Abstract
Bladder carcinoma (BC) is one of the most common malignancies of the urinary system in developed countries, with a high number of recurrences. The secondary lymphoid organs (SLO) are crucial for initiating the adaptive immune response. They are developed as a part of a genetically preprogrammed process during embryogenesis. However, SLO’s organogenesis can be reduplicated de novo in other tissues by a process termed lymphoid neo-genesis, giving rise to tertiary lymphoid structures (TLS). These well-organized lymphoid structures in cancer are essential modulators of cancer immunologic response, and the histological examination of TLS gave a new strategy for cancer immunotherapy. This review explores the biological and histological characteristics of TLS in muscle non-invasive and invasive BC.
Keywords: Tertiary lymphoid structures, TRL, immunotherapy, bladder carcinoma, urothelial carcinoma, immunology
Introduction
It is well-known that the immune system’s organs are made up of lymph tissue and composed of a heterogeneous population of cells. Secondary lymphoid organs, SLO (lymph nodes and spleen), play a critical role in adaptive immunity [1]. SLOs develop during embryogenesis in the first few weeks after birth, which is controlled and coordinated by various homeostatic chemokines, cytokines, and growth factors that attract hematopoietic cells to the site of the future lymphoid organ development and promote their differentiation [2].
SLOs are integral to the initiation and development of adaptive immune responses to exogenous pathogens. They offer an environment for interactions between antigen-specific lymphocytes and antigen-presenting cells recruited from locally tumorigenic or inflamed tissues [3].
The importance of SLOs in the mediation of lymphocyte proliferation and rapid response to repeated antigens has long been recognized [4,5].
It is also well established that SLOs contribute to peripheral immune tolerance to self-antigens and commensals by controlling the trafficking of the immunosuppressive subpopulation Foxp3+ regulatory T cells (Tregs), major cell players in establishing the peripheral tolerance by promoting the deletion of self-reactive T cells by SLO-resident extrathymic Air-expressing cells [6,7].
The immune system plays a vital function in the incidence and growth of cancers. Once activated, the human body’s highly adaptive immune system can recognize and kill tumor cells and prevent cancer from growing. However, the effective anti-tumor immune response is a complex multi-stage process, the study of which ensures the development of a rational immunotherapeutic strategy. Although an immune checkpoint blockade effectively increases anti-tumor immune response in different tumor types, tumor immunotherapy also faces several challenges.
Most solid tumors have infiltrating immune cells; the existence of antigen-presenting dendritic cells (DCs) and lymphoid cells in situ suggests that such solid tumors might be identified as foreign and induce an immune reaction [8]. Furthermore, the presence of high numbers of tumor-infiltration lymphocytes (TILs) has been regarded as a prognostic factor. The rational background of existing immunotherapy therapies is that the immune system in tumor patients is compromised, and there is no adequate immune surveillance [9].
Simultaneously, immune cells provide suitable settings for tumorigenesis, cancer development, and metastases. The pro-tumoral activity of immune cells relies on the inflammatory environment mediated by inflammatory cells, including recruitment and induction of alternatively activated macrophages, myeloid-derived suppressor cells (MDSCs), and Tregs. Emerging data has shown that even the same subpopulation of TILs often has separate or opposite effects on the patient’s outcomes, which is the most significant barrier to every tumor-immunotherapy strategy. However, the processes have not been completely elucidated to date [10]. Recent data demonstrated that the immune reaction might be activated, independently of SLO, in the so-called tertiary lymphoid organs or structures (TLS) that form under chronic inflammatory conditions, such as autoimmune disease, chronic inflammation, chronic grafting rejection, and tumors [11].
Our interest in the presence of TLS in the bladder - apart from the general biological point of view is increased due to the possibility that the study of TLS gives us, both in the field of inflammatory pathology (for example, in follicular cystitis) and research with PD-L1 in tumor pathology of the bladder. There are many articles in the field of TLS and their significance in various carcinomas. Still, literature data on the presence of TLS in urothelial bladder cancer and their association with the degree of invasion, tumor stage, and patient survival are scarce. On the other hand, the presence of tumor-associated TLS in BC correlate with positive expression of PD-L1. PD-L1 positive patients are treated with a potential novel personalized immunotherapy with checkpoint inhibitors and showed a favorable clinical outcome.
