Abstract
Different organ perturbation and multiple complications might occur after cardiopulmonary bypass (CPB). A variety of solutions might be used for pump priming with different advantages and disadvantages. The advantage of fresh frozen plasma (FFP) inclusion in pump prime has been shown in post-CPB coagulation management. Acquired hypogammaglobulinemia is the disadvantage of albumin (ALB) pump prime. Our aim was to assess the impact of FFP prime on the post-pump serum level of immunoglobulin G (IgG) and its subclasses. Fifty-six patients under the age of 5 years old who were scheduled for cardiac surgery on CPB were randomly primed with FFP or ALB. Any innate or acquired immune deficiency was considered as exclusion criteria. The pre-CPB and 24-hour post-CPB collected blood samples were analyzed by the nephelometric method for the plasma level of IgG and its four subclasses. Twenty-two patients (mean age and weight of 13 months and 6.8 kilograms) in the ALB prime group and 26 patients (mean age and weight of 15 months and 8.1 kilograms) in the FFP prime group completed the study. Using paired t-test and repeated measures ANOVA test, patients in the ALB prime group had a significant drop in the post-CPB serum level of total IgG (597±138 mg/dL to 379±179 mg/dL, P value <0.001) and its two subclasses of IgG1 and IgG3. In contrast, there was a slight elevation in the serum level of total IgG (549±207 mg/dL to 630±180 mg/dL, P value =0.008) and its two subclasses of IgG2 and IgG4 in patients who had FFP prime solution. In conclusion, compared to the ALB prime solution, FFP inclusion in prime could hamper the pediatric post-CPB induced hypogammaglobulinemia.
Keywords: Cardiopulmonary bypass, fresh frozen plasma, albumin, prime, immunoglobulin G
Introduction
Immune dysregulation after cardiopulmonary bypass (CPB) has been described in children and adults [1-3]. Acquired hypogammaglobulinemia after cardiac surgery has been reported as the impact of CPB on the humoral immune system [4,5]. CPB might be primed with a variety of solutions including albumin (ALB), artificial colloids and FFP [6,7]. There is still debate on the advantages of the different prime solutions [8-10]. The advantage of FFP priming in reducing the risk of hemorrhage and coagulopathy has been shown in the literature [9,10]. In a retrospective case study, Rhodes et al showed the reducing trend of serum immunoglobulin G (IgG) level in patients undergoing cardiac surgery with CPB primed by ALB [1]. While Ryhanen et al [11] found the post-CPB depression in the serum level of all three immunoglobulin classes, Parker et al [12] described the unchanged level of serum IgM and IgA after cardiac surgery. Moreover, hypogammaglobulinemia has been associated with a variety of clinical adverse effects including the increased risk of post-cardiac surgery infection [1-3,5]. However, in our practice we encountered patients with FFP prime solution whose serum IgG level did not show the typical post-CPB reducing trend. To the best of our knowledge the effect of FFP pump priming on the fate of post-CPB serum immunoglobulin levels has not yet been studied. Therefore, we decided to conduct a study to assess the impact of FFP pump priming on the total serum IgG level and more specifically its four subclasses after pediatric cardiac surgery.
Materials and methods
Patients and pump priming
Fifty-six patients with congenital heart disease who were scheduled to undergo heart surgery on CPB enrolled in a parallel-group prospective study from March 2019 to January 2020. Patients less than 5 years old were randomly divided into two groups of CPB with either ALB or FFP as the prime solution. The ALB prime pump included albumin 20% 1 g/kg, mannitol 20% 0.5 g/kg and packed red blood cells to achieve the target hematocrit. Calcium 60 mg/kg, MgSO4 50 mg/kg, unfractionated heparin 150 IU/kg and 1 meq/kg of sodium bicarbonate to reach a PH of 7.4 were added in addition to the serum Ringer. Randomizing in half of the patients (FFP group), 30 to 40 ml/kg of FFP was added to the prime solution instead of albumin. Exclusion criteria included a preexisting immune deficiency and syndromes affecting the immune system such as DiGeorge syndrome and trisomy 21. Reoperation (previous thymectomy), preoperative phlebotomy and transfusion of blood products (including IVIG) and postoperative bleeding requiring surgical hemostasis were other exclusion criteria. Data related to technical errors in sample collection and storing were also excluded.
Serum total IgG and subclasses measurement
Pre-CPB and 24-hour post-CPB plasma level of IgG and its four subclasses were analyzed by the nephelometric method. The collected blood samples for measuring the serum immunoglobulin level were centrifuged for 10 minutes at 2500 rpm and frozen to -20° Celsius.
Anesthesia and perioperative management
Anesthesia was induced by either ketamine or propofol and maintained by continuous intravenous infusion of midazolam, fentanyl and cisatracurium. Custodiol cardioplegia was used for myocardial protection. All patients received ultrafiltration for zero balance. Heparin neutralization by protamine was done according to the protocol of anticoagulation profile. Peritoneal dialysis catheter was implanted for neonates and patients with complex congenital heart disease. All patients received prophylactic antibiotic regimens of either cefazolin or vancomycin in combination with amikacin at the onset of ICU admission.
