seek medical treatment for clinical manifestations of non-hematological diseases. Presently, T-ALL chemotherapy is often used for the treatment of T-LBL/ALL internationally. With the application of high-intensity standard chemotherapy, the efficacy and prognosis of T-LBL/ALL are still not optimistic. The authors present a young male patient with facial and neck edema as the initial symptoms. This young patient of T-LBL/ALL was found to have a mediastinal mass after CT examination and he was finally diagnosed as highly malignant T-LBL/ALL. Unfortunately, after undergoing three standard courses of high-intensity chemotherapy, the young male patient eventually died of white blood cell stasis and severe infection caused by hyperleukocytosis. To this end, we find that the prognosis of T-LBL/ALL with multiple gene mutations or fusions and hyperleukocytosis, is extremely poor, and probably becomes a medical problem worthy of continuing resolution in the field of hematology and oncology.
Keywords: T lymphoblastic lymphoma/leukemia, hyperleukocyte, PICALM-MLLT10, prognosis
Introduction
Lymphoblastic lymphoma (LBL), also known as pro-lymphocyte lymphoma, is a kind of highly malignant non-Hodgkin’s lymphoma (NHL) common in young men. T-LBL/ALL often shows common symptoms including mediastinal lymphadenopathy and pleural effusion. It is usually misdiagnosed clinically and pathologically with more than 70% of patients diagnosed at the stage of phases III/IV [1]. This patient with “pre-upper mediastinal occupying position” initially reported to the thoracic surgery department of our hospital and was treated with the following procedures: “supervenous venography + superior vena cava stenting”, “right thoracic closed drainage”, “thoracoscopic pleural biopsy + lymph node biopsy”, and later “bone marrow puncture + pathological biopsy” and other series of diagnoses measures to confirm the diagnosis of the disease before treatment. During the treatment, this patient had poor chemotherapy response, high white blood cell count, and severe infection, accompanied by the possibility of multiple organ failure. The above conditions were fatal to this young male patient. Perhaps there are few or no studies that have reported a case of hyperleukocytic T lymphoblastic lymphoma/leukemia complicated with multi-gene fusion and mutation in the past. We believe that this case report may be considered as a reference material in guiding future clinical practice involving patients with similar clinical features.
Case presentation
A 33-year-old male patient complained of “facial and right neck edema for 3 weeks” and the cause of his illness was not clear. In addition to these clinical signs, he also showed symptoms of dry cough. The lung CT results from his local hospital showed: 1. Anterior superior mediastinum irregular soft tissue occupied space and multiple mediastinal lymph nodes, probably suggestive of mediastinal lymphoma with the possibility of being malignant. A contrast-enhanced CT examination was recommended; 2. An appearance suspicious of right pneumonia Infection; 3. Presence of right pleural effusion and thickening of the right pleura. The outpatient clinic admitted the patient to the thoracic surgery department of our hospital for clinical assessment and treatment. The physical examination results showed the following: temperature of 36.8°C, face slightly puffy with no wrinkles, and ecchymosis. A mass of 3.5 cm×2.5 cm on the left clavicle with no tenderness and adhesion to the local tissues. The mass showed good activity, tough texture, and shallowness. Swollen lymph nodes were present. The lungs were clear, and there was no obvious dry and wet sound. The heart rhythm was even and at a steady pace. The abdomen was soft with no tenderness. There was no swelling of the liver and spleen and no edema was detected in both lower extremities.
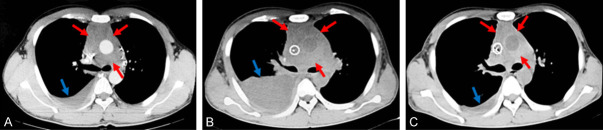
On February 15 of 2019, investigative analysis and examination of the patient revealed the following: WBC 74.20×109/L, Hb 159 g/L, Plt 330×109/L. Differential white blood cell count revealed 10% of lobular nuclei, 2% of lymphocytes, 88% of immature cells, and common platelet distribution. Blood biochemistry investigation revealed 714 IU/L LDH and no abnormalities in the remaining parameters. Abdominal ultrasound investigation revealed: 1. right hepatic hyperechoic plaque (hemangioma?); 2. splenomegaly; 3. prostate enlargement. However, a CT chest scan + enhancement previously conducted showed (Figure 1A): 1. Multiple mediastinal lymph nodes in the mediastinum, right hilar palpitations, and anterior superior mediastinum clumps around the large blood vessels, probably suspicious of lymphoma 2. Right lung inflammation with a small amount of pleural effusion in the right thoracic cavity.