Also, the contribution of immune responses is poorly understood in BC. There are still many unclear mechanisms in our understanding of TLS biology. For example, not all types of neoplastic disease seem to exhibit a positive correlation between the density of TLS and survival [12].
We investigated 104 cases of non-invasive and invasive urothelial BCs, where we found statistical significance between the presence of TLS with the degree of invasion (P = 0.021) and between the presence of TLS and WHO grading system by 1973 and 2016 (P = 0.024/P = 0.018) (unpublished our data).
This review explores the biological and histological characteristics of TLS in muscle non-invasive and invasive BC.
Characteristics of tertiary lymphoid structures
The central morphological unit of the lymphatic system, the lymphatic tissue, in addition to the primary and secondary lymphoid organs, can form de novo structures in various tissues under pathological circumstances - a process termed lymphoid neogenesis. This process results in the formation of ectopic tertiary lymphatic structures (TLS) that have lymph node-like characteristics [1,3]. Besides, the described process follows similar molecular steps to lymphoid organogenesis during embryonic development [13].
TLSs are ectopic lymphoid organizations that form in non-lymphoid tissues at sites of chronic inflammation, including tumors. Some similar characteristics were identified between secondary lymphoid organogenesis and the neogenesis of TLS. For example, in tumors, TLSs occur under various maturation states, resulting in germinal centers’ development. The mechanisms underlying the role of TLSs in the immune response to adaptive anti-tumors are being deciphered [14].
An association between the existence of TLS and the therapeutic efficiency in cancer patients indicated that TLSs might be a prognostic and predictive factor for the patients’ outcome. This observation has led to a strong interest in investigating the role of TLSs in tumors. The present challenge is how to use TLSs to facilitate lymphocyte penetration in tumors, tumor antigen activation, and differentiation to improve immune response to cancer [14].
TLSs also display all the features of lymph node structures associated with the generation of the adaptive immune response, including a T-cell zone with mature dendritic cells (DC), a germinal center with follicular dendritic cells (FDC) and proliferating B-cells, and a high endothelial venule (HEV) [15].
However, there are some differences between the lymph node and TLS. The lymph nodes are encapsulated, while the TLSs represent immune and stromal cells’ aggregates within an organ or tissue [16]. The second significant difference is that while the development of SLOs is genetically predisposed with a programmed process that occurred during ontogenesis, TLSs are formed in response to chronic inflammatory and antigenic stimuli [17]. Besides, secondary lymphoid structures are formed at well-defined anatomical sites, and TLS are usually formed temporarily in non-lymphoid organs and disappear after antigen elimination [3].
TLSs have long been studied in transplantology, autoimmune and idiopathic diseases, non-neoplastic chronic inflammatory conditions, etc. Moreover, TLSs are potential auto- and alloreactive lymphocyte activation factors contributing to disease exacerbation, failure of therapy, and resistance [18].
TLS also possess characteristics of SLOs such as T and B cell areas, the involvement of follicular dendritic cell, HEV, and lymphoid fibroblasts, and use mechanisms to promote local adaptive immune responses to locally activated antigens. TLS tissue identification is also associated with poor prognosis of cancer, autoantibody development, and malignancy [18].
Formation of B-cell follicles and germinal centers (GCs) has also been identified in TLSs, thus, giving the name of the process as “ectopic lymphangiogenesis”. As we mentioned above, TLS form in target organs of chronic inflammatory/autoimmune processes, localized infections, and in areas surrounding solid tumors. In tumor pathology, these ectopic lymphoid structures provide a more favorable outcome in cancer patients [18,19]. Nevertheless, since TLSs are formed by prolonged inflammatory and neoplastic stimulation, they successfully replicate the function of SLOs, especially as immunological modulators in the tumor environment [11,20,21].
The predictive value of these observations is being addressed. TLS presence in target organs in autoimmune disorders is historically associated with the persistence of disorder and worsen clinical symptoms. On the contrary, TLS in solid tumors has been correlated with the anti-tumor response. However, in some cases, tumor cells’ ability to induce Tregs and suppress host immune response has been compromised [17].
TLS is not present in embryonic development but develops later in adult life to promote local lymphocyte aggregation in the disease’s target organ. Other used terms, like “ectopic lymphoid systems” or “ectopic germinal centers”, show the significance of these structures for immunity. However, they can be utilized where the composition of GC is histologically defined within the ectopic lymphoid tissue [17], although the differences, there is a crossover between TLS and SLO that has been examined by some groups and described in recent publications. The word “tertiary lymphoid” in the literature dated back to 1992 and was used by Louis Picker and Eugene Butcher [22] to characterize the development of extra-lymphoid sites where memory lymphocytes and/or precursors may be re-stimulated by antigen to cause more clonal expansion or terminal effector reactions. By contrast, TLS exists in tissues whose key role is not the generation of immune cells or the development of an adaptive immune response. This excludes bone marrow and thymus (as primary lymphoid organs) and spleen, lymph nodes, and Peyer patches (defined as SLOs) [18].
The liver, heart, pancreas, synovium, and salivary glands are not embryologically predisposed to host lymphoid tissue. Hence lymphocyte assembly at these locations should be called TLS. The liver has hematopoietic activity during embryonic development. Still, this activity is lost postnatally, including this organ among those hosting TLS in adult life [16].
TLS is developed in response to a sequence of pro-inflammatory cytokines and TNF receptor family members following local cross-talk between inflammatory immune cells and resident stromal cells. Fibroblasts, perivascular myofibroblasts, and resident mesenchymal cells have been involved differently in the production of TLS [18,23]. Possibly formed before SLO, TLS may have grown in ectopic tissues to satisfy the survival needs prior to the placentation and evolution of SLOs. As such, the capacity of TLS to begin independently from lymphotoxin (LT) on the release of inflammatory cytokines and in the absence of lymphoid tissue inducer cells (LTi) may have remained a heritage of their developmental ancestors [16].
Physiologically, the generation of humoral response requires the physical interaction of naive B cells with antigen-experienced T cells in the enclosed space of a microenvironment rich in survival and chemotactic factors. Lymphocytes are recruited from the bloodstream to the SLO in response to a chemotactic gradient. CXCL13 and CCL19/CCL21, ligands for the CXCR5 and CCR7 chemokinetic receptors, respectively, control the recirculation of naive B cells between the inner part of the B cell follicle and the outer part of the T/B cell boundary, thereby allowing B cells to come into contact with antigen-experienced, activated T cells. Within the follicles, the antigen-experienced B cells migrate to the GC’s dark zone, a highly hypoxic CXCL12-rich region. In this region, centroblasts become highly proliferating and upregulate the enzyme activation-induced cytidine deaminase (AID) that regulates the intestine leading to the introduction of single base-pair substitutions of antibody gene segments in the immunoglobulin (Ig) variable-region genes, in a process defined as somatic hypermutation [3].
Cancer immunity and TLS in cancers
T cell lymphocytes recognize tumor cells as foreign and differentiate to a population of cytotoxic T lymphocytes that have the ability to migrate, infiltrate, and lyse the tumor [24]. This process is expressed by cell proliferation of T and B lymphocytes, plasma cells, macrophages, dendritic cells, etc. It is an expression of the immune response of the organism carrying the tumor information [15]. These cells proliferate to form a discrete CD8+ T cell and CD20+ B cell zone, involving mature CD83+ dendritic cells within the T-zone and a blood vessel with histological characteristics of a single post-capillary venule with elongated cylindrical cells [25]. These single venules are structurally different blood vessels that develop during embryonic and neonatal life in all secondary lymphoid organs except the spleen [26]. They are highly specialized blood vessels that generally settle in the paracortical area of the lymph node [3,27]. The term ‘TLS’ can refer to structures with a different organization, from ‘simple’ clusters made up of lymphocytes to those resembling lymph follicles observed in secondary lymphoid organs [3]. The structures containing all three components are referred to as “classic” TLS [1].
For many years in the morphology of tumors, an important place is occupied by the steep reaction, representing cellular proliferation of inflammatory cells as an expression of the organism’s immune response carrying the tumor lesion. The more massive reaction is a sign of slower progression of the neoplastic process and vice versa - the scarce and/or missing steep reaction is a manifestation of immune deficiency. The presence of persistent antigenic stimulation leads to T cell accumulation in non-lymphoid areas, where it initiates lymphoid neogenesis. This lymphoid neogenesis is the hallmark of the active immune response that sometimes leads to the formation of TLS [28].
Recently, the active study of TLS has begun due to their role in local and systemic anti-tumor immunity. According to literature, numerous cases of TLS in the stroma of most carcinomas have been reported. In the last two to three years, a number of new hypotheses have accumulated in the literature on the correlation between the density of tertiary lymphoid infiltrates and the clinical outcome studied in more than ten different types of carcinomas [29].
