Abstract
Objective: Overweight/obesity has predicted cardiovascular disease (CVD) risk for long with its standard measure of body mass index (BMI), which later was found to mis-classify risk oftentimes. This is because it does not differentiate between fat and whole body mass. The finding that fat especially visceral fat was more culpable shifted attention to ectopic fat as a more precise measure of CVD risk. Peri-renal fat (PRF) is one such ectopic foci, which is hardly used despite the relative ease of assessment. We assessed PRF to correlate it with carotid intima media thickness (CIMT) to see if there was any significance in order to obviate need for heavy equipment in CVD risk assessment. Methods: This is secondary analysis of data generated in the course of studying sub-clinical atherosclerosis in apparently normal individuals. Subjects underwent routine anthropometry to determine BMI. They then underwent abdominal ultrasound studies wherein PRF was measured as the size of the echogenic strip between the posterior part of the liver and the right kidney. The CIMT was measured using the same equipment but a different transducer, as the distance between the intima and medial layers of the right common carotid artery 1 cm proximal to the carotid bulb. Results: The 221 subjects (82 M, 139 F) had mean ages of 37.01±10.97 and 36.86±11.62 years respectively. PRF correlated significantly with CIMT, age and all anthropometric measures. A PRF level of 0.26 cm turned out to be a significant value that determined presence of sub-clinical atherosclerosis deriving from the receiver operating characteristic curve analysis. Conclusion: PRF has shown to be correlated significantly with indices that predict atherosclerosis. Being an ectopic fat focus, its local and systemic effects on the kidney increase systemic vascular resistance and CVD. Since it can easily be measured on abdominal ultrasound, a test readily available and requiring lower level skills it should be used to advantage. Levels above 0.26 cm should prompt initiation of curative or preventive action to control CVD in the population.
Keywords: Peri-renal fat, atherosclerosis, cardiovascular disease, risk, prediction
Introduction
Overweight and obesity have long been acknowledged to predict cardiovascular disease (CVD). The standard index of measure, the body mass index (BMI), has served the purpose until it was noticed to misclassify CVD risk in patients; especially in sub-Sahara Africa (SSA) [1]. This is because it considers both fat and non-fat components of body weight whereas the culprit for CVD is fat. Recently the distribution of fat especially intra-abdominally was found to be most predictive [2]. This is because the fat related to the viscera in the abdomen is most active metabolically; thus-fueling development of hypertension and other cardio metabolic diseases [3].
Measuring visceral fat accurately requires costly and heavy equipment like magnetic resonance and computer tomographic imaging [4]. Additional encumbrances include time consumed and exposure to radiation or contra-indications like medical devices inside the individuals. They are also not appropriate for repetitive measurements [5]. Waist circumference (WC) measurement, though practical and inexpensive is confounded by sub-cutaneous fat and at times reproducibility [6]. The abdominal height (AH) measured by the newly conceptualized appliance called abdominometer seems to overcome this; but its use is still at rudimentary stages [7]. Epicardial fat, which has been found to be a useful measure of CVD risk, requires highly skilled personnel to perform [8]. In our climes, skills for abdominal ultrasound is more readily available, as personnel lower on the skill ladder undertake it. Therefore, peri-renal fat (PRF) data could more readily be available and should be latched upon to predict CVD risk in asymptomatic individuals. PRF is one of the most metabolically active intra-abdominal ectopic fat foci [9]. It has been shown to be associated with hypertension [10] and atherosclerosis in adults [11].
So far, the relationship of PRF measured on abdominal ultrasound to current CVD risk assessment tools in primary care has not been explored in our environment. The purpose of this study was to define the relationship of PRF measured at ultrasound to sub-clinical atherosclerosis indexed by carotid intima-media thickness and other indirect measures of cardio metabolic disease risk.
Materials and methods
This was a secondary analysis of data generated as part of a larger study undertaken in our facility on sub-clinical atherosclerosis in apparently normal individuals. The main study was done in Jos University Teaching Hospital after obtaining ethical clearance from the Research and Ethics committee communicated in a memo JUTH/DCS/ADM/127/XIX/6257 of 25th July 2015. It involved 221 apparently healthy adults in Jos from among senior and junior staff of the hospital, relations of patients and students; who all gave written informed consent. To be included they had to have no previous diagnosis of any chronic cardio metabolic disease nor on any medication related to these. Only adults 18 years or older were included. Pregnancy and puerperium served as exclusion criteria. The sample size was determined statistically by using the formula N = Z2pq/X2 where p is the estimated proportion which has the prevalence of the disease. As there is dearth of data on sub-clinical atherosclerosis in Nigeria we used 15%, the mid-point of the 10-20% prevalence of hypertension in Nigeria as reported by Ike and Ikeh [12] as p. The q is (1-p), X is the desired level of normally taken as 0.05 (95%) confidence interval and Z value corresponding to 1.96. This gave a minimum sample size of 196 but to improve power, we raised it by 12.8% to arrive at 221.
Anthropometry: Body weight was measured using a Hana bathroom-weighing scale to the nearest 0.5 kg with subjects wearing light clothing as much as possible with empty pockets; standing erect on a scale with both legs well placed on it without shoes. The scale was checked for zero error before each measurement. The height measurement was taken as subject stood erect without shoes with a stadiometer in metres. Waist and hip circumferences were measured in standard fashion with a non-flexible tape to the nearest 0.1 cm. Body mass index was generated from the weight and height measurements in kilogram per metre squared using the quotient of weight and square of height as derivation formula. The waist to height and waist to hip ratios (WhtR and WHR respectively) were determined from the values derived from each subject. Abdominal height (AH) was measured using the novel appliance called the Abdominometer in cm as enunciated in a previous study [13-15].
Demographic, behavioral and other risk factors were obtained by interviewer-administered questionnaire and each participant signed an informed consent form prior to commencement of clinical examinations. Pregnant women were excluded from the study and only apparently healthy healthy adults above 18 years were included. The subjects were stratified to include all BMI classifications. BMI < 18.50 kg/m2 defined underweight, 18.50-24.99 kg/m2 defined normal, 25.00-29.99 kg/m2 defined overweight and ≥ 30.00 kg/m2 defined obese. All the information obtained from the physical examination such as body weight, height, waist and hip circumferences including AH were used to calculate anthropometric indices namely: BMI, WHtR, WHR, WC and AH.
Ultrasonography: After satisfactory completion of calibration tests for resolutions and distance measurements with an ultrasound test phantom on the General Electric Logiq 5 Expert Duplex Ultrasound machine, the PRF was measured using a 3.5 MHz probe. This is the echogenic strip between the posterior part of the liver and right kidney; which was measured using the in-built electronic calipers. Using the same equipment but now with a 7.5 MHz transducer with the patient appropriately positioned the carotid artery intima-media thickness (CIMT) was measured as the distance between the intima and media layers of the vessel. An average of 2 measurements was considered and taken at a site 1.0 cm proximal to the carotid bulb. CIMT risk values were generated by assigning 0 to those with CIMT less than 0.078 cm and 1 to those with CIMT values greater than or equal to 0.078; using the sub-clinical atherosclerotic risk cut off value introduced in a previously published work [16].
Statistics: All these information were transferred from Microsoft Excel 2013 into a data sheet of IBM SPSS Statistics (Version 22) software package to perform the statistical and Receiver Operating Curve (ROC) analysis to determine the cut off value of PRF for atherosclerotic risk. Selecting descriptive statistics, the descriptive values (mean ± SD of all the study parameters were obtained. Bi-variate Pearson correlations were performed for all the selected data groups to determine the correlation coefficients and their p values. These include PRF with age, CIMT, and anthropometric indices as well as CIMT with anthropometric indices. Using the compare means section of the software package, paired-sample t test was performed to find the significance between means of study parameters according to gender. For the ROC analysis PRF values were used as the test variable and CIMT rIsk values as the state variable. The value of state variable was used as 1 (presence of sub-clinical atherosclerosis) for the plotting of the ROC curve. The cut-off value of of PRF was determined alongside the area under the curve (AUC) with associated 95% confidence intervals. A level of p ≤ 0.05 was set for statistical significance.
Results
Socio-demographic characteristics: Of the 221 subjects studied, 82 were males and 129 females with mean ages of 37.01±10.97 and 36.86±11.62 respectively. There was no significant difference in age from the perspective of gender. Only few patients smoked, 3 males and 2 females. The difference in proportion was not statistically significant (chi squared of 1.15; P = 0.28). For alcohol use, more males (22) than females (11) admitted to indulgence. The difference in proportion was higher in males than females (chi-squared value of 14.53; P = 0.00).
Anthropometry: All the anthropometric measures differed between the sexes, some more in males than females; but for PRFT and CIMT there was no difference (See Table 1).
Table 1.
Gender influence on anthropometric variables
| Variable | Male Mean ± SD | Female Mean ± SD | P-value |
|---|---|---|---|
| Age (Years) | 37.01±10.97 | 36.86±11.62 | 0.925 |
| PRFT (cm) | 0.27±0.07 | 0.27±0.08 | 0.994 |
| CIMT (cm) | 0.05±0.02 | 0.05±0.01 | 0.528 |
| BMI (kg/m2) | 25.32±4.20 | 28.65±5.93 | 0.000 |
| WHR | 0.92±0.07 | 0.89±0.09 | 0.018 |
| WHtR | 0.53±0.07 | 0.60±0.10 | 0.000 |
| WC (cm) | 89.73±11.77 | 94.51±15.49 | 0.016 |
| AH (cm) | 23.28±3.95 | 24.85±4.95 | 0.015 |
CORRELATIONS: PRFT correlated significantly with CIMT, age and the anthropometric indices namely WHtR, WHR, WC and AH (See Table 2). CIMT also correlated significantly with all the anthropometric indices used in this study (See Table 3).
Table 2.
Bivariate pearson correlations of prft with cimt, age and anthropometric indices
| PARAMETERS | CORRELATION (MALE/FEMALE) | CORRELATION (TOTAL) |
|---|---|---|
| PRFT Vs CIMT | 0.188/0.203* | 0.195** |
| PRFT Vs AGE | 0.242*/0.282** | 0.269** |
| PRFT Vs WHR | 0.223*/0.399** | 0.346** |
| PRFT Vs BMI | 0.323**/0.435** | 0.388** |
| PRFT Vs AH | 0.341**/0.436** | 0.404** |
| PRFT Vs WHtR | 0.316**/0.487** | 0.415** |
| PRFTT Vs WC | 0.328**/0.460** | 0.419** |
Data are correlation coefficients.
significance at p < 0.01;
significance at P < 0.05.
Table 3.
Bivariate pearson correlations of cimt with anthropometric indices
| PARAMETERS | CORRELATION (MALE/FEMALE) | CORRELATION (TOTAL) |
|---|---|---|
| CIMT Vs BMI | 0.365**/0.313** | 0.296** |
| CIMT Vs WHR | 0.452**/0.289** | 0.342** |
| CIMT Vs WHtR | 0.453**/0.372** | 0.349** |
| CIMT Vs WC | 0.475**/0.364** | 0.382** |
| CIMT Vs AH | 0.470**/0.370** | 0.386** |
Data represent correlation coefficients.
significance at P < 0.01.
Receiver operating characteristic analysis: Using the receiver operating characteristic curve analysis and taking risk of sub-clinical atherosclerosis as CIMT ≥ 0.078 cm [15]; the cut off value of PRFT for sub-clinical atherosclerosis turned out to be 0.26 cm with 80% sensitivity and 44% specificity. Area under the curve with 95% confidence intervals of lower and upper boundaries of 0.457 and 0.690 respectively (See Figure 1).
Figure 1.
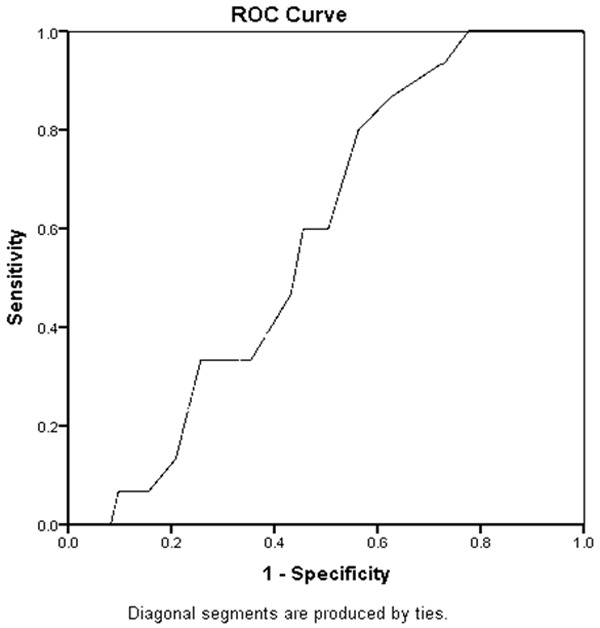
Roc curve for prft and sub-clinical atherosclerotic cvd risk. Using CIMT ≥ 0.078 cm.
Discussion
In this study, PRFT has shown positive correlation with indices that predict atherosclerosis namely: CIMT, WHtR, WHR, WC and AH. This is in alignment with findings in a pediatric population by Bassols et al [9]. This implies that it has a bearing like the other indices on atherosclerosis. Ectopic fat deposition generally has been incriminated in the pathophysiology of diabetes mellitus, insulin resistance and cardiovascular diseases [17]. Visceral adipose tissue (VAT) generally increases lipolysis and with increased delivery of free fatty acids (FFA) to the liver, insulin resistance results [18]. Low-grade inflammation is high in instances of high VAT. Inflamed fat produces cytokines that promote atherosclerosis [19].
Uniquely, when the fat is around the kidneys, consequent microalbuminuria has been shown to be a grave risk factor for CVD [20]. After adjusting for BMI in patients with type 2 DM, estimated glomerular filtration rate (GFR) and uric acid levels were associated with para and peri-renal fat [21]. These are factors associated with atherosclerosis. From animal studies, accumulation of fat in the peri-renal sinus compresses low-pressure vessels and ureters [22]. These structural and functional pertubations in the kidney increase systemic vascular resistance and CVD [23]. In humans as well, intra-renal pressures rise in the presence of peri-renal fat; impairing pressure natriuresis and increasing blood pressure [24]. Therefore, when fat is around the kidneys, intra-renal pressures, cytokines and uric acid levels rise; causing high blood pressure, increased vascular resistance and microalbuminuria. These accelerate atherosclerosis and CVD.
This work is limited in being a single centre study and among only Nigerians, which affects generalization of findings. Its strength however is in the fact that without full gamut of work-up, one-off abdominal ultrasound study carries information that will help in preventing CVD morbi-mortality. In conclusion, for our resource-constrained population where high-tech equipment to detect atherosclerosis are not commonplace, PRFT in excess of 0.25 cm detected from abdominal ultrasound study should lead to initiation of action to prevent or mitigate atherosclerotic CVD.
Acknowledgements
Authors wish to thank all the apparently normal subjects who volunteered for this study and staff of Radiology Department Jos University Teaching Hospital who assisted with logistics at different stages of the study.
Disclosure of conflict of interest
None.
References
- 1.Murphy GAV, Asiki G, Nsubuga RN, Young EH, Seeley J, Sandhu MS, Kamali A. The use of anthrpometric measures for cardiometabolic risk identification in a rural African population. Diabetes Care. 2014;37:e64–e65. doi: 10.2337/dc13-2096. [DOI] [PubMed] [Google Scholar]
- 2.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenaut B, Cuevas A, Hu FB, Griffin BA, Zambon A, Barter P, Fruchart JC, Eckel RH, Matsuzawa Y, Despres JP. Waist circumference as a vital sign in clinical practice: a concensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. 2020;16:177–189. doi: 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassan M, Latif N, Yacoub M. Adipose tissue: friend or foe? Nat Rev Cardiol. 2012;9:689–702. doi: 10.1038/nrcardio.2012.148. [DOI] [PubMed] [Google Scholar]
- 4.Kang SM, Yoon JW, Ahn HY, Kun SY, Lee KH, Shin H, Choi SH, Park KS, Jang HC, Lim S. Android fat depot is more closely associated with metabolic syndrome than abdominal visceral fat in elderly people. PLoS One. 2011;6:e27694. doi: 10.1371/journal.pone.0027694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner DT, Hall EJ. Computed tomography-an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 6.Whaley-Connel A, Sowers JR. Indices of obesity and cardiometabolic risk. Hypertens. 2011;58:991–993. doi: 10.1161/HYPERTENSIONAHA.111.180406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okeahialam BN, Diala UM, Uwakwe J, Ejeh I, Ozoilo U. Utility of the abdominometer. A novel contribution to cardiovascular anthropometry. Food Nutr Sci. 2015;6:1202–1207. [Google Scholar]
- 8.Nelson MR, Mookadam F, Thota V, Emani U, Al-Harthi M, Lester SJ, Che S, Stepanek J, Hurst RT. Epicardial fat: an additional measurement for sub-clinical atherosclerosis and cardiovascular risk stratification? J Am Soc Echocardiogr. 2011;24:339–345. doi: 10.1016/j.echo.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Bassols J, Martinez-Calcerrada JM, Prats-Puig A, Carrreras-Badosa G, Xargay-Torrent S, Lizarraga-Mollinedo E, Feliu-Alsima M, Riera-Perez E, Osiniri I, de Zegher F, Ibanez L, Lopez-Bermejo A. Perirenal fat is related to carotid intima-media thickness in children. Int J Obes (Lond) 2018;42:641–647. doi: 10.1038/ijo.2017.236. [DOI] [PubMed] [Google Scholar]
- 10.De Pergola G, Campobasso N, Nardecchia A, Triggiani V, Caccavo D, Gesualdo L, Silvestre F, Mamo C. Para and peri renal ultrasonographic fat thickness is associated with 24 -hours mean diastolic blood pressure levels in overweight and obese subjects. BMC Cardiovasc Disord. 2015;15:108. doi: 10.1186/s12872-015-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grima P, Guido M, Zizza A, Chiavaroli R. Sonographically measured perirenal fat thickness: an early predictor of atherosclerosis in HIV 1 infected patients receiving highly active antiretroviral therapy? J Clin Ultrasound. 2010;38:190–195. doi: 10.1002/jcu.20664. [DOI] [PubMed] [Google Scholar]
- 12.Ike S, Ikeh V. The prevalence of diastolic dysfunction in adult hypertensive Nigerians. Ghana Med J. 2006;40:55–60. doi: 10.4314/gmj.v40i2.36018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okeahialam BN, Diala UM, Uwakwe J, Ejeh I, Ozoilo U. Abdominal height measures cardiometabolic risk better than body mass index: result of a preliminary study. J Med Res. 2016;2:149–151. [Google Scholar]
- 14.Sirisena AI, Okeahialam BN, Ike EE, Pam SD, Barki JL. Abdominometer: a novel instrument to determine the level of risk for cardio-metabolic diseases. Nig J Cardiol. 2018;15:41–44. [Google Scholar]
- 15.Sirisena AI, Okeahialam BN, Ike EE, Chagok NMD, Pam SD, Ani CC. Clinical significance of the abdominal height measured with a locally made abdominometer for the risk evaluation of sub-clinical atherosclerosis. AJMP. 2019;2:15–20. [Google Scholar]
- 16.Ssinabulya I, Kayima J, Longenecker C, Luwedde M, Semitala F, Kambugu A, Ameda F, Bugeza S, McComsey G, Freers J, Nakanjako D. Sub-clinical atherosclerosis among HIV/AIDS care at two large HIV clinics in Uganda. PLoS One. 2014;9:e89537. doi: 10.1371/journal.pone.0089537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trouwborst I, Bowser SM, Goosens GH, Black EE. Ectopic fat accumulation in distinct insulin resistance phenotypes; targets for personalized nutritional interventions. Front Nutr. 2018;5:77. doi: 10.3389/fnut.2018.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zoniga FA. Association between insulin resistance and the development of cardiovascular diseases. Cardiovasc Diabet. 2018;17:122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fatkhullina AR, Peshkova IO, Koltsova EK. The role of cytokines in the development of atherosclerosis. Biochemistry (Mosc) 2016;81:1358–1370. doi: 10.1134/S0006297916110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho H, Kim JH. Prevalence of microalbumiuria and its associated cardiometabolic risk factors in Korean youths: data from the Korean National Health and Nutrition Examination Survey. PLoS One. 2017;12:e0178716. doi: 10.1371/journal.pone.0178716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang Y, Xu Y, Yang Y, Lin C, Zhao D, Ke J. The relationship between peri renal fat thickness and reduced glomerular filtration rate in patients with type 2 diabetes. J Diabetes Res. 2020;2020:6076145. doi: 10.1155/2020/6076145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roever L, Rensende ES, Veloso FC, Diniz A, Penha-Silva N, Casell-Fillo A, Dourado P, Chagas A. Perirenal fat and association with metabolic risk factors. Medicine (Baltimore) 2015;94:e1105. doi: 10.1097/MD.0000000000001105. [DOI] [PubMed] [Google Scholar]
- 23.Gruzdera O, Borodkina D, Uchesova E, Dyleva Y, Barbarash O. Localisation of fat depots and cardiovascular risk. Lipids Health Dis. 2018;17:218. doi: 10.1186/s12944-018-0856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poirier P. Cardiovascular outcome in patients with hypertension: phenotype versus genotype, there is no small risk (Editorial Commentary) Hypertens. 2015;66:278–279. doi: 10.1161/HYPERTENSIONAHA.115.05329. [DOI] [PubMed] [Google Scholar]