The study was approved by the ethics committee of Tehran University of Medical Sciences with the code of IR.TUMS.CHMC.REC.1398.136. Informed consent was obtained for blood sample extraction to check the serum immunoglobulin level and also having access to the patients’ medical files.
Statistical analysis
SPSS Software version 19.0 (SPSS, Inc, Chicago, III) was used for statistical analysis. All normally distributed data were expressed as mean ± standard deviation. The significance of the difference between 2 groups was assessed by t-test, repeated measures ANOVA (RM-ANOVA) test, Chi-square (χ 2) test and Fisher’s exact test as appropriate. T-test was used to compare the means of two sets of different data, while RM-ANOVA as the extension of dependent t-test was used for the related groups. Fisher’s exact test was applied to the sets of categorical data as well as Chi-square test, especially when the sample size was small. Pearson’s and Spearman’s correlation coefficients were used to measure the strength of association between parametric and nonparametric variables, respectively. The P value of less than 0.05 was considered significant.
Results
Forty-eight out of 56 patients who were enrolled in the study have completed the exam. One patient in the FFP prime group and 2 patients in the ALB prime group were excluded because of failure to be separated from CPB. Technical error in sample storing resulted in the removal of one patient in each group. Moreover, 3 additional patients were excluded from the ALB prime group because of massive hemorrhage requiring surgical hemostasis. Table 1 shows the demographic characteristics of 26 patients in the group of FFP prime compared to the matched 22 patients in the group of ALB prime. The details of both groups’ congenital heart disease pathology are also shown in Table 1. The risk of postoperative mortality regarding the risk adjustment for congenital heart surgery score (RACHS-1) [13,14] was similar for both groups (Table 2). No significant difference was found between the means of CPB time, aortic cross clamp time, duration of mechanical ventilation and ICU-stay of the two groups (Table 3). Patients in FFP prime group had lower mean chest tube drainage over the first 24-hour admission in the ICU compared to the ALB prime group; 10±5 ml/kg vs. 19±14 ml/kg, t-test, P value =0.004 (Table 3). The comparison of the first 24-hour total intake and output in both groups is also shown in Table 3. The mean of 24-hour net fluid balance was negative for both groups without any significant statistical difference (t-test, P value =0.72, Table 3). Peritoneal dialysis was done in 2 patients in the ALB prime group and in one patient in the FFP prime group.
Table 1.
Demographic characteristics and cardiac structural abnormalities in ALB and FFP prime groups
| Variable | ALB prime N=22 | FFP primeN=26 | P value, X2/t-test |
|---|---|---|---|
| Weight (kg) | 6.8±2.7 | 8.1±4.2 | 0.21 |
| Age (month) | 13±11.4 | 15±14.1 | 0.45 |
| Male gender, N (%) | 10 (45) | 11 (42) | 0.82 |
| Diagnosis N (%) | |||
| ALCAPA | 0 | 1 (4) | |
| Aortic stenosis | 1 (4.5) | 1 (4) | |
| ASD | 1 (4.5) | 1 (4) | |
| AVSD | 3 (13.5) | 3 (11) | |
| Cor triatriatum | 1 (4.5) | 1 (4) | |
| HLHS | 1 (4.5) | 0 | |
| Tricuspid Atresia | 1 (4.5) | 1 (4) | |
| Tetralogy of the Fallot | 5 (23) | 6 (23) | |
| TAPVC | 1 (4.5) | 2 (8) | |
| TGA+VSD | 1 (4.5) | 0 | |
| TGA | 0 | 1 (4) | |
| VSD+COA | 0 | 1 (3.8) | |
| VSD | 7 (32) | 8 (30) |
ALB, Albumin; FFP, Fresh frozen plasma; X2, Chi-square; ALCAPA, Anomalous origin of left coronary artery from the pulmonary artery; ASD, Atrial septal defect; AVSD, Atrioventricular septal defect; HLHS, Hypoplastic left heart syndrome; TAPVC, Total anomalous pulmonary venous connection; TGA, Transposition of the great arteries; VSD, Ventricular septal defect; COA, Coarctation of aorta.
Table 2.
| Prime groups | RACHS -1 score in risk category | P value, χ2 test | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| ALB prime N=22 | 1 | 14 | 5 | 1 | 0 | 1 | 0.8 |
| FFP prime N=26 | 1 | 18 | 7 | 0 | 0 | 0 | |
ALB, Albumin; FFP, Fresh frozen plasma; RACHS, Risk adjustment for congenital heart surgery; X2, Chi-square.
Table 3.
Perioperative variables and fluid balance in ALB and FFP prime groups
| Variable | ALB prime N=22 | FFP prime N=26 | P value, t-test |
|---|---|---|---|
| CPB time (minutes) | 113±41 | 107±39 | 0.58 |
| Cross-clamp time (minutes) | 76±26 | 73±38 | 0.75 |
| Mechanical ventilation (hours) | 70±130 | 44±109 | 0.45 |
| ICU-stay (days) | 7.4±5.7 | 6.5±4.3 | 0.58 |
| First 24-hour intake (ml/kg) | 100±33 | 85±26 | 0.07 |
| First 24-hour output (ml/kg) | 159±90 | 146±42 | 0.50 |
| First 24-hour chest tube drainage (ml/kg) | 19±14 | 10±5 | 0.004 |
| First 24-hour net fluid balance (ml/kg) | -79±94 | -71±59 | 0.72 |
ALB, Albumin; CPB, Cardiopulmonary bypass; FFP, Fresh frozen plasma; ICU, Intensive care unit.
Perioperative lab exams
The means of pre and post-pump total protein, ALB, BUN and creatinine are shown in Table 4 without any significant statistical difference except for the increased serum level of post-CPB total protein in the FFP prime group (5.3±0.76 g/dL vs. 5.8±0.47 g/dL, t-test, P value =0.03). Table 4 also demonstrates the pre and post-pump WBC count, lymphocyte count, neutrophil count, neutrophil/lymphocyte ratio (NLR), platelet count and the acute phase reactants of C-reactive protein (CRP) and procalcitonin (only post-pump) without prominent difference among the two groups. Also shown in Table 4, the means of pre and post-pump partial thromboplastin time (PTT) did not differ between the ALB and FFP prime groups, however the post-pump values of prothrombin time (PT) (23±9.4 sec vs. 15±3.4 sec, t-test, P value =0.002) and INR (1.9±0.61 vs. 1.3±0.24, t-test, P value <0.001) were significantly higher in the ALB prime group.
Table 4.
Pre and post-CPB laboratory variables in ALB and FFP prime groups
| Variable | ALB prime N=22 | FFP prime N=26 | P value, t-test |
|---|---|---|---|
| Pre-CPB | |||
| Albumin (g/dL) | 3.4±0.65 | 3.8±0.99 | 0.06 |
| Total protein (g/dL) | 4.9±0.90 | 5.4±1.27 | 0.10 |
| BUN (mg/dL) | 10±4.9 | 9±3.5 | 0.38 |
| Cr (mg/dL) | 0.58±0.12 | 0.52±0.14 | 0.06 |
| CRP (mg/L) | 2±2.9 | 2±2.6 | 0.59 |
| WBC (103/µL) | 10.71±3.09 | 9.92±2.51 | 0.33 |
| Neutrophil (103/µL) | 3.83±1.85 | 3.36±1.5 | 0.34 |
| Lymphocyte (103/µL) | 5.81±2.24 | 4.87±2.61 | 0.19 |
| NLR | 0.8±0.48 | 0.8±0.89 | 0.81 |
| Hct (%) | 40±6.6 | 39±8.3 | 0.63 |
| Platelet (103/µL) | 331±88.8 | 304±98.9 | 0.33 |
| PT (seconds) | 14.5±1.47 | 15.0±1.72 | 0.28 |
| PTT (seconds) | 37±7.9 | 37±6.5 | 0.89 |
| INR | 1.2±0.21 | 1.3±0.19 | 0.39 |
| Post-CPB | |||
| Albumin (g/dL) | 3.9±0.63 | 4.0±0.39 | 0.42 |
| Total protein (g/dL) | 5.3±0.76 | 5.8±0.47 | 0.03 |
| BUN (mg/dL) | 12±5.0 | 11±4.8 | 0.70 |
| Cr (mg/dL) | 0.55±0.20 | 0.54±0.14 | 0.84 |
| CRP (mg/L) | 36±22.7 | 38±17.39 | 0.80 |
| Procalcitonin (ng/ml) | 5.5±4.9 | 5.9±6.5 | 0.81 |
| WBC (103/µL) | 15.83±5.83 | 12.77±5.24 | 0.06 |
| Neutrophil (103/µL) | 10.71±4.77 | 8.53±4.50 | 0.11 |
| Lymphocyte (103/µL) | 4.39±2.21 | 3.52±1.37 | 0.10 |
| NLR | 3±1.4 | 3±2.2 | 0.67 |
| Hct (%) | 36±10 | 37±6.2 | 0.60 |
| Platelet (103/µL) | 90±10.6 | 85±11.6 | 0.18 |
| PT (seconds) | 23±9.4 | 15±3.4 | 0.002 |
| PTT (seconds) | 43±22.2 | 33±4.8 | 0.07 |
| INR | 1.9±0.61 | 1.3±0.24 | <0.001 |
ALB, Albumin; BUN, Blood urea nitrogen; CPB, Cardiopulmonary bypass; Cr, Creatinine; CRP, C-reactive protein; FFP, Fresh frozen plasma; Hct, Hematocrit; INR, International normalized ratio; NLR, Neutrophil/Lymphocyte ratio; PT, Prothrombin time; PTT, Partial thromboplastin time; WBC, White blood cell.
Serum total IgG and subclasses
Table 5 shows the serum total IgG and its four subclasses concentration before and after CPB in the two groups. Using paired t-test and repeated measures ANOVA test, pre-CPB serum total IgG and its subclasses were balanced between the two groups. The mean of post-CPB serum total IgG was significantly dropped in the ALB prime group (597±138 mg/dL to 379±179 mg/dL, paired t-test, P value <0.001) compared to the slight elevation of concentration in the FFP prime group (549±207 mg/dL to 630±180 mg/dL, paired t-test, P value =0.008) (Figure 1). The significant drop in ALB prime group was seen in the subclasses of IgG1 (395±110 mg/dL to 223±134 mg/dL, paired t-test, P value <0.001) and IgG3 (59±35 mg/dL to 33±16 mg/dL, paired t-test, P value <0.001). Interestingly, the serum levels of IgG2 (118±60 mg/dL to 190±53 mg/dL, paired t-test, P value <0.001) and IgG4 (10±11 mg/dL to 24±12 mg/dL, paired t-test, P value <0.001) increased in the group that FFP was added to the prime solution (Figure 2).
Table 5.
Comparison of pre and post-CPB serum Immunoglobulin G (IgG) level and its subclasses within and between two groups of ALB and FFP prime
| Serum IgG (mg/dL) | ALB prime N=22 | FFP prime N=26 | P value, between groups RM-ANOVA test | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Pre-CPB | Post-CPB | P value Paired t-Test | Pre-CPB | Post-CPB | P value Paired t-Test | ||
| IgG1 | 395±110 | 223±134 | <0.001 | 375±158 | 374±130 | 0.95 | <0.001 |
| IgG2 | 125±43 | 109±62 | 0.34 | 118±60 | 190±53 | <0.001 | <0.001 |
| IgG3 | 59±35 | 33±16 | <0.001 | 45±25 | 40±17 | 0.09 | 0.003 |
| IgG4 | 17±19 | 12±14 | 0.13 | 10±11 | 24±12 | <0.001 | <0.001 |
| IgG total | 597±138 | 379±179 | <0.001 | 549±207 | 630±180 | 0.008 | <0.001 |
ALB, Albumin; CPB, Cardiopulmonary bypass; FFP, Fresh frozen plasma; RM-ANOVA test, Repeated measures analysis of variance.
Figure 1.
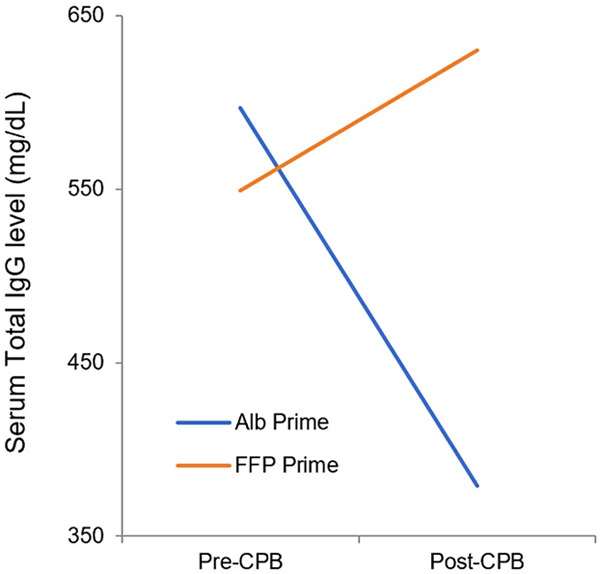
Comparison of pre and post-CPB serum level of total IgG in the ALB and FFP prime groups (ALB, Albumin; CPB, Cardiopulmonary bypass; FFP, Fresh frozen plasma; IgG, Immunoglobulin G).
Figure 2.
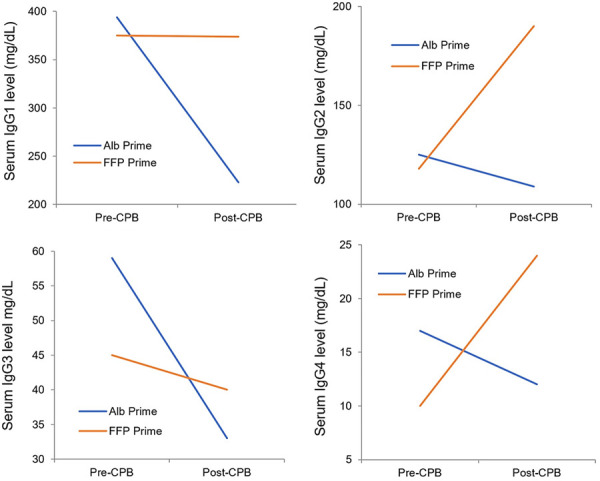
Comparison of pre and post-CPB serum level of IgG subclasses in the ALB and FFP prime groups (ALB, Albumin; CPB, Cardiopulmonary bypass; FFP, Fresh frozen plasma; IgG, Immunoglobulin G).
Blood products donor exposure
We also checked the donor exposure per patient from the transfusion of blood products in both groups as seen in Table 6. FFP prime group had significantly higher donor exposure from FFP transfusion (t-test, P value <0.001) while in the ALB prime group, this was significant for the packed red blood cell and cryoprecipitate transfusion (t-test, P value <0.001). No difference was seen for platelet transfusion (t-test, P value =0.15). Overall, the ALB prime group had a significantly higher donor exposure compared to the FFP prime group (5.3±2.12 U/Patient vs 3.9±1.22 U/Patient, t-test, P value =0.006).
Table 6.
Donor exposure from blood products per patient in ALB and FFP prime groups
| Variable | ALB prime N=22 | FFP prime N=26 | P value, t-test |
|---|---|---|---|
| FFP | 0.6±0.64 | 1.8±0.85 | <0.001 |
| Packed red blood cell | 2.7±0.74 | 1.6±0.56 | <0.001 |
| Platelet | 1.3±0.46 | 1.1±0.30 | 0.15 |
| Cryoprecipitate | 1.9±0.78 | 1±0.0 | <0.001 |
| Total | 5.3±2.12 | 3.9±1.22 | 0.006 |
ALB, Albumin; FFP, Fresh frozen plasma.
Serum IgG and perioperative variables correlation
The univariate correlation between perioperative variables and post-CPB serum total IgG level was demonstrated in Table 7. Post-CPB serum total IgG in the ALB prime group was inversely correlated with CPB time, aortic cross clamp time and C-reactive protein (Pearson’s Correlation Coefficient, r=-0.67, P value =0.006, and r=-0.65, P value =0.001, r=-0.51, P value =0.01 respectively). Moreover, the RACHS-1 score was inversely correlated with post-CPB serum total IgG either (Spearman’s correlation coefficient, r=-0.78, P value <0.001). However, no significant correlation was found in the group of FFP prime (Table 7).
Table 7.
The correlation of total Immunoglobulin G and perioperative variables in ALB and FFP prime groups
| ALB prime N=22 | FFP prime N=26 | |||
|---|---|---|---|---|
|
|
|
|||
| Correlation* | P value | Correlation* | P value | |
| Cardiopulmonary bypass time (minutes) | -0.67 | 0.006 | -0.12 | 0.56 |
| Cross clamp time (minutes) | -0.65 | 0.001 | 0.05 | 0. 81 |
| RACHS-1 score risk category | -0.78₤ | <0.001 | -0.18₤ | 0.39 |
| First 24-hour net fluid balance (ml/kg) | 0.10 | 0.65 | -0.07 | 0.74 |
| Postoperative CRP (mg/L) | 0.51 | 0.01 | 0.36 | 0.07 |
ALB, Albumin; CRP, C-reactive protein; FFP, Fresh frozen plasma; RACHS, Risk adjustment for congenital heart surgery;
Pearson’s correlation coefficient;
Spearman’s correlation coefficient.
Morbidity and mortality
There was only one culture proven infection with staphylococcus epidermidis in the group of ALB prime who had palliative Senning procedure. We also had one mortality in the ALB prime group in a neonate with hypoplastic left heart syndrome because of acute kidney injury after the stage I Norwood procedure.
Discussion
Hypogammaglobulinemia as the impact of CPB on the humoral immune system after cardiac surgery has been documented in several studies [1,2,4,5]. Hemodilution, denaturation, sequestration and extravasation are different possible mechanisms that lower the level of serum IgG after CPB [4,5,12,15]; Moreover proteinuria and protein loss from the drains have been addressed as other causes [16,17]. Importantly the post-CPB hypogammaglobulinemia was associated with the increased risk of postoperative infection. Patients are predisposed to pulmonary infection [2]. Several post-CPB pan-resistant gram negative pneumonia leading to death has been reported [2,5]. Specific infections associated with IgG subclass deficiency are summarized in Table 8 [18-22].
Table 8.
| IgG subclass deficiency | Total IgG% | Common infection and pathogen |
|---|---|---|
| IgG1 | 60% | Sinopulmonary infection, Septicemia, Osteomyelitis, Diarrhea, Skin infection, Meningitis |
| IgG2 | 32% | Sinopulmonary infection, Infection with streptococcus pneumonia, Haemophilus influenzae type b, Neisseria meningitidis |
| IgG3 | 4% | Sinopulmonary infection with Moraxella catarrhalis, Streptococcus pyogenes, Gastrointestinal infection, Lymphocytic meningitis, Herpes simplex infection, Erysipelas |
| IgG4 | 4% | Pulmonary infection, Mucocutaneous candidiasis |
lgG, Immunoglobulin G.
