Abstract
Cancer is a highly proliferative disease, which is caused due to the loss of regulation of cell cycle and apoptosis, DNA damage, faulty repair system etc. The cancer microenvironment plays a pivotal role in disease progression as they contain different types of innate and adaptive immune cells. The most important molecules that establish a correlation between inflammation, innate immunity, adaptive immunity, and cancer are the molecules released by inflammatory cells in cancer microenvironment. These molecules secreted by the immune cells, which might activate a pro-tumorigenic and anti-tumorigenic response in cancer. In inflammatory microenvironment, the equilibrium state of immunosuppressive and immunostimulatory signals are important in tumor suppression. The immunotherapeutic approaches could be more effective in cancer treatment. However, advancement in immunobiology and cancer are improving the prospects of immunotherapy alone and/or in combination with the conventional therapies. Thus, the review attempts to highlight a promising and futuristic immunotherapeutic approach in combination with conventional treatment modalities.
Keywords: Cancer, innate immunity, adaptive immunity, immunotherapies, oncolytic viruses and bacteria, tumor-immuno printing strategy
Introduction
The immune system plays a crucial role in infection. It acts in a cascade manner to counter the pathogenic response both by the innate and adaptive immune systems [1]. They work in tandem to protect the host by specialized immune cells acting in the tumor microenvironment [1,2]. Innate immunity is the forefront protector in our body that generally protects the host by combating harmful microbes and helps in tissue repairing. Adaptive immunity comes into play when innate immunity breaks down and not capable to protect the body, which is based on antigen-specific receptors expressed on clonally expanded B and T lymphocytes. When innate immunity recognizes an infection or tissue injury, it recruits cells like macrophages, fibroblast, mast cell, dendritic cells, and leukocytes (monocytes and neutrophils) [2], which recognizes pathogenic determinants by PAMPs present on microbial nucleic acids, lipoprotein and carbohydrates. It also recognizes intracellular damage by DAMPs, released from injured tissues, with the help of intracellular and surface-expressed PRRs present on these cells. Furthermore, the activated PRRs then activate downstream transcription factors like NF-ĸB, AP-1, CREB, IRF etc. which gets activated and recruit leukocytes at the site of injury to repair microenvironment around the damaged tissue [2]. Thus, the activated leukocytes secrete pro-inflammatory cytokines (TNFα and IL1) and various chemokine’s that initiate the downstream effector cells, which are required for acute or chronic inflammation. Normally, anti-inflammatory cytokines are released after pro-inflammatory cytokines, which combat the effect of pro-inflammatory cytokines. Inflammation has pro-tumorigenic effects as well as anti-tumorigenic effects which are used in cancer immunotherapy [3]. Host defense response normally shares the process of acute inflammation while chronic inflammation is a prolonged inflammation that can lead to cancer [4]. Nearly onethird of cancers are found to be linked with chronic inflammation [5]. A deregulated molecular pathways maintains the connection between the immune system and cancer in the tumor microenvironment; while considering the role of the immune system, inflammation and cancer are well documented [6]. This review provides holistic insights on the role of immune response in cancer and its futuristic manipulations highlighting the scope of immunotherapeutics in prevention and management of cancers.
Origin of immunotherapy and cancer
In 1909, Paul Ehrlich first suggested the idea of cancer immunotherapy and demonstrated that antibodies might have the ability to directly combat cancer cells [7]. Later, in 1950s, Burnet and Thomas hypothesized the concept of immune surveillance, according to which the immune system destroys malignant cells from primary cancer site before they become detectable tumors [8]. However, in 2001, Robert D Schreiber and his colleagues first used the term immunoediting in the light of cancer research to describe the phenomenon wherein tumors are characterized by the immune environment in which they form. In their study, they suggested that the immune response prohibited the development of carcinogen-induced sarcomas and spontaneous epithelial tumors. Besides, they also demonstrated that the tumor suppressor activity of the immune system is crucially reliant on IFN-γ, which partially helps in regulating the immunogenicity of tumor cells. Schreiber and his group provided in experimental evidence supporting the concept of immune surveillance for cancer. However, they had also suggested that tumors developed in the presence of healthy immune system are less immunogenic compared to those that are developed in an immunocompromised host makes the immune system paradoxical in favoring the eventual growth of tumors leading to the escape of the immune response that is better able to escape the immune response [9]. The immune system has four basic tumor eradication strategies: 1) The host is protected from virus-induced tumors by immune shedding of viral load. 2) In case of inflammation, the rapid clearance of pathogens and response of inflammation prevents the inflammatory microenvironment from advancing into the tumor. 3) The immune system identifies explicitly TAAs or molecules secreted by cells under stress to kill tumors. 4) The immune system identifies precancerous and cancerous cells and eradicates them before the damage occurs [10]. As we all know, nothing is perfect in this world likewise, our body’s defense mechanism is not as perfect as it should be able to eradicate the cancer cells. As a result, some tumor cells take advantage and escape the immune surveillance to promote proliferation of the cancer cells. In addition, these tumors are less immunogenic to evade the immune response [11].
The genetic and/or epigenetic alterations in a normal cell transform them into cancer cells. Whereas, it is important to understand the biology of cancer cells which has two standard characteristic features: an uncontrolled cell division and their invasive ability either locally or at distant sites. It is well established that if oncogenes regulate cancer initiation then their progression is further guided by tumor microenvironment. In addition, the inflammatory cells can also influence cancer progression in the tumor microenvironment by distorting the metastatic ability of tumor cells [8]. The six known characteristic features of cancer are: unrestricted replication, predetermined growth signals, insensitivity to growth inhibitors, circumvents programmed cell death, blood vessel development, tissue invasion, and metastasis [9]; where in addition to these, cancer-related inflammation is now becoming seventh [12].
Recently, immunotherapy has shown positive patient outcomes in various clinical trials [13] wherein various exogenously modified immune molecules (interferons, interleukins and monoclonal antibodies) are being manipulated to provide better immune response over conventional therapies, such as chemotherapy/radiotherapy or both along with surgery. Immunotherapies are also recently used with adjuvants, which are termed as neo-adjuvant therapies. These therapies either encourage the activities of specific cells of the immune system or deactivate the signals produced by the cancer cells that help in suppressing the immune response. Therapies, including the endogenous immune mechanisms against cancer will act as a potent determinant to recognize the malignant cells as foreign agents. However, in order to achieve this, multiple immune pathways should be targeted simultaneously, which may offer better clinical outcomes.
Role of immune cells in cancer
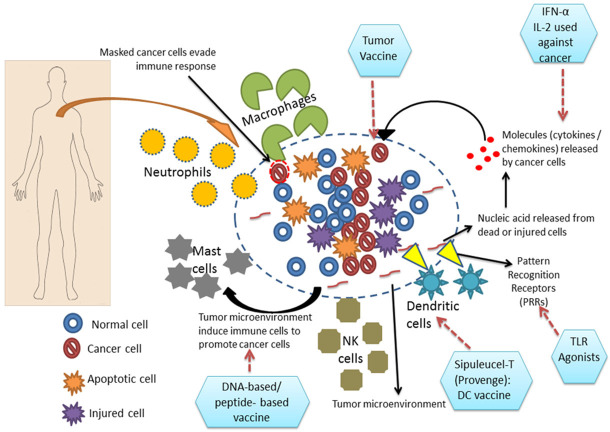
As described in the previous section, the immune cells play a crucial role in carcinogenesis. Both innate immune cells (myeloid progenitors) and adaptive immune cells (lymphoid progenitors) participate either in cancer progression or cancer suppression. The innate immune cells act as the first line of defense against any pathogen which includes dendritic cells, macrophages, neutrophils, mast cells and natural killer cells, whenever, microenvironment around the normal tissue gets disturbed these cells secrete various cytokines, chemokines, growth factors and proteases which hampers the cascade of events that leads to inflammation. Also, the adaptive immune cell-like T-cells and B-cells react to tumor microenvironment, thus making a favorable environment for inflammatory response. These innate and adaptive immune cells in the tumor microenvironment communicate with the cancer cells and the surrounding stromal cells (mesenchymal cells) by autocrine and/or paracrine mechanism(s). In an aggressive and established tumor, the immune response generates towards the pro-inflammatory signaling which results in regression of these tumors very rarely. Both pro-tumor and anti-tumor immune responses co-exist with each other but which way the tumor has to progress is dependent on the tumor microenvironment [14]. Most of the immune cells are involved in the tumor microenvironment, where tumor-associated macrophages (TAMs) and T cells are mainly present in the area where the tumor is present. TAMs mostly promote tumor growth, angiogenesis, invasion and migration and their increased infiltration leads to the poor prognosis of cancer [15]. Mature T-cells are broadly categorized into two major types based on the presence of T cell receptors (TCR): γδ and αβ. αβ-T cells can be further divided into various subgroups like CD8+ cytotoxic T cells (Tc) and CD4+ helper T cells (Th). These Th cells further include Th1, Th2, Th17 and Treg, as well as NK cells. T cells can utilize both pro-tumor and anti-tumor effects [16], where an increase in T cell numbers can activate an increased population of Tc and Th cells which sometimes help in better survival of patients suffering from various types of cancers, melanoma, invasive colon cancer, multiple myeloma, and pancreatic cancers [10]. Sometimes, the lower number of Tc cell involvement increases the susceptibility in experimental animal models towards spontaneous or chemical carcinogenesis [9]. It has also been observed that in case of solid tumors, various T cell types (including CD8+, Th1, Th2, Th17 cells) cause tumor progression [17]. Till now it has been reported that NK cells lack pro-tumorigenic role. Similarly, TAMs and lymphocytes also play a major role in tumor progression including Treg cells which act in a pro-tumorigenic manner by suppressing the antitumor immune responses [18]. Leucocytes forming the major group of the immune cells due to which these can be one of the important determinants among hallmarks of cancer as cancer-related inflammation is also considered as the seventh hallmark of cancer [19,20]. Previously, it was believed that the leucocytes help in immune surveillance to eradicate the tumor, but their diverse role has changed the concept in carcinoma-induced sarcomas and spontaneous epithelial carcinomas where they have shown protection against lymphocytes and IFN-γ [9]. In breast cancer, the occurrence of TILs with a high number of CD4+/CD8+ and the Th2/Th1 ratio is one of the indicators of poor cancer prognosis [21]. Progression and metastasis of mammary cancer is stimulated by Th2 CD4+ T cells by targeting TAMs, giving rise to pro-angiogenic and pro-metastatic factors [22]. Similarly like these immune cells, breast cancer cells also produce several pro-tumorigenic cytokines and chemokines like IL-4, IL-6, IL-8 and CXCR-4, CCL-2, CCL-5 respectively; which cause tumor progression [23]. Till now, the degeneracy of T cell is not clear which arises various queries regarding the factors that determine the fate of T cell whether it will act as anti- or pro-tumorigenic in different types of cancers. As a consequence, these factors are one of the significant factors in immunotherapeutics. The above-mentioned phenomena may be collectively called as “tumorimmuno printing strategy” (TIPS); where both the innate and adaptive immune cells (dendritic cells, macrophages, neutrophils, mast cells, natural killer cells and lymphocytes) infiltrate the tumor stroma and making it more favorable for tumor progression and escape from a further immune response in the tumor microenvironment, and which may have a beneficial impact on both the diagnostic and prognostic approach for cancer management. TIPS may also be beneficial for clinicians and researchers to identify the purpose of various immune cells infiltrated in the tumor stoma, which may eventually dictate the tumor contents for advanced stages of carcinoma (Figure 1).
Figure 1.
The schematic representation demonstrates “tumor-immune printing strategy” (TIPS) the tumor microenvironment developing inside a human body and also representing the implication of immunotherapeutics to combat cancer. In the figure, the hexagonal boxes were used to express the various immunotherapies applied against various target molecules in the tumor microenvironment.
Immune cell infiltration and tumor microenvironment: from immune surveillance to immune editing
Recent studies have highlighted that the immune system may promote the emergence of primary tumor tissues and evade the immune selection process, rather than acting as a suppressor of the disease that might lead to the progression of cancer. Immune surveillance is known to regulate not only in host protection but also the advancement of the tumor in three major steps including elimination, equilibrium, and escape [24]. The process starts when the normal cells are induced to change into transformed cells. The first phase-elimination helps the cancer immune surveillance using extrinsic tumor suppressor response to clear out those transformed cells, thus, giving protection against cancer which is mostly T-cell dependent [10]. If the elimination process fails to clear the transformed cells, then the second phase, i.e., equilibrium comes in action, where cancer persistence occurs due to the genetic instability and immune response [25]. In which the transformed cells maintain their favorable microenvironment to expand their number for the maintenance of cancerous condition [24]. Tumor cells having reduced immunogenicity can survive better in an immunocompetent host however maintenance leads to the escape from immunological surveillance which allows third phase to act resulting in growth of the cancer [22]. During this phase, immune-edited cells grow uncontrollably through immune pressure determining as invasive tumors whereas in other models, a tumor-mediated active immunosuppression is found to enhance the tolerance level of tumor-specific T cell as a dominant immune escape mechanism [23]. In cancer patients, immunoediting shows the main effect of the “triple E” theory (elimination, equilibrium and escape) where clinically seeming tumors inherit the immune response resistance by escaping the adaptive immunity [24]. It can help the process of complete inalterability of most immunotherapies and vaccines for cancer therapy, in a small population of patients with even immunogenic diseases such as melanoma [25]. Neoplastic cells also have capability to enter the inflammatory pathways leading to tumor development by recruiting leukocytes, however, the underlying mechanisms in tumor-mediated inflammatory responses is still unclear. The innate immune cells belonging to myeloid lineage composed of TAMs and immature myeloid cells are found to be involved intrinsically [26]. These cells produce various chemokines, cytokines, proteases and several growth factors, which may promote tumor growth; and mediate local or systemic immunosuppression by inducing angiogenesis and tissue remodeling.
Advancement in cancer therapies
The most conveniently used therapies in cancer are chemotherapy/radiotherapy or both and surgery, that have shown moderate success in the treatment of advanced carcinomas. Despite the use of these conventional therapies as bridges, there is a gap in cancer therapeutic strategies for relapse-free survival of patients [26]. So to combat these drawbacks in the therapeutic strategies there is a need in the advancement of cancer therapy. In the current scenario, immunotherapy is emerging as a new strategy and various other types of immunotherapies are underway for treatment (Table 1). Immunotherapy is combined with conventional therapies or used with adjuvants called neoadjuvant therapies. Therefore, cancer research is leading towards a better advancement in developing contemporary immunotherapies along with modified adjuvants. Adjuvant therapy is a process that includes improvement in a patient’s relapse free long-term survival chances after the patient undergoes primary therapy. Majorly, adjuvant therapies are considered to be systemic, where a substance travels through bloodstream targeting cancer cells in different parts of the body. The process involves chemotherapy, hormone therapy, radiation therapy, and a combination of various other therapies. Among several adjuvant therapies, such as adjuvant chemotherapy, in which drugs kills targeted cancer cells. Previous studies have shown that adjuvant chemotherapy may help in preventing cancer recurrence in early-stage breast cancer patients [27]. Generally, when more than one drug is given during adjuvant chemotherapy, it is known as combination chemotherapy [25] whereas, the hormonal therapy has been proven to be eminent in case of epithelial cancer like breast carcinoma. Tamoxifen has shown to reduce the level of estrogen, and it is already known that estrogen is suitable for the growth of the breast cancer cells. Previous studies have shown that tamoxifen helps in preventing the relapse of breast cancer [28]. Postmenopausal women are usually treated with aromatase inhibitors before or after tamoxifen treatment and instead of tamoxifen; some women are treated with trastuzumab, a monoclonal antibody that helps in reducing the level of Her2 [29]. Generally, radiation therapy is given after mastectomy or lumpectomy, but it is not given at the time of chemo or hormonal therapy. Neo-adjuvant chemotherapy refers to medicines given before surgery to treat breast cancer. Sometimes, it is used in women with large tumors who would have needed a mastectomy but may become a candidate for lumpectomy by reducing the size of invasive tumor before surgery. Both the adjuvant and neo-adjuvant therapies may have side effects depending on patient’s body physiology. Neo-adjuvant therapies are now being used more frequently than adjuvant therapies.
Table 1.
Different types of immunotherapy with their respective examples applied in various cancers
| Types of immunotherapy | Examples | |
|---|---|---|
| Cancer immunotherapy | Naked monoclonal antibodies | Alemtuzumab is used to treat some patients suffering from Chronic Lymphocytic leukemia |
| Conjugated antibodies | Brentuximab vedotin binds to CD30 antigen, used against Hodgkin’s lymphoma | |
| Bispecific monoclonal antibodies | Blinatumomab, whose one half binds to CD19 and the other half binds to CD3, used to treat acute lymphocytic leukemia | |
| Immune checkpoint inhibitors | Monoclonal antibody blockade of the cytotoxic T-lymphocyte antigen-4 (CTLA-4) with ipilimumab | |
| Non-specific immunotherapy | Some interleukins and interferons are used | |
| • IFN-α for use against hairy cell leukemia; | ||
| • IL-2 is approved to treat advanced kidney cancer and metastatic melanoma | ||
| Targeted vaccine therapy | Sipuleucel-T (Provenge) is a dendritic cell vaccine, used to treat advanced prostate cancer | |
| • Tumor cell vaccine | ||
| • Antigen vaccine | ||
| • Peptide-based vaccine | ||
| • DNA-based vaccine | ||
| • Dendritic cell vaccine |
Immunotherapy
It is also known as biological therapy or biotherapy, which utilizes body’s own defense system to fight against diseases such as cancer. In immunotherapy, inhibitors of immune checkpoints are used to abolish the immune tolerance opted by some tumor cells [30]. Several types of immunotherapies have been routinely used such as monoclonal antibodies, cancer vaccines and non-specific immunotherapies. Various kinds of monoclonal antibodies are used in cancer treatment, like nude monoclonal antibodies, which work independently and no drug or radiolabeled substances are attached to it. For example, alemtuzumab is used to treat some patients suffering from Chronic Lymphocytic leukemia. It binds to CD25 antigen. Conjugated antibodies are targeted with chemotherapeutic drugs, radiolabeled toxic substances or any drug capable of killing cancer cells. For example, Brentuximab vedotin binds to CD30 antigen, and used against Hodgkin’s lymphoma. Bispecific monoclonal antibodies are made up of two parts that allows it to bind with two different proteins simultaneously. For example, Blinatumomab, which has two parts; one half binds to CD19 and the other half binds to CD3, and used to treat acute lymphocytic leukemia. Presently, immune inhibitors including monoclonal antibody are in use; for example, CTLA-4 is blocked with ipilimumab; and blocking of PD-1 receptor and the PD-1 ligand by antibodies like Nivolumab (BMS-936558) and MK-3475 (Merck) [31].
The understanding between the immune system and tumor is gradually improving. To withstand self-tolerance and restoration of homeostasis, a family of T-cells assists T-cell activation [32]. DCs and T-cells are the essential immune cells; thus, the recent approaches utilizing TLR signaling via TLR ligands to enhance the anti-tumor immune response [33]. In tumor microenvironment, TLR agonists induce Th1 antibody response and tumor antigen-specific CD8+ T cells to reduce the effect of cancer cells but then also the survivability and clinical response in the cancer patients is very poor; like utilization of TLR agonist against melanoma [34]. Therefore, the utilization of TLR agonist to treat cancer still needs research efforts and improvement to enhance its efficiency.
Targeted vaccination therapy
Previous studies have demonstrated that the regulation of tumor development is well performed by the immune system. It is also reported that adaptive immunity acts as a facilitator of “spontaneous” worsening of tumor cells [35]. The immune system has the notable quality to identify variety of antigens located on tumor cell surfaces, like TAAs [36]. Many predictions have been done by researchers and scientists regarding the vaccination therapy which suggests that this more reliable than standard therapies. From handling the cancer patients to their relapse-free survival, the immune system is necessary to produce a long-term and effective immune response against cancer cells by administering a vaccine. The tumor vaccination may also prove to be a promising strategy. The ideal tumor vaccine should have the ability to induce strong and long-lasting immune response against a broad spectrum of tumor antigens [24]. Scientists are also trying to develop therapy-based vaccinations against cancer cells that might activate the immune system to check the cancer cells. In addition, vaccines are also improvised against cancer cells with peptide-based vaccines, DNA-based vaccines, whole cell-based vaccines, dendritic cells-based vaccines, Anti-HER-2 vaccines, Anti-MUC-1 and Anti-CEA vaccines, and Anti-hTERT vaccines [37]. Cancer can also be caused due to viral and bacterial infections. So, in some cases vaccination might prevent infections that are causing cancers [38]. There are some strains of High-Risk Human Papilloma Virus (HR-HPVs), which causes cervical and other cancers [39,40]. Also, chronic infection, in patients with HBV has a higher chances of developing liver cancer [41,42], and chronic carrier state of Salmonella typhi has also been reported to be associated with gallbladder cancer [43]. But most of the cancers like colorectal, prostate, lung, and breast cancers, are not supposed to be caused due to infections. Doctors are not certain yet regarding the preparation of the vaccine against such type of cancers. Despite of being very promising, the availability of such vaccine will take longer time. Combining with other substances mainly with adjuvants result in enhancing the immune responses up to greater extent. As the immune system has special cells for memory, so researchers are predicting that it might continue to work for long from the time it is given. For example, FDA officially accepted Sipuleucel-T (Provenge®) as the sole vaccine for cancer treatment, thereby abandoning use of Hormonal therapy for advanced prostate cancer [44].
Non-specific cancer immunotherapies and adjuvants
This type of immunotherapies are not specific in their action against cancer cells, but stimulates a general immune system to work against cancer cells. Some cytokines, interleukins and interferons are used in this type of immunotherapy. In melanoma and renal carcinoma, a synthetically made IL-2 is allowed to treat the disease. Thus, IL-2 can be used alone or in combination with chemotherapy or with other cytokines such as IFN-α. Similarly, IL-2 and IFN-α is also taken up for the treatment of cancer. It may facilitate immune cells to win the battle against cancerous cells by hampering the growth process of cancer cells or inhibiting the blood vessels to help in providing nutrition to the tumor cells. The FDA approved some molecules for use in various types of cancer; for example, IFN-α can be used for the treatment of chronic myeloid leukemia, non-Hodgkin’s follicular lymphoma, cutaneous T-cell lymphoma, hairy cell leukemia, kidney cancer, melanoma and Kaposi’s sarcoma. Despite of having advantages of the above-mentioned drugs, they have their own side effects, which can be fatal for the survival of the cancer patients [45].
Advancement in immunotherapy: variety of cancer treating vaccines and emergence of oncolytic viruses and bacteria as an immunotherapeutic tool
Researchers are constantly working in the field of vaccine preparation that are assumed to treat various types of cancers. Among various types of such vaccines; the tumor cell vaccine would be highly promising, as it is being formed of cancer cells from the cancer patients and modified to be attacked by the patient’s immunological system and then injected back into the patient. The immune system targets these or any similar type of cells if persisting in the body. Antigen based vaccines are another type of vaccines that provokes the immune system utilizing only one or few groups of antigens, rather than exploiting the whole tumor cells. As these vaccines are mainly composed of peptides, they are also known as peptide-based vaccines, which are known to activate the immune response (including antibodies, Tc-cells and Th-cells) using antigenic epitopes derived from TAAs. Vaccines wherein DNA encoding the TAAs is taken up by APCs, are termed as DNA-based vaccines [46]. These DNAs will be delivered alone or in combination with other molecules through vectors, nanoparticles or lipoproteins. But in this case, researchers are still figuring out the selection of right vector because of its cumbersome delivery [47]. Dendritic cell vaccines utilize the DCs to raise both class-I and class-II immune response, which further activates the co-stimulatory molecules. This type of immune response can fight against the multiple targets in cancer [48]. Currently in the field of Immunotherapeutics, dendritic cell vaccines are proven to be one of the most successful molecule in treating cancer. For example; Sipuleucel-T (Provenge) is a dendritic cell vaccine, used to treat advanced prostate cancer [49].