Figure 1.
Three lung CT scans during hospitalization. A. Before diagnosis: anterior superior mediastinal mass with a small amount of pleural effusion; B. Second visit to our hospital: anterior superior mediastinal mass with massive pleural effusion on the right side, incomplete expansion of the right lung; C. After the patient receiving VICLP chemotherapy and antibiotics treatment, the anterior superior mediastinal mass became smaller than before, and the right lung was re-expanded. The right lung inflammation and right pleural effusion decreased (Note: the red arrow shows mass; the blue arrow shows pleural effusion location).
On February 18 of 2019, “supervenous venography + superior vena cava stenting” procedure was carried out on the patient, and the “right thoracic closed drainage” procedure was carried out the following day. Finally, on February 20, 2019, “thoracic pleural biopsy + lymph node biopsy”, surgery, and postoperative recovery procedures were carried out on the patient until February 22, 2019, when he was transferred to the hematology department for further treatment. On February 26, 2019, bone marrow puncture + pathological biopsy was carried out on the patient. The pathological results of lymph nodes and pleural nodules showed: (LN on left clavicle), (right pleural nodule) T lymphoblastic lymphoma. Immunohistochemical results showed that CD79a, bcl-2, CD43, CD34, CD99, CD7 were positive, CD19, CD20 showed lymphocyte positive, CD3, CD5, CD8, CD2, CD4 showed T lymphocyte positive, CD21, CD23 showed FDC network destruction, bcl-6, CD10, Cyclin D1, CKpan, Mum-1, ALP80, Pax-5, CD1a, CD117 were negative. In situ hybridization examination indicated EBER negative. Bone marrow routine examination led to the diagnosis of the patient to be acute lymphoblastic leukemia. Bone marrow pathological examination of patient samples revealed the following (Figure 2): HE and PAS staining examination confirmed the patient condition to be acute leukemia and further immunoassay investigation confirmed it was acute T lymphocytic leukemia, MF-0 to 1. Immunohistochemistry examination also revealed the following (Figure 3): CD2-, CD3±, CD5±, CD7+++, PAX5-, CD99+++, CD34+++, TdT+, MPO+, CD117-, CD20±. Bone marrow flow cytometry analysis showed 91.0% of nucleated cells expressed HLA-DR, CD34, CD7, CD33, CD15dim, and CD38, probably suspicious of acute leukemia. The fusion gene analysis report indicated negative or below detection sensitivity and the peripheral blood gene rearrangement test showed TCRD positive, TCRβ, and TCRγ were negative. Chromosome analysis revealed 46, XY (8), showing 8 normal metaphase karyotypes. The patient was eventually diagnosed with “T lymphoblastic lymphoma/leukemia”.
Figure 2.
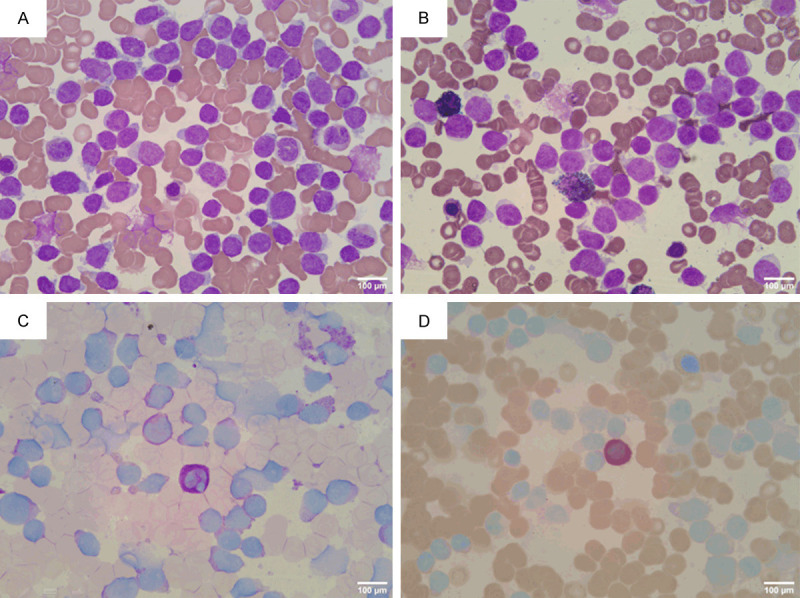
Bone marrow smear: (A) Bone marrow nucleated cell proliferation was markedly active, granulocyte and erythroid hyperplasia were reduced, lymphocytes accounted for 90.5%. Wright-Giemsa stain, ×400; (B) Peroxidase stain was negative (POX ×400); (C) PAS-positive staining rate was 19% (PAS ×400); (D) Naphthol AS-D Chloroacetate stain was negative (NAS-DCE ×400).
Figure 3.
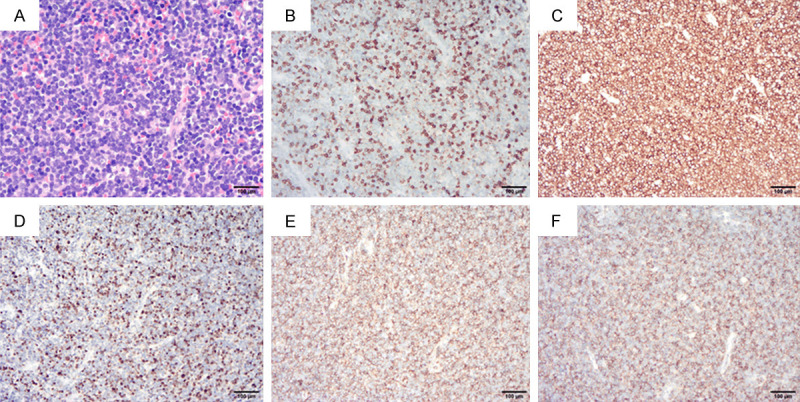
Immunohistochemical examination of bone marrow pathology. (A) Hematoxylin-eosin, ×200; (B) CD3 (±); (C) CD7 (+++); (D) TdT (+); (E) CD34 (+++); (F) CD99 (+++) (B-F. Immunohistochemical staining ×100).
On March 6 of 2019, the patient’s routine blood test showed: WBC 210.60×109/L, Hb 131 g/L, Plt 163×109/L; differential white blood cell count revealed 2% of lobulated nucleus, 98% of immature cells, and platelet distribution was common. Blood biochemistry investigation revealed: ALB 33.9 g/L, LDH 890 IU/L, and no abnormalities were detected in the remaining parameters. The following day the patient was given a chemotherapy regimen of “Hyper-CVAD” (CTX 0.5 g q12 h d1-3; dexamethasone 40 mg d1-4; vindesine 4 mg d4; liposome doxorubicin 20 mg d4 and 40 mg d5), combined with mezlocillin sulbactam antibiotics given orally. These treatments greatly improved the patient symptoms and he was later discharged. However, after his discharge, he did not pay attention to the medical advice to later return for a review, but rather resorted to the use of oral Chinese herbal medicine, specifics of which are unknown.
On April 21 of 2019, the patient again complained of “cough, shortness of breath and fever”. Samples of the patient were taken for the following investigations. The blood routine examination revealed: WBC 575.72×109/L, Hb 58.0 g/L, and Plt 37×109/L; differential white blood cell count revealed 100% of naive cells and platelet distribution was rare. Biochemistry analysis revealed: GLU 2.46 mmol/L, LDH 1285 IU/L, and the remaining parameters indicated no obvious abnormalities. The test of plasma lactate levels indicated 7.90 mmol/L of lactic acid and 207 pg/ml of NT-proBNP. The CT scan of the lungs showed (Figure 1B): There were multiple enlarged lymph nodes in the mediastinum and right hilar palpitations. The anterior superior iliac crest was surrounded by large blood vessels. There was an increase in the right lung inflammation and the right pleural effusion, and the right lung was not inflated as well. The results of the genetic and chromosome examination performed during the patient’s last admission to the hospital were checked: Leukemia fusion gene detection analysis conducted on the patient revealed PICALM-MLLT10 positive, indicating a poor prognosis. Mutation detection reports showed: WT1, KDM6A, PHF6, SH2B3, NOTCH1, and RUNX1 gene mutations. The immunophenotype of acute T lymphocytic leukemia showed the abnormal cell population accounted for 98.14% of nucleated cells and expressed CD34, CD7, CD38, CD33, and CD123. A small number of the cells expressed CD15, and some expressed cytoplasmic CD3, weak expression of HLA-DR, consistent with T lymphoblastic leukemia. After his admission, the leukocytes were treated with leukocyte apheresis. We used “CP” (CTX 200 mg qd d1-3, dexamethasone 10 mg qd d1-3) for the pretreatment chemotherapy, and then “VICLP” (Vindesine 4 mg qd d1, d8, idarubicin 10 mg qd d1-3, d8, CTX 0.6 g qd d1, aspartase 3750 u qd d5, dexamethasone 10 mg qd d1-11, 7.5 mg qd d12-13, 5 mg d14) chemotherapy regimen. At the same time, the patient was as well treated with antibiotics. His fever, cough, shortness of breath, and the other symptoms greatly improved. After the above series of treatments, we again conducted a CT examination of the patient’s lungs. The results as showed in Figure 1C indicate: 1. There were multiple enlarged lymph nodes in the axillary, mediastinum, and right hilum, the anterior superior mediastinal mass became smaller than before, and the right lung was re-expanded well. 2. The right lung inflammation and right pleural effusion decreased. On May 7, 2019, the patient’s blood routine examination showed: WBC 25.06×109/L, Hb 61.0 g/L, Plt 29×109/L; differential white blood cell count revealed: lymphocytes 71%, immature cells 29%, and platelet distribution was rare. The patient was discharged on May 8, 2019, and he never returned to our hospital for chemotherapy again.
We did a follow-up on the patient’s family on June 6th, 2019, and learned that the patient died of severe infection at their local hospital in his hometown on May 25, 2019. We did not know the results of the clinical examinations, investigations, and treatments given to the patient before his death at his local hospital.
Discussion
Lymphoblastic lymphoma (LBL) is a type of highly invasive malignant NHL. When the ratio of naive lymphocytes (lymphoblasts) in the bone marrow is >25%, it can be defined as T-ALL. The WHO defined LBL and acute lymphoblastic leukemia (ALL) to represent the same disease entity in 2008 [2]. Depending on the cellular immunophenotyping, it can be divided into T lymphoblastic lymphoma/leukemia (T-LBL/ALL) and B lymphoblastic lymphoma/leukemia (B-LBL/ALL). T-LBL/ALL has a high recurrent rate, poor prognosis, often involving peripheral blood, bone marrow, and central nervous system with no standard treatment regimens, it accounts for about 85% to 90% of cell lymphoma, 30% of childhood NHL, and <2% of NHL [3].
The incidence of adult high leukocyte acute lymphoblastic leukemia (HALL) accounts for about 10% to 30% of leukemia, and event-free survival (EFS) of adult HALL is low with poor prognosis and high rates of mortality and recurrence [4]. It also presents severe complications such as leukocyte stagnation, diffuse coagulopathy (DIC), and tumor lysis syndrome [5]. We thought that early leukocyte apheresis clinical intervention is necessary to effectively reduce early mortality. Also, the peripheral blood WBC ≥300×109/L at the time of the initial diagnosis of children with ALL is prone to central nervous system infiltration. Children have low EFS, a high recurrence rate, and poor prognosis [6]. Similarly, in adult or elderly acute myeloid leukemia, high leukocyte acute myeloid leukemia (HAML) usually manifests as fast-growing bone marrow failure and is accompanied by serious complications such as abnormal blood coagulation and leukocyte stagnation, and the risk of death is extremely high. Administration of Individualized induction chemotherapy usually improves survival rate [7,8]. A case of chronic myeloid leukemia (CML) with WBC 640.34×109/L in a young male patient was reported, the patient developed microcirculation stasis due to the extremely high white blood cells, which lead to “Leukostasis retinopathy”. Thus, high white blood cell CML may be an indication of a critical and irreversible non-blood system organ involvement [9].
The PICALM-MLLT10 fusion gene (formerly known as CALMAF10), formed from the translocation of t (10;11) (p12-13; q14-21), was originally used to describe diffuse histiocytic lymphoma. It is common in acute T-lymphocytic leukemia (T-ALL) and accounts for about 7% of T-ALL patients, often associated with recurrence and poor prognosis [10]. PICALM-MLLT10 fusion of mature lymphocytic leukemia often expresses CD5, TCRγ/δ, CD4, and CD8, while immature T lymphocytic leukemia expresses CD13, CD33, and CD34. T-ALL and ETP-ALL patients with PICALM-MLLT10 fusion gene-positive had a shorter EFS and OS than PICALM-MLLT10 fusion gene-negative patients [11], this indicates that patients with PICALM-MLLT10 fusion have a poor prognosis. We believe that monitoring of minimal residual disease (MRD) of PICALM-MLLT10 during early high-intensity chemotherapy and post-remission maintenance therapy is one of the hallmarks for assessing recurrence and prognosis to adjust clinical outcomes promptly to maximize patient outcomes.
WT1, KDM6A (UTX) mutations can be found in most acute myeloid leukemia (AML) cases and they were regarded as an unfavorable indicator of poor prognosis. Bordin et al. [12] report the incidence of WT1 mutations in T-ALL and AML cases to be about 10%, prone to drug resistance, with a high risk of recurrence and low long-term survival rate. KDM6A gene is regarded as a driving gene for X-linked T-ALL and its mutation leads to drug resistance in AML therapy [13]. Li et al. [14] reports a 27.1% incidence of PHF6 mutation in adult Asians with T-ALL.%, PHF6 mutation was commonly found in lymphoma of T cell lineage, with significant racial differences. A combination of PHF6 and NOTCH1 mutations resulted in patient’s EFS to be significantly decreased with a poor prognosis. SH2B3 is a negative regulator of the JAK-STAT signaling pathway. The mutation and deletion of the SH2B3 gene is associated with poor prognosis and recurrence in myeloproliferative neoplasms (MPN) and B-ALL. It commonly results in central nervous system (CNS) infiltration and a decreased EFS and OS [15]. RUNX1 mutation, found in myeloid tumors and ETP-ALL, is associated with rapid disease progression and poor clinical efficacy. The dynamic monitoring of RUNX1a and RUNX1b/c isoform expression levels is used as an effective tool for MRD monitoring after chemotherapy. This is used to assess the efficacy of treatment and early detection of recurrence risk [16].
In summary, the early lymphoma symptoms of T lymphoblastic lymphoma/leukemia of the patient reported in this case report are atypical with rapid progress in disease. The patient was diagnosed as highly malignant T-LBL/ALL after extensive diagnoses and treatments. The results of the patient’s bone marrow gene mutation screening suggest the presence of multiple loci mutations such as WT1, KDM6A, PHF6, etc., with poor prognosis, easy relapse, and low overall survival. Besides, given the highly invasive nature of T-LBL/ALL, in the US the NCCN guidelines support that patients with stage I or stage IV are treated according to ALL regimen, for patients with suitable stem cell transplant donors, chemotherapy becomes less necessary and Allo-HSCT is recommended [1]. In the case of this current patient with very high white blood cell count, we thought it wise to treat him with leukocyte apheresis and pre-chemotherapy. We as well ensured hydration, alkalized and diuretics were included in the therapy regimen. We tried to avoid tumor lysis syndrome caused by direct high-intensity chemotherapy, but each course treatments of the Hyper-CVAD and VICLP regimens did not yield CR in the patient. Unfortunately, the young patient and his family abandoned Allo-HSCT and epigenetic targeted drug therapy due to financial reasons. His disease prognosis was very poor with a low survival rate. There was no recommended treatment plan available at the time, and hence a clinical trial was recommended. Perhaps, the use of traditional high-intensity chemotherapy combined with epigenetic targeted drugs may be a better option for the treatment of patients with epigenetic abnormalities to avoid transplantation.
Acknowledgements
This study received funding from the construction project of Fujian medical center of hematology (Min 201704) and sponsored by the National and Fujian Provincial Key Clinical Specialty Discipline Construction Program. The patient’s family had read and approved the contents of this case report and given written informed consent for its publication.
Disclosure of conflict of interest
None.
References
- 1.Guan W, Jing Y, Yu L. Prognostic value of recurrent molecular genetics and epigenetics abnormity in t lymphoblastic lymphoma/leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017;25:587–591. doi: 10.7534/j.issn.1009-2137.2017.02.050. [DOI] [PubMed] [Google Scholar]
- 2.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 3.Bassan R, Maino E, Cortelazzo S. Lymphoblastic lymphoma: an updated review on biology, diagnosis, and treatment. Eur J Haematol. 2016;96:447–460. doi: 10.1111/ejh.12722. [DOI] [PubMed] [Google Scholar]
- 4.Thapa N, Pham R, Cole C, Meinershagen M, Bowman PW, Ray A. Therapeutic leukocytapheresis in infants and children with leukemia and hyperleukocytosis: a single institution experience. J Clin Apheresis. 2018;33:316–323. doi: 10.1002/jca.21610. [DOI] [PubMed] [Google Scholar]
- 5.Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7:1–12. doi: 10.1038/bcj.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong SG, Seo JH, Jun SE, Lee BK, Lim YT. Childhood acute lymphoblastic leukemia with hyperleukocytosis at presentation. Blood Res. 2014;49:29–35. doi: 10.5045/br.2014.49.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giammarco S, Chiusolo P, Piccirillo N, Di Giovanni A, Metafuni E, Laurenti L, Sica S, Pagano L. Hyperleukocytosis and leukostasis: management of a medical emergency. Expert Rev Hematol. 2017;10:147–154. doi: 10.1080/17474086.2017.1270754. [DOI] [PubMed] [Google Scholar]
- 8.Brandwein JM, Zhu N, Kumar R, Leber B, Sabloff M, Sandhu I, Kassis J, Olney HJ, Elemary M, Schuh AC. Treatment of older patients with acute myeloid leukemia(AML): revised Canadian consensus guidelines. Am J Blood Res. 2017;25:30–40. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen B, Yan X, Zhang X, Yang H. Leukostasis retinopathy: an uncommon visual threatening complication of chronic myeloid leukemia with severe hyperleukocytosis - a case report and review of the literature. Indian J Ophthalmol. 2018;66:1871–1874. doi: 10.4103/ijo.IJO_627_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borel C, Dastugue N, Cances-Lauwers V, Mozziconacci MJ, Prebet T, Vey N, Pigneux A, Lippert E, Visanica S, Legrand F, Rault JP, Taviaux S, Bastard C, Mugneret F, Collonges Rames MA, Gachard N, Talmant P, Delabesse E, Récher C. PICALM-MLLT10 acute myeloid leukemia: a French cohort of 18 patients. Leukemia Res. 2012;36:1365–1369. doi: 10.1016/j.leukres.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Abdelali RB, Asnafi V, Petit A, Micol JB, Callens C, Villarese P, Delabesse E, Reman O, Lepretre S, Cahn JY, Guillerm G, Berthon C, Gardin C, Corront B, Leguay T, Béné MC, Ifrah N, Leverger G, Dombret H, Macintyre E. The prognosis of CALM-AF10-positive adult T-cell acute lymphoblastic leukemias depends on the stage of maturation arrest. Haematologica. 2013;98:1711–1717. doi: 10.3324/haematol.2013.086082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bordin F, Piovan E, Masiero E, Ambesi-Impiombato A, Minuzzo S, Bertorelle R, Sacchetto V, Pilotto G, Basso G, Zanovello P, Amadori A, Tosello V. WT1 loss attenuates the TP53-induced DNA damage response in T-cell acute lymphoblastic leukemia. Haematologica. 2018;103:266–277. doi: 10.3324/haematol.2017.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stief SM, Hanneforth AL, Weser S, Mattes R, Carlet M, Liu WH, Bartoschek MD, Moreno HD, Oettle M, Kempf J, Vick B, Ksienzyk B, Tizazu B, Rothenberg-Thurley M, Quentmeier H, Hiddemann W, Vosberg S, Greif PA, Metzeler KH, Schotta G, Bultmann S, Jeremias I, Leonhardt H, Spiekermann K. Loss of KDM6A confers drug resistance in acute myeloid leukemia. Leukemia. 2020;34:50–62. doi: 10.1038/s41375-019-0497-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, Xiao L, Xu J, Zhang R, Guo J, Olson J, Wu Y, Li J, Song C, Ge Z. Co-existence of PHF6 and NOTCH1 mutations in adult T-cell acute lymphoblastic leukemia. Oncol Lett. 2016;12:16–22. doi: 10.3892/ol.2016.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu Y, Han Q, McGrath M, Song C, Ge Z. Clinical significance of novel SH2B3 mutations in adult Chinese acute lymphoblastic leukemia patients. Leukemia Res. 2018;72:67–70. doi: 10.1016/j.leukres.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins CE, Gusscott S, Wong RJ, Shevchuk OO, Rana G, Giambra V, Tyshchenko K, Islam R, Hirst M, Weng AP. RUNX1 promotes cell growth in human T-cell acute lymphoblastic leukemia by transcriptional regulation of key target genes. Exp Hematol. 2018;64:84–96. doi: 10.1016/j.exphem.2018.04.008. [DOI] [PubMed] [Google Scholar]