In consistent with Rhodes study [1] our patients in the ALB prime group showed a prominent drop in the serum level of post-CPB gammaglobulin, dominantly in two subclasses of IgG1 and IgG3. However, adding FFP to the prime solution in values of more than 30-40 ml/kg instead of albumin aborted the post-CPB drop of serum total IgG and its subclasses. Conversely, our patients in the FFP prime group had an elevated serum level of IgG2 and IgG4. Opposite to the finding of Acunas et al that claiming FFP transfusion in neonatal sepsis does not increase the serum IgG levels (except for IgA and IgM levels) [23], we found that FFP exposure especially in prime solution will prevent the postoperative hypogammaglobulinemia.
Acute kidney injury post-CPB might increase the immunoglobulin filtration in the urine [17,24]. Although the serum creatinine in 8 patients with total IgG level of less than 300 mg/dL was higher than those with lesser decline in serum IgG, the statistical difference was not meaningful. Furthermore, the similarity of serum creatinine level as a marker of acute kidney injury in both groups, ruled out the renal injury as the major initial cause for hypogammaglobulinemia. It is noteworthy that our findings did not support the theory of hemodilution as the mechanism of hypogammaglobulinemia either [5], because the net fluid balance in the first 24 hours was similar in both groups. Moreover, the serum albumin level that leaves the intravascular space in case of capillary leak event [15] was not statistically different between the two groups. It has been shown that hypogammaglobulinemia might prolong the duration of mechanical ventilation and ICU stay and increase the incidence of fluid overload [25], however our findings were not in the same direction (Table 3).
Although one might speculate that the different antioxidant capacity of the FFP and ALB prime solutions is the cause of opposite response in the IgG serum levels, Molicki et al showed that both solutions had a very low capacity of antioxidants [26]. Neutrophils act as the trigger for direct cell injury and activation of inflammatory cascade [27,28]. Despite the less elevation in post-pump neutrophil count in FFP prime group, the inclusion of FFP in prime solution could not prevent the post-CPB changes in absolute count of neutrophil and neutrophil/lymphocyte ratio (Table 4). There was an inverse correlation between CRP and post-CPB serum IgG level in the ALB prime group that was aborted by FFP inclusion in pump prime solution (Table 7). Since the level of CRP and procalcitonin as the indirect evidence of systemic inflammatory response syndrome [29,30] were the same in both prime groups, one can postulate that instead of FFP inflammatory suppressive characteristic, it is the globulin content of FFP that keeps elevated the serum IgG level after CPB.
The clinical benefit of FFP prime in promoting the hemostatic function and reducing the risk of bleeding after CPB has been shown in several studies [9,10,31]. However, in a recent trial by Dieu et al [8], there was no significant difference in the postoperative bleeding and the need for allogeneic blood product transfusion between the crystalloid and FFP primed groups. In consistent with McCall et al study [31] we showed that including FFP in the prime solution would reduce the risk of blood product donor exposure (Table 6). Moreover, the less need for packed red blood transfusion results in maintained post-pump serum immunoglobulin level. It is assumed that postoperative replacement of bleeding solely by the packed red blood cell devoid of globulins will worsen the magnitude of hypogammaglobulinemia [2,31]. If pump prime with FFP inhibits post-CPB immunoglobulin depletion, then the risk of postoperative infections due to hypogammaglobulinemia will decline as an advantage of pump priming with FFP. Although we did not find any evidence of postoperative infection in the group of FFP prime, the small sample size of our study did not let us conclude statistically that FFP in prime would reduce the risk of postoperative infection.
Limitation of study
Since we only measured the first 24-hour serum immunoglobulin level after CPB, the potential ongoing loss of IgG in pleural and peritoneal fluids, urine output and intestinal extravasation could not be judged. The immunoglobulin concentration in the fluid loss was not measured either. Moreover, our study suffers from the lack of measuring the pro-inflammatory cytokines of IL-6, IL-8 and IL-12 and TNF-Alpha to assess the impact of the capillary leak on the IgG balance.
In conclusion, our results were in favor of avoiding hypogammaglobulinemia post-CPB in case of pump priming with FFP. The reduced risk of bleeding and less risk of blood product donor exposure are other beneficial aspects of CPB prime with FFP. However, a larger sample size of patients with a more sophisticated method of study including interleukins and other inflammatory cytokines measurement is necessary to compare the impact of different CPB prime solutions on the immune system post pediatric cardiac surgery.
Acknowledgements
This study was supported by a grant from Tehran University of Medical Sciences.
Disclosure of conflict of interest
None.
Abbreviations
- ALB
albumin
- CPB
cardiopulmonary bypass
- CRP
C-reactive protein
- FFP
Fresh frozen plasma
- IgG
immunoglobulin G
- NLR
neutrophil/lymphocyte ratio
- RACHS
risk adjustment for congenital heart surgery
References
- 1.Rhodes LA, Robert SM, Atkinson TP, Dabal RJ, Mahdi AM, Alten JA. Hypogammaglobulinemia after cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 2014;147:1587–1593. e1. doi: 10.1016/j.jtcvs.2013.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rankin JS, Oguntolu O, Binford RS, Trochtenberg DS, Muhlbaier LH, Stratton CW. Management of immune dysfunction after adult cardiac surgery. J Thorac Cardiovasc Surg. 2011;142:575–580. doi: 10.1016/j.jtcvs.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 3.Hauser GJ, Chan MM, Casey WF, Midgley FM, Holbrook PR. Immune dysfunction in children after corrective surgery for congenital heart disease. Crit Care Med. 1991;19:874–881. doi: 10.1097/00003246-199107000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Eskola J, Salo M, Viljanen MK, Ruuskanen O. Impaired B lymphocyte function during open-heart surgery. Effects of anaesthesia and surgery. Br J Anaesth. 1984;56:333–338. doi: 10.1093/bja/56.4.333. [DOI] [PubMed] [Google Scholar]
- 5.van Velzen-Blad H, Dijkstra YJ, Schurink GA, Verbrugh HA, Verhoef J, Zegers BJ, Ballieux RE. Cardiopulmonary bypass and host defense functions in human beings: I. Serum levels and role of immunoglobulins and complement in phagocytosis. Ann Thorac Surg. 1985;39:207–211. doi: 10.1016/s0003-4975(10)62578-7. [DOI] [PubMed] [Google Scholar]
- 6.Hosseinzadeh Maleki M, Derakhshan P, Rahmanian Sharifabad A, Amouzeshi A. Comparing the effects of 5% albumin and 6% hydroxyethyl starch 130/0.4 (voluven) on renal function as priming solutions for cardiopulmonary bypass: a randomized double blind clinical trial. Anesth Pain Med. 2016;6:e30326. doi: 10.5812/aapm.30326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Himpe D. Colloids versus crystalloids as priming solutions for cardiopulmonary bypass: a meta-analysis of prospective, randomised clinical trials. Acta Anaesthesiol Belg. 2003;54:207–215. [PubMed] [Google Scholar]
- 8.Dieu A, Rosal Martins M, Eeckhoudt S, Matta A, Kahn D, Khalifa C, Rubay J, Poncelet A, Haenecour A, Derycke E, Thiry D, Gregoire A, Momeni M. Fresh frozen plasma versus crystalloid priming of cardiopulmonary bypass circuit in pediatric surgery: a randomized clinical trial. Anesthesiology. 2020;132:95–106. doi: 10.1097/ALN.0000000000003017. [DOI] [PubMed] [Google Scholar]
- 9.Lee JW, Yoo YC, Park HK, Bang SO, Lee KY, Bai SJ. Fresh frozen plasma in pump priming for congenital heart surgery: evaluation of effects on postoperative coagulation profiles using a fibrinogen assay and rotational thromboelastometry. Yonsei Med J. 2013;54:752–762. doi: 10.3349/ymj.2013.54.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver WC Jr, Beynen FM, Nuttall GA, Schroeder DR, Ereth MH, Dearani JA, Puga FJ. Blood loss in infants and children for open heart operations: albumin 5% versus fresh-frozen plasma in the prime. Ann Thorac Surg. 2003;75:1506–1512. doi: 10.1016/s0003-4975(02)04991-3. [DOI] [PubMed] [Google Scholar]
- 11.Ryhänen P, Leinonen M, Koskela M, Hollmén A, Nuutinen L, Pihlajaniemi R, Saarela E. Are changes in serum immunoglobulin and complement levels following open heart surgery influenced by oxygenator type or postoperative parenteral nutrition? Ann Clin Res. 1979;11:9–12. [PubMed] [Google Scholar]
- 12.Parker DJ, Cantrell JW, Karp RB, Stroud RM, Digerness SB. Changes in serum complement and immunoglobulins following cardiopulmonary bypass. Surgery. 1972;71:824–827. [PubMed] [Google Scholar]
- 13.Nina RV, Gama ME, Santos AM, Nina VJ, Figueiredo Neto JA, Mendes VG, Lamy ZC, Brito LM. Is the RACHS-1 (risk adjustment in congenital heart surgery) a useful tool in our scenario? Rev Bras Cir Cardiovasc. 2007;22:425–431. doi: 10.1590/s0102-76382007000400008. [DOI] [PubMed] [Google Scholar]
- 14.Thiagarajan RR, Laussen PC. Risk adjustment for congenital heart surgery -1 (RACHS-1) for evaluation of mortality in children undergoing cardiac surgery. In: Barach PR, Jacobs JP, Lipshultz SE, Laussen PC, editors. Pediatric and congenital cardiac care: volume 1: outcomes analysis. London: Springer London; 2015. pp. 327–336. [Google Scholar]
- 15.Seghaye MC, Grabitz RG, Duchateau J, Busse S, Däbritz S, Koch D, Alzen G, Hörnchen H, Messmer BJ, Von Bernuth G. Inflammatory reaction and capillary leak syndrome related to cardiopulmonary bypass in neonates undergoing cardiac operations. J Thorac Cardiovasc Surg. 1996;112:687–697. doi: 10.1016/s0022-5223(96)70053-3. [DOI] [PubMed] [Google Scholar]
- 16.El Mashad GM, El Hady Ibrahim SA, Abdelnaby SAA. Immunoglobulin G and M levels in childhood nephrotic syndrome: two centers Egyptian study. Electron Physician. 2017;9:3728–3732. doi: 10.19082/3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fivush BA, Case B, May MW, Lederman HM. Hypogammaglobulinemia in children undergoing continuous ambulatory peritoneal dialysis. Pediatr Nephrol. 1989;3:186–188. doi: 10.1007/BF00852907. [DOI] [PubMed] [Google Scholar]
- 18.Escobar-Pérez X, Dorta-Contreras AJ, Interián-Morales MT, Noris-García E, Ferrá-Valdés M. IgG2 immunodeficiency: association to pediatric patients with bacterial meningoencephalitis. Arq Neuropsiquiatr. 2000;58:141–145. doi: 10.1590/s0004-282x2000000100021. [DOI] [PubMed] [Google Scholar]
- 19.Siber GR, Schur PH, Aisenberg AC, Weitzman SA, Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980;303:178–182. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- 20.Schur PH, Borel H, Gelfand EW, Alper CA, Rosen FS. Selective gamma-g globulin deficiencies in patients with recurrent pyogenic infections. N Engl J Med. 1970;283:631–634. doi: 10.1056/NEJM197009172831205. [DOI] [PubMed] [Google Scholar]
- 21.Kalfa VC, Roberts RL, Stiehm ER. The syndrome of chronic mucocutaneous candidiasis with selective antibody deficiency. Ann Allergy Asthma Immunol. 2003;90:259–264. doi: 10.1016/S1081-1206(10)62152-7. [DOI] [PubMed] [Google Scholar]
- 22.de Moraes Lui C, Oliveira LC, Diogo CL, Kirschfink M, Grumach AS. Immunoglobulin G subclass concentrations and infections in children and adolescents with severe asthma. Pediatr Allergy Immunol. 2002;13:195–202. doi: 10.1034/j.1399-3038.2002.00058.x. [DOI] [PubMed] [Google Scholar]
- 23.Acunas BA, Peakman M, Liossis G, Davies ET, Bakoleas B, Costalos C, Gamsu HR, Vergani D. Effect of fresh frozen plasma and gammaglobulin on humoral immunity in neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 1994;70:F182–187. doi: 10.1136/fn.70.3.f182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan CJ, Zappitelli M, Robertson CM, Alton GY, Sauve RS, Joffe AR, Ross DB, Rebeyka IM. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr. 2013;162:120–127.e121. doi: 10.1016/j.jpeds.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 25.Sampaio TZ, O’Hearn K, Reddy D, Menon K. The influence of fluid overload on the length of mechanical ventilation in pediatric congenital heart surgery. Pediatr Cardiol. 2015;36:1692–1699. doi: 10.1007/s00246-015-1219-0. [DOI] [PubMed] [Google Scholar]
- 26.Molicki JS, Draaisma AM, Verbeet N, Munneke R, Huysmans HA, Hazekamp MG, Berger HM. Prime solutions for cardiopulmonary bypass in neonates: antioxidant capacity of prime based on albumin or fresh frozen plasma. J Thorac Cardiovasc Surg. 2001;122:449–456. doi: 10.1067/mtc.2001.115422. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Sun Y, Zhang S. The Relationship between neutrophil to lymphocyte ratio and clinical outcome in pediatric patients after cardiopulmonary bypass surgery: a retrospective study. Front Pediatr. 2019;7:308. doi: 10.3389/fped.2019.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber JN, Hilkin BM, Hook JS, Brophy PD, Davenport TL, Davis JE, Colaizy TT, Moreland JG. Neutrophil phenotype correlates with postoperative inflammatory outcomes in infants undergoing cardiopulmonary bypass. Pediatr Crit Care Med. 2017;18:1145–1152. doi: 10.1097/PCC.0000000000001361. [DOI] [PubMed] [Google Scholar]
- 29.Rey C, Los Arcos M, Concha A, Medina A, Prieto S, Martinez P, Prieto B. Procalcitonin and C-reactive protein as markers of systemic inflammatory response syndrome severity in critically ill children. Intensive Care Med. 2007;33:477–484. doi: 10.1007/s00134-006-0509-7. [DOI] [PubMed] [Google Scholar]
- 30.Minami E, Ito S, Sugiura T, Fujita Y, Sasano H, Sobue K. Markedly elevated procalcitonin in early postoperative period in pediatric open heart surgery: a prospective cohort study. J Intensive Care. 2014;2:38. doi: 10.1186/2052-0492-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCall MM, Blackwell MM, Smyre JT, Sistino JJ, Acsell JR, Dorman BH, Bradley SM. Fresh frozen plasma in the pediatric pump prime: a prospective, randomized trial. Ann Thorac Surg. 2004;77:983–987. doi: 10.1016/j.athoracsur.2003.09.030. discussion 987. [DOI] [PubMed] [Google Scholar]