Tertiary lymphoid systems were initially identified in the sense of non-neoplastic chronic inflammatory conditions, including autoimmune diseases, infections, and idiopathic disorders [13,30].
Indeed, neoplastic malignancies have many symptoms associated with systemic inflammation, as well as local chronic inflammation. However, malignant tumors vary from chronic inflammatory environments in one important feature that many would believe may inhibit the development of TLSs: the extremely immunosuppressive microenvironmental tumor [20,31].
However, TLSs with various degrees and organization have been identified in patients with several primary and metastatic cancer forms. Tumor’s TLSs were associated mainly with a better therapeutic prognosis for various types of solid tumors in patients. However, the relation between tumor-associated TLSs and patient outcomes continues to depend on several factors, including the type and degree of cancer and disease.
Foxp3+ Tregs is the leading cell population repeatedly indicated to play a regulatory role in ectopic TLS and/or HEV neogenesis. Tregs are highly immunosuppressive T cells that preserve immune homeostasis and encourage immune tolerance to self-antigens. Tregs avoid autoimmunity by keeping the recruitment and proliferation of over-reactive immune cells under regulation, thereby restricting undue and adverse immune responses [32,33].
Therefore, the depletion of immunosuppressive Tregs is a prerequisite for the production of isolated intratumoral HEVs in some sarcomas, meaning that Tregs can inhibit HEV neogenesis and probably TLSs in tumors [34].
Other studies related IL-17 and/or TH17 cells to TLS neogenesis [35,36]. Interestingly, the IL-17-producing potential of LTi cells is one of the characteristics that has recently been demonstrated as an ancestral association between them [37]. However, the identity of a specific TLS-inducing cell group remains elusive, with B cells and TNF-producing myeloid cells serving as candidates [38].
It has been proposed that, similar to SLOs, TLSs act mainly to stimulate the local immune response at the site of development. TLSs will also have the ability to worsen or regulate the disease, depending on the pathology type. The existing consensus indicates that there is justification for therapeutically potentiating TLS development in the sense of microbial invasion and malignancy, where local immunity may contribute to infection clearance or tumor rejection. Simultaneously, the immune system may inhibit the formation of TLS in chronic inflammation and autoimmunity, where increased local immune responses will result in increased local immune responses [19].
However, TLSs are mostly recorded in anatomy through histological analysis of tissues, preventing conclusive evidence for their practical implications. The bulk of existing research relating to TLSs with prognosis in patients or disease development in animals is by default linked. The prognostic relationship of tumor-associated TLSs or HEVs in the absence of TLSs may be complicated because their neogenesis often occurs in the sense of a vigorous immune response; the correlation between these ectopic lymphoid structures and a beneficial clinical outcome may be indirect and show the presence of effector T cells. Conclusive functional results are substantially missing. We can not be sure of the exact location of these ectopic lymphoid constructs within pathological foci, a prerequisite for successful therapeutic targeting [3].
Examination of TLS in urothelial carcinoma of the bladder
Urothelial carcinoma (UC) is a widespread disease worldwide with a high recurrence rate and the need for active monitoring and clinical follow-up. However, clinical control and outcome of this disease remain a problem. The contribution of the immune response to the outcome in UC patients is still unclear. However, lymphoid neogenesis is a hallmark of an active immune response against urothelial carcinoma, which in some cases leads to the formation of TLS similar to lymph follicles with GCs observed in SLOs [28,39].
Research also reveals a direct link between urothelial bladder cancer and tumor immunology, especially considering BCG immunotherapy, a significant tool in managing and treating non-invasive UCs for more than 40 years. For two to three years, great attention has been paid, and efforts have been made to the study of tumor immunology in relation to tumor-associated lymphoid structures, which are prognostic indicators for the successful therapeutic use of checkpoint inhibitors to improve prognosis [28,39].
It is generally accepted that non-invasive UCs with a low potential of malignancy are biologically different from invasive urothelial carcinomas with a high potential of malignancy and different clinical outcomes. Therefore, it is vital to conduct research on the presence of TLS and compare them in non-invasive and invasive UCs with varying degrees of malignancy.
Essential efforts in cancer immunology have been geared towards the correlation of tumor infiltration lymphocytes (TILs) with cancer prognosis, both prognostic and predictive significance throughout cancers [40]. To this end, recent studies on immunopathological tumor tests have presented substantial evidence of correlations in particular spatiotemporal interactions of TILs within the tumor microenvironment among various types of cancer [28].
Given their main functions in anti-tumor immunity and therapeutic consequences, most attempts have been made to examine the cytotoxic CD8+ TIL populations through cancers that have led to the creation of prognostic markers such as “Immunoscore” in addition to the effective therapeutic utilization of immune checkpoint blockade therapies [28].
Bladder tumors are synonymous with widespread lymphocytic invasion. Considering the role of immunotherapy in both localized and systemic diseases, it may tend to be a successful model to research the immunopathological spread of adaptive immune cells. Therefore, it is of great importance to compare and contrast the microenvironment of the tumor, particularly the tertiary lymphoid structures that indicate the humoral anti-tumor response in these two divergent stages of the bladder UC. This is the first research to examine the existence and characteristics of TLS in bladder UC with a focus on comparing and contrasting TLS development in the treatment of naive, low- and high-grade bladder tumors [28].
In addition, the degree of TLS development and maturity can be correlated with the aggressiveness of the disease and the severity of the stromal inflammatory reaction it evokes. It may also be hypothesized that the enrichment or evolution of tumors during the progression to active disease stages is permissible for B cell receptors’ maturation inside these TLS inside tumors. These issues will preferably be answered by characterizing antibodies secreted from B cells derived from fresh tumor specimens, combined with confirming additional features associated with events such as somatic hypermutation and antibody affinity maturation [28].
This is the first research to report the existence of TLS in bladder tumors with novel insights into the phases associated with the tumor grade. The characteristics of the stages associated with the development of well-formed TLS are detectable by H&E staining. Still, they could be verified by immunohistochemistry (IHC). An in-depth examination of the B cells derived from these tumors will provide novel knowledge on the neo-epitopes that these B cells are likely to support their functional involvement in UBC as well as their therapeutic implications. Future experiments in larger cohorts are required to validate the relationship between TLS, disease recurrence, and overall survival to determine their prognostic significance in bladder cancer.
The study of TLS is performed by pathologists initially by conventional staining with hematoxylin-eosin (H&E) and by immunohistochemical examination with detection of CD31, CD34, CD34-blasts, CD10, CD23, CD4, CD8, CD20, CD68, pS-100, C-kit, smooth muscle actin, desmin, vimentin, podoplanin, Ki-67, ckAE1/AE3, IgG4 and PD-L1 [28,39].
According to modest literature data on the association of TLS with the stage of UC of the bladder, performed on a group of 28 cases of UCs using a broad panel of antibodies, reveals different organization and stages of development, following strict criteria for reporting and classification of classical tertiary lymphoid structures in the studied cohort [28].
The realization of precise morphological analysis, taking into account the differences in the architecture of the tertiary lymphoid structures, is carried out by categorizing them into four types (degrees) [41], along with presenting pictures from our experience.
Type 0 - no significantly significant lymphoid stromal response: < 20 lymphoid cells;
Type 1 (1st degree) - the presence of mild lymphoid infiltrate: from 20 to 40 lymphoid cells, with diffuse and/or nodular arrangement - a precursor of TLS (Figures 1 and 2).
Figure 1.

TLS of type 1 (1st degree (→)) in the stroma of invasive urothelial. carcinoma of the bladder with high malignant potential. H&E × 200.
Figure 2.
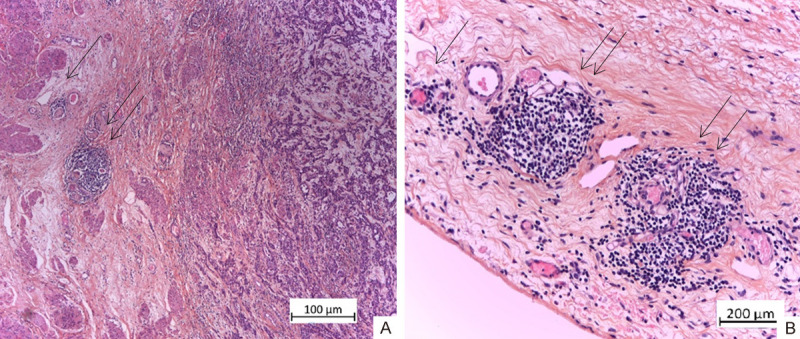
A and B. TLS of type 1 and 2 (1st degree (1→) and 2nd degree (2→)) in the subserosa of invasive UC of the bladder with high malignant potential. H&E × 100, × 200.
Type 2 - the presence of a more pronounced lymphoid infiltrate: from 40 - 60 lymphoid cells grouped around the post-capillary venule - initial forms of TLS (Figure 2).
Type 3 - the presence of pronounced lymphoid infiltrate: > 60 lymphoid cells - with well-formed germinal centers - a terminal form of TLS (= lymph follicles) (Figure 3).
Figure 3.
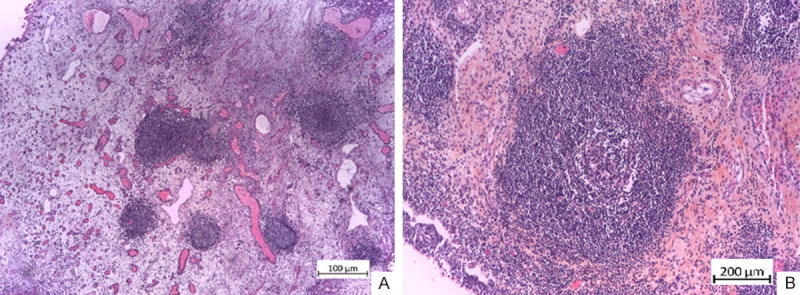
A and B. TLS of type 3 (3th degree) in the stroma of non-invasive UC of the bladder with low malignant potential. H&E × 100, × 200.
Conclusion
According to the literature data and our observations, we suggest that TLS in BC may be a promising histological feature to maximize the therapeutic strategy with checkpoint inhibitors and improve the clinical outcome for patients. They appear likely to be a part of an adaptive immune response. They may help diagnose and understand the BC’s immunobiology and could also guide patient selection for immunotherapy because tumor-associated TLS are the essential modulators of cancer immunologic microenvironment.
Besides, the determination of TLS can be easily quantified by simple histological methods, such as H&E staining. Thus, TLS can be applied in routine practice to predict overall survival.
Disclosure of conflict of interest
None.
References
- 1.Teo YM, Rosenberg EJ. Nivolumab for the treatment of urothelial cancers. Expert Rev Anticancer Ther. 2018;18:215–221. doi: 10.1080/14737140.2018.1432357. [DOI] [PubMed] [Google Scholar]
- 2.Randall TD, Carragher DM, Rangel-Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol. 2008;26:627–650. doi: 10.1146/annurev.immunol.26.021607.090257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colbeck EJ, Ager A, Gallimore A, Jones GW. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front Immunol. 2017;8:1830. doi: 10.3389/fimmu.2017.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 5.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 6.Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, Anderson MS. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 8.Gardner A, Ruffell B. Dendritic cells and cancer immunity. Trends Immunol. 2016;37:855–865. doi: 10.1016/j.it.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postow MA, Callahan MK, Wolchok JD. Immune check-point blockade in cancer therapy. J. Clin. Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, Liu Z, Dong M, Hu X, Ouyang W, Peng J, Zhang Z. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169:1342–1356. e16. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Lin L, Hu X, Zhang H, Hu H. Tertiary lymphoid organs in cancer immunology: mechanisms and the new strategy for immunotherapy. Front Immunol. 2019;10:1398. doi: 10.3389/fimmu.2019.01398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silina K, Kroeger D. Editorial: immune outposts on the inflammatory frontier: tertiary lymphoid structures as targets for immunotherapy of cancer and autoimmunity. Front Immunol. 2019;10:993. doi: 10.3389/fimmu.2019.00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 14.Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19:307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 15.Dieu-Nosjean MC, Goc J, Giraldo NA, Sautès-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35:571–580. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Barone F, Gardner DH, Nayar S, Steinthal N, Buckley CD, Luther SA. Stromal fibroblasts in tertiary lymphoid structures: a novel target in chronic inflammation. Front Immunol. 2016;7:477. doi: 10.3389/fimmu.2016.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones GW, Hill DG, Jones SA. Understanding immune cells in tertiary lymphoid organ development: it is all starting to come together. Front Immunol. 2016;7:401. doi: 10.3389/fimmu.2016.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pipi E, Nayar S, Gardner DH, Colafrancesco S, Smith C, Barone F. Tertiary lymphoid structures: autoimmunity goes local. Front Immunol. 2018;9:1952. doi: 10.3389/fimmu.2018.01952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14:447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- 20.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M, Validire P, Remark R, Hammond SA, Cremer I, Damotte D, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74:705–715. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 21.Nayar S, Campos J, Smith CG, Iannizzotto V, Gardner DH, Mourcin F, Roulois D, Turner J, Sylvestre M, Asam S, Glaysher B, Bowman SJ, Fearon DT, Filer A, Tarte K, Luther SA, Fisher BA, Buckley CD, Coles MC, Barone F. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local pathology. Proc Natl Acad Sci U S A. 2019;116:13490–13497. doi: 10.1073/pnas.1905301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Ann Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- 23.Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 24.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy-inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18:6580–6587. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 25.Dieu-Nosjean MC, Giraldo NA, Kaplon H, Germain C, Fridman WH, Sautès-Fridman C. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol Rev. 2016;271:260–275. doi: 10.1111/imr.12405. [DOI] [PubMed] [Google Scholar]
- 26.Jones E, Gallimore A, Ager A. Defining high endothelial venules and tertiary lymphoid structures in cancer. Methods Mol Biol. 2018;1845:99–118. doi: 10.1007/978-1-4939-8709-2_7. [DOI] [PubMed] [Google Scholar]
- 27.Ager A. High endothelial venules and other blood vessels: critical regulators of lymphoid organ development and function. Front Immunol. 2017;8:45. doi: 10.3389/fimmu.2017.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koti М, Xud АS, Ren KYM, Visram K, Rena R, Berman DM, Siemens DR. Tertiary lymphoid structures associate with tumour stage in urothelial bladder cancer. Bladder Cancer. 2017;3:259–267. doi: 10.3233/BLC-170120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fridman SC, Lawand M, Giraldo AN, Kaplon H, Germain C, Fridman HW, Dieu-Nosjean CM. Tertiary lymphoid structures in cancers: prognostic value, regulation, and manipulation for therapeutic intervention. Front Immunol. 2016;7:407. doi: 10.3389/fimmu.2016.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 31.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Attridge K, Walker LS. Homeostasis and function of regulatory T cells (Tregs) in vivo: lessons from TCR-transgenic Tregs. Immunol Rev. 2014;259:23–39. doi: 10.1111/imr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hindley JP, Jones E, Smart K, Bridgeman H, Lauder SN, Ondondo B, Cutting S, Ladell K, Wynn KK, Withers D, Price DA, Ager A, Godkin AJ, Gallimore AM. T-cell trafficking facilitated by high endothelial venules is required for tumor control after regulatory T-cell depletion. Cancer Res. 2012;72:5473–5482. doi: 10.1158/0008-5472.CAN-12-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deteix C, Attuil-Audenis V, Duthey A, Patey N, McGregor B, Dubois V, Caligiuri G, Graff-Dubois S, Morelon E, Thaunat O. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol. 2010;184:5344–5351. doi: 10.4049/jimmunol.0902999. [DOI] [PubMed] [Google Scholar]
- 36.Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, Wucherpfennig K, Turley S, Carroll MC, Sobel RA, Bettelli E, Kuchroo VK. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35:986–996. doi: 10.1016/j.immuni.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O’Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, Eberl G. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med. 2011;208:125–134. doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfannstiel C, Strissel PL, Chiappinelli KB, Sikic D, Wach S, Wirtz RM, Wullweber A, Taubert H, Breyer J, Otto W, Worst T, Burger M, Wullich B, Bolenz C, Fuhrich N, Geppert CI, Weyerer V, Stoehr R, Bertz S, Keck B, Erlmeier F, Erben P, Hartmann A, Strick R, Eckstein M BRIDGE Consortium, Germany; BRIDGE Consortium, Germany; BRIDGE Consortium, Germany; BRIDGE Consortium, Germany. The tumor immune microenvironment drives a prognostic relevance that correlates with bladder cancer subtypes. Cancer Immunol Res. 2019;7:923–938. doi: 10.1158/2326-6066.CIR-18-0758. [DOI] [PubMed] [Google Scholar]
- 40.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 41.Restucci B, Dipineto L, Martano M, Balestrieri A, Ciccarelli D, Russo TP, Varriale L, Maiolino P. Histopathological and microbiological findings in buffalo chronic mastitis: evidence of tertiary lymphoid structures. J Vet Sci. 2019;20:e28. doi: 10.4142/jvs.2019.20.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]