Therapies other than radiation, chemo, hormonal, anti-angiogenic and targeted therapies; virotherapy is emerging as one of the promising immunotherapeutic approach in cancer treatment. Those viruses are termed as “oncolytic viruses” which target cancerous cells by evading the normal cells [50]. Examples of such oncolytic viruses contain herpes, measles, adenovirus, coxsackie virus, reovirus, poliovirus, poxviruses, and Newcastle disease viruses, which are part of clinical and preclinical development for cancer therapy [51]. Till date, the Food and Drug Administration (FDA) has approved only one oncolytic virus, a genetically modified form of a herpesvirus, for the treatment of melanoma. However, several viruses are being evaluated as a potential cancer treatment in clinical trials [52]. After infecting tumor cell, the virus copies itself multiple times until the tumor cell burst out and releases substances, such as tumor antigens, that allow the immune system to identify cancer. Thus, certain researchers consider tumor viruses to be a form of immunotherapy. The first oncolytic virus to receive FDA approval was for a skin cancer treatment known as Talimogene Lairbarybvic (Emilic®) or T-VEC; when injected into tumors produces a protein that stimulates the production of immune cells in the body and reduces the risk of developing herpes [53,54]. When it comes to oncolytic bacteria, attenuated Salmonella typhimurium and Clostridium novyi are being used in clinical trials to attack various types of cancer [55].
Cost-effectiveness of immunotherapy
Immunotherapy has proved to help in cure and manage many cancers like melanoma, lymphoma, lung, kidney, and bladder cancers. Doctors have witnessed that with the help of these immunotherapeutic drugs; patients were sent under remission for years rather than dying in short notice of time. But the main drawback of immunotherapy is their high cost, approximately $100,000 per patients that is a major hindrance in the field of immunotherapy. The average cost of cancer immunotherapy drugs has increased from $50,000 per patient in mid1990 to $250,000 today [56]. But when the medical facilities were added up with immunotherapy the prices soared up around $850,000 per patient. Most of the drug companies agree that their investment is too high to prepare these drugs in their R&D laboratories. For example, the making of drug “Kymriah”, Novartis is around $1 billion, but according to researchers at University of Pennsylvania, the total cost for removing, reprogramming, and injecting into the cells in each patient is less than $60,000 which is way less than the so-called high price tags. “Kymriah™ (CT019)” is a first ever artificially prepared CAR T-cell (Chimeric Antigen Receptor (CAR) T-Cell) therapy drug approved by FDA, and produced by Novartis for treating patients up to the age of 25 years with B-cell precursor or acute lymphoblastic leukemia (B-ALL) [57].
Over the past two years, the FDA has approved eight new immunotherapy drugs (MABS and NIBS), but the cost is still so high that it’s not affordable for normal people (https://blog.onco.com/immunotherapy-in-india-cancer-patients). In India, oncologists also agree on the cost-effectiveness of immunotherapy, the first therapy which is between 1-1.3 lakh depending on the patient’s weight, complemented with another therapy usually required after 21 days and stretching for 3-6 months depending on the patient’s condition [58].
Conclusion and future prospects
With the intervention of cost-effective and potential therapeutics, new strategies can be developed to bridge the lacunae surrounding the grey areas in the field of Immunotherapy. Moreover, with the advancement in strategies and technologies, immune cells can lead to the development of cost-effective immunotherapeutic strategies, which can be further used to develop personalized medicine based on tumor immune profiles of patients. Although, adjuvant therapies and other vaccinations are proving to be effective for treating metastatic carcinomas, furthermore there is a huge scope of developing vaccines with very limited side effects. Apart from the conventional methods of surgery, radiation and chemotherapy, cancer immunotherapies are highly expected to emerge as one of the efficient treatment options among all. This has also fueled conventional methods to increase long-term tumor reduction possibility among cancer patients, thereby creating an impactful treatment options.
Acknowledgements
Indian Council of Medical Research (ICMR), New Delhi, India.
Disclosure of conflict of interest
None.
Abbreviations
- NF-ĸB
nuclear factor kappa-light-chain-enhancer of activated B cells
- AP1
Activator protein 1
- CREB
cAMP response element binding protein
- IRF
Interferon regulatory factor
- PAMP
pathogen-associated molecular pattern
- DAMP
damage-associated molecular pattern
- PRR
pattern recognition receptors
- TAA
tumor-specific antigens
- TIPS
tumor-immuno printing strategy
- TAM
tumor-associated macrophages
- Treg
T regulatory cells
- NK
natural killer cells
- TIL
tumor-infiltrating lymphocytes
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- PD1
programmed cell death-1
- HPV
human papillomavirus
- HBV
hepatitis B virus
- APC
antigen-presenting cells
- CAR T-cell
Chimeric Antigen Receptor (CAR) T-Cell
References
- 1.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal G, Pradeep PV, Aggarwal V, Yip CH, Cheung PS. Spectrum of breast cancer in Asian women. World J Surg. 2007;31:1031–1040. doi: 10.1007/s00268-005-0585-9. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 10.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adam JK, Odhav B, Bhoola KD. Immune responses in cancer. Pharmacol Ther. 2003;99:113–132. doi: 10.1016/s0163-7258(03)00056-1. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Gholizadeh Z, Tavakkol-Afshari J, Nikpoor AR, Jalali SA, Jaafari MR. Enhanced immune response induced by P5 HER2/neu-derived peptide-pulsed dendritic cells as a preventive cancer vaccine. J Cell Mol Med. 2018;22:558–567. doi: 10.1111/jcmm.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prendergast GC, Metz R, Muller AJ. Towards a genetic definition of cancer-associated inflammation: role of the IDO pathway. Am J Pathol. 2010;176:2082–2087. doi: 10.2353/ajpath.2010.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 16.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallimore AM, Simon AK. Positive and negative influences of regulatory T cells on tumour immunity. Oncogene. 2008;27:5886–5893. doi: 10.1038/onc.2008.269. [DOI] [PubMed] [Google Scholar]
- 19.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 21.Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005;2:e284. doi: 10.1371/journal.pmed.0020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gingras I, Azim HA Jr, Ignatiadis M, Sotiriou C. Immunology and breast cancer: toward a new way of understanding breast cancer and developing novel therapeutic strategies. Clin Adv Hematol Oncol. 2015;13:372–382. [PubMed] [Google Scholar]
- 24.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 26.Payne KK, Toor AA, Wang XY, Manjili MH. Immunotherapy of cancer: reprogramming tumor-immune crosstalk. Clin Dev Immunol. 2012;2012:760965. doi: 10.1155/2012/760965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chew HK. Adjuvant therapy for breast cancer: who should get what? West J Med. 2001;174:284–287. doi: 10.1136/ewjm.174.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freedman OC, Verma S, Clemons MJ. Using aromatase inhibitors in the neoadjuvant setting: evolution or revolution? Cancer Treat Rev. 2005;31:1–17. doi: 10.1016/j.ctrv.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira AR, Saini KS, Metzger-Filho O. Treatment of early-stage HER2+ breast cancer-an evolving field. Ecancermedicalscience. 2015;9:523. doi: 10.3332/ecancer.2015.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahzari M, Liu D, Arnaout A, Lochnan H. Immune checkpoint inhibitor therapy associated hypophysitis. Clin Med Insights Endocrinol Diabetes. 2015;8:21–28. doi: 10.4137/CMED.S22469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villaruz LC, Kalyan A, Zarour H, Socinski MA. Immunotherapy in lung cancer. Transl Lung Cancer Res. 2014;3:2–14. doi: 10.3978/j.issn.2218-6751.2013.10.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 33.Molenkamp BG, Sluijter BJ, van Leeuwen PA, Santegoets SJ, Meijer S, Wijnands PG, Haanen JB, van den Eertwegh AJ, Scheper RJ, de Gruijl TD. Local administration of PF-3512676 CpG-B instigates tumor-specific CD8+ T-cell reactivity in melanoma patients. Clin Cancer Res. 2008;14:4532–4542. doi: 10.1158/1078-0432.CCR-07-4711. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, Wang B, Shivji GM, Toto P, Amerio P, Tomai MA, Miller RL, Sauder DN. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J Invest Dermatol. 2000;114:135–141. doi: 10.1046/j.1523-1747.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 35.Burnet FM. Immunological aspects of malignant disease. Lancet. 1967;1:1171–1174. doi: 10.1016/s0140-6736(67)92837-1. [DOI] [PubMed] [Google Scholar]
- 36.Anderson KS. Tumor vaccines for breast cancer. Cancer Invest. 2009;27:361–368. doi: 10.1080/07357900802574421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimitrov DS. Therapeutic antibodies, vaccines and antibodyomes. MAbs. 2010;2:347–356. doi: 10.4161/mabs.2.3.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bharadwaj M, Hussain S, Nasare V, Das BC. HPV & HPV vaccination: issues in developing countries. Indian J Med Res. 2009;130:327–333. [PubMed] [Google Scholar]
- 39.Das BC, Hussain S, Nasare V, Bharadwaj M. Prospects and prejudices of human papillomavirus vaccines in India. Vaccine. 2008;26:2669–2679. doi: 10.1016/j.vaccine.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 40.Shukla S, Bharti AC, Mahata S, Hussain S, Kumar R, Hedau S, Das BC. Infection of human papillomaviruses in cancers of different human organ sites. Indian J Med Res. 2009;130:222–233. [PubMed] [Google Scholar]
- 41.De Flora S, La Maestra S. Epidemiology of cancers of infectious origin and prevention strategies. J Prev Med Hyg. 2015;56:E15–E20. [PMC free article] [PubMed] [Google Scholar]
- 42.Bharadwaj M, Roy G, Dutta K, Misbah M, Husain M, Hussain S. Tackling hepatitis B virus-associated hepatocellular carcinoma-the future is now. Cancer Metastasis Rev. 2013;32:229–268. doi: 10.1007/s10555-012-9412-6. [DOI] [PubMed] [Google Scholar]
- 43.Nagaraja V, Eslick GD. Systematic review with meta-analysis: the relationship between chronic Salmonella typhi carrier status and gall-bladder cancer. Aliment Pharmacol Ther. 2014;39:745–750. doi: 10.1111/apt.12655. [DOI] [PubMed] [Google Scholar]
- 44.Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104:599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shefet-Carasso L, Benhar I. Antibody-targeted drugs and drug resistance--challenges and solutions. Drug Resist Updat. 2015;18:36–46. doi: 10.1016/j.drup.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Prud’homme GJ. DNA vaccination against tumors. J Gene Med. 2005;7:3–17. doi: 10.1002/jgm.669. [DOI] [PubMed] [Google Scholar]
- 47.Liu Z, Lv D, Liu S, Gong J, Wang D, Xiong M, Chen X, Xiang R, Tan X. Alginic acid-coated chitosan nanoparticles loaded with legumain DNA vaccine: effect against breast cancer in mice. PLoS One. 2013;8:e60190. doi: 10.1371/journal.pone.0060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Ma B, Zhou Y, Zhang M, Qiu X, Sui Y, Zhang X, Ma B, Fan Q. Dendritic cells fused with allogeneic breast cancer cell line induce tumor antigen-specific CTL responses against autologous breast cancer cells. Breast Cancer Res Treat. 2007;105:277–286. doi: 10.1007/s10549-006-9457-8. [DOI] [PubMed] [Google Scholar]
- 49.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 50.Hemminki O, Dos Santos JM, Hemminki A. Oncolytic viruses for cancer immunotherapy. J Hematol Oncol. 2020;13:84. doi: 10.1186/s13045-020-00922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2016;15:660. doi: 10.1038/nrd.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eissa IR, Bustos-Villalobos I, Ichinose T, Matsumura S, Naoe Y, Miyajima N, Morimoto D, Mukoyama N, Zhiwen W, Tanaka M, Hasegawa H, Sumigama S, Aleksic B, Kodera Y, Kasuya H. The current status and future prospects of oncolytic viruses in clinical trials against melanoma, glioma, pancreatic, and breast cancers. Cancers (Basel) 2018;10:356. doi: 10.3390/cancers10100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White RR, Stanley WE, Johnson JL, Tyler DS, Seigler HF. Long-term survival in 2,505 patients with melanoma with regional lymph node metastasis. Ann Surg. 2002;235:879–887. doi: 10.1097/00000658-200206000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vähä-Koskela MJ, Heikkilä JE, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Guo W, Wu X, Zhang Y, Mannion C, Brouchkov A, Man YG, Chen T. Oncolytic Bacteria and their potential role in bacterium-mediated tumour therapy: a conceptual analysis. J Cancer. 2019;10:4442–4454. doi: 10.7150/jca.35648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verma V, Sprave T, Haque W, Simone CB 2nd, Chang JY, Welsh JW, Thomas CR Jr. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer. 2018;6:128. doi: 10.1186/s40425-018-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ali S, Kjeken R, Niederlaender C, Markey G, Saunders TS, Opsata M, Moltu K, Bremnes B, Grønevik E, Muusse M, Håkonsen GD, Skibeli V, Kalland ME, Wang I, Buajordet I, Urbaniak A, Johnston J, Rantell K, Kerwash E, Schuessler-Lenz M, Salmonson T, Bergh J, Gisselbrecht C, Tzogani K, Papadouli I, Pignatti F. The european medicines agency review of kymriah (tisagenlecleucel) for the treatment of acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Oncologist. 2020;25:e321–e327. doi: 10.1634/theoncologist.2019-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Misra P, Singh S. Role of cytokines in combinatorial immunotherapeutics of non-small cell lung cancer through systems perspective. Cancer Med. 2019;8:1976–1995. doi: 10.1002/cam4.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]