Abstract
Introduction: HspB5 (αB-crystallin) is known to be involved in a variety of cellular functions, including, protection of cells from oxidative damage and inhibiting apoptosis. Neural stem/progenitor cells (NSPCs) have significant therapeutic value, especially in the NSC/NPC transplantation therapy. However, the viability of the transplanted NSPCs remains low because of various factors, including oxidative stress. Objective: The current investigation explored the possible role of HspB5 in the protection of mouse NSPCs (mNSPCs) against paraquat-induced toxicity. Methods: The recombinant human HspB5 was expressed in E.coli and was purified using gel filtration and Ion-exchange chromatography. The biophysical characterization of HspB5 was carried out using DLS, CD, and Analytical Ultracentrifugation (SV); the chaperone activity of HspB5 was determined by alcohol dehydrogenase aggregation assay. We have subjected the mNSPCs to paraquat-induced oxidative stress and monitored the protective ability of HspB5 by MTT assay and Hoechst-PI staining. Furthermore, increase in the expression of the anti-apoptotic protein, procaspase-3 was monitored using western blotting. Results: The recombinant HspB5 was purified to its homogeneity and was characterized using various biophysical techniques. The externally added FITC-labeled HspB5 was found to be localized within the cytoplasm of mNSPCs. Our Immunocytochemistry results showed that the externally added FITC-labeled HspB5 not only entered the cells but also conferred cytoprotection against paraquat-induced toxicity. The protective events were monitored by a decrease in the PI-positive cells and an increase in the procaspase-3 expression through Immunocytochemistry and Western blotting respectively. Conclusion: Our results clearly demonstrate that exogenously added recombinant human HspB5 enters the mNSPCs and confers protection against paraquat toxicity.
Keywords: Neural stem/progenitor cells, αB-crystallin, HspB5, paraquat, cytoprotection
Introduction
Neural stem cells (NSCs) are self-renewing, multipotent cells derived from the ectoderm during the early embryonic stages; they give rise to neurons and glial cell types [1]. NSCs were isolated from different parts of the brain [2] and can be cultured as Neurospheres, which mimic the neurogenic niche [3,4]. Neurospheres are three-dimensional floating aggregates containing Neural Stem Cells/Neural Progenitor Cells (NSPCs) [5].
NSC transplantation has opened new avenues in the field of Neurosurgery. It is a promising therapeutic approach and has shown beneficial effects, such as improving the neural microenvironment and cognitive behavior in various animal models of Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, and other neurodegenerative diseases [6]. However, the transplanted NSCs have a poor survival rate in the new and hostile environment, majorly due to oxidative stress. Exposure to oxidative reagents such as H2O2 drastically reduced the cell viability in cultured rat NSCs [7] and adult spinal cord-derived NSCs/neural progenitor cells [8]. Increasing the survival of NSCs would be beneficial, especially in case of NSC transplantation therapy.
Paraquat is used globally as an herbicide. It is used in many laboratory studies to induce oxidative stress. Several mechanisms are proposed to explain the cytotoxicity of paraquat [9]. However, the consensus is that paraquat induces oxidative stress, leading to an increase in ROS, ultimately leading to cellular damage and apoptosis [9,10].
Heat shock proteins (Hsps) protect cells from oxidative stress [11]. Heat shock, prior to the exposure of paraquat, rescues the immortalized rat brain neuroblasts (E18) from cell death; the heat shock response leads to elevated levels of HspB1 and Hsp70, which directly correlates with cellular protection [12].
Heat shock proteins bind to non-native proteins and prevent aggregation and the consequent cellular damage. In addition, they also exhibit chaperone activity, help in many cellular processes and confer cytoprotection against environmental stresses viz. heat stress, oxidative stress, chemical stress, etc., [13]. Among Hsps, small Hsps (sHsps) are a group of heat shock proteins characterized by the presence of an alpha-crystallin domain (ACD) in their structures; their monomeric molecular sizes range between 12 kDa to 43 kDa [14].
αB-crystallin (HspB5), one of the member proteins of the sHsp family is found in many tissues and is also reported to be present at low concentrations in the extracellular space/fluid. Earlier studies have shown the translocation of HspB5 from the cytoplasm into the nucleus upon stress. The reason for HspB5’s nuclear translocation is unclear, but it interacts with proteins such as intranuclear lamin A/C and splicing factor SC-35 [15]. HspB5 works majorly as a molecular chaperone that binds to misfolded proteins and prevents them from aggregation [14]. HspB5 is shown to be associated with stress-induced apoptosis, cell differentiation, cell cycle, cancer, and angiogenesis [14,16]. HspB5 has a protective role in CNS conditions such as Alzheimer’s disease, multiple sclerosis, cerebral ischemia, and Alexander disease [17-21]. HspB5 protects the cells from apoptosis induced by oxidative agents such as H2O2, drugs such as Staurosporine, and cytokines such as TNF-α [14,16]. Earlier findings demonstrated that HspB5 protects myoblasts against TNFα-induced cytotoxicity by activating NFκB [22]. Brownell et al. have listed the role of HspB5 in neurological disease and its neuroprotective characteristics [23]. Cell penetration peptide (CPP) tagged recombinant HspB5 protects the human lens epithelial cells from oxidative stress and heat-induced cell death [24].
Intravenously administered recombinant HspB5, in a spinal cord injury (SCI) mouse model, was shown to be taken up by oligodendrocytes and glial cells at the injury site and help in the recovery [25]. Intravenously administered recombinant HspB5 is known to promote axonal regeneration [26], rescue the optic nerve oligodendrocytes [27], and increase the survival of retinal ganglion cells (RGC) in optic nerve crush experiments in rats [28].
In the current study, we have investigated the protective ability of exogenously added recombinant human HspB5 in the context of mNSPC. Our results demonstrate that exogenously added HspB5 not only gains entry into the cultured mNSPCs but also confers protection against paraquat-induced apoptosis. Such protective ability might prove useful in NSC transplantation therapy.
Material and methods
Overexpression, purification, and FITC-labeling of human recombinant HspB5
Human recombinant HspB5 was purified using the procedure described earlier [8]. The protein was then buffer exchanged from 1xTNE to PBS (20 mM phosphate buffer with 100 mM NaCl, pH 7.4). After purification, the protein was labeled with fluorescein isothiocyanate (FITC) (F7250, Sigma). Briefly, 50 µL of 1 mg/mL FITC was added to 1 mL of protein solution (1 mg/mL) and incubated overnight at 4°C under constant stirring. The FITC-tagged protein was separated from the untagged protein by using a Sephadex G-25 desalting column. The protein concentration was determined using the extinction coefficient of HspB5 (1 mg/mL protein gives an OD of 0.693 at 280 nm). The purified and labeled proteins were stored at 4°C until further experiments were carried out.
Biophysical characterization
Dynamic Light Scattering (DLS)
Photocor DLS Instrument (Photocor Instruments Inc., College Park, MD) fitted with a 633 nm, 25 mW laser was used for the determination of the hydrodynamic radii (Rh) of HspB5 (1 mg/mL) in 20 mM phosphate buffer with 100 mM NaCl, pH 7.4 at 25°C by measuring the scattering at 90° angle. Protein samples were filtered through a 0.22 μm syringe filter before the experiment. DYNALS v2.0 software was used to analyze the data.
Circular dichroism (CD)
Far UV-CD spectra of the protein (0.2 mg/mL protein in a 0.05 cm pathlength cuvette) were recorded at room temperature at a step size of 0.2 nm and an approximate scan time of 5 min using an Applied Photophysics instrument, Chirascan Plus. An average of four accumulations was reported as the final spectra. The spectrum was recorded from 195 nm to 260 nm. All the ellipticity values obtained were transformed to mean residue mass ellipticity.
[θ] MRM (deg. cm2. dmol-1) = θ x MRW/10cl
θ: Ellipticity in mdeg; MRW: mean residue weight; c: Concentration in mg/mL, and l: path length in cm.
Sedimentation-velocity measurements
Sedimentation-velocity (SV) measurement was performed using an Optima XL-I analytical ultracentrifuge (Beckman Coulter, Fullerton, CA, USA). A 1 mg/mL (50 μM) sample of HspB5 in 20 mM phosphate buffer, pH 7.4, containing 100 mM NaCl, was subjected to centrifugation at 24,000 rpm at 20°C in a rotor. The sample was scanned for absorbance at 280 nm. The data were analyzed using the ‘SEDFIT’ program, and the sedimentation coefficient S20w and the molecular mass of the protein were calculated as described earlier [29,30]. ‘SEDFIT’ uses nonlinear regression fitting of the sedimenting boundary profile with the Lamm equation.
dc/dt = (1/r)·(d/dr)·[(rD dc/dr) - sω2r2c]
c(r, t) stands for concentration distribution of a species with diffusion coefficient D and sedimentation coefficient s, and ω2r represents the centrifugal field.
Chaperone assay
In this study, we have used the thermal aggregation of alcohol dehydrogenase (ADH; 0.2 mg/mL in 20 mM phosphate buffer with 100 mM NaCl, pH 7.4) as a model to study the chaperone-like activity of HspB5. The aggregation of the target protein at 48°C, either in the absence or in the presence of HspB5 (0.0125 mg/mL), was monitored as right-angle light scattering using a Hitachi F-7000 fluorescence spectrophotometer. The cuvettes containing the samples were placed in a thermostated-cuvette holder; the excitation and emission wavelengths were set at 465 nm, and the excitation and emission bandpass were set at 2.5 nm. The response time was set at 2 seconds, and the light-scattering was measured as a function of time.
Isolation and maintenance of mNSPCs
Pregnant female BALB/c mice were used to obtain embryonic day 14 (E14) embryos (from CCMB animal house facility (IEC 117/2016)). The mice were euthanized by cervical dislocation, and E14 embryos were collected. The cortical, hippocampal, and striatal tissues were aseptically dissected from fetal brains and were homogenized by passing them through a sterile Pasteur pipette several times. The detached cells were further centrifuged at 1,000 g, and the cell pellet was resuspended in NeuroCultTM NSC Basal Medium (05700, Stem cell technologies) supplemented with NeuroCultTM NSC Proliferation Supplement (05701, Stem cell technologies) and 20 ng/mL epidermal growth factor (EGF) (E4127, Sigma). The cultures were then maintained in a humidified atmosphere with 5% CO2 at 37°C, with media replacement every 3-4 days until healthy neurospheres were obtained. The mNSPCs were stained for stem cell markers.
Localization of exogenously added HspB5 in mNSPCs
In order to study whether the mNSPCs take up the exogenously added HspB5, the mNSPCs were seeded on poly L-Ornithine (0.1 mg/mL; Sigma P4707) -coated coverslips and incubated with different concentrations of FITC-tagged protein for 24 hours. After incubation, the coverslips were taken, washed with PBS, counterstained with DAPI, and mounted on to grease-free slides; the slides were then analyzed by confocal microscopy.
Anti-apoptotic activity of HspB5 against paraquat-induced oxidative stress
To study the protective effect of HspB5 against the oxidative stress-induced apoptosis, the mNSPCs were seeded on poly L-Ornithine (0.1 mg/mL; Sigma P4707) -coated plates and pre-treated with HspB5 (100 µg/mL) for 24 hours and then treated with 3 mM paraquat (PQ2+) for 4 hours.
Hoechst-propidium iodide staining
Hoechst 33258 dye (Sigma) and propidium iodide (PI; Sigma) staining was used to evaluate the cell viability of paraquat-treated mNSPCs (PQ2+), HspB5 pre-treated mNSPCs exposed to paraquat (HspB5-PQ2+), and control mNSPCs. In brief, Hoechst dye was added to the culture medium (10 µg/mL final concentration), and the samples were incubated at 37°C for 30 minutes. PI (50 µg/mL final concentration) was added immediately before imaging with a fluorescence microscope. Hoechst-positive + PI-positive cells were counted as dead cells, and Hoechst-positive + PI-negative cells were counted as live cells.
MTT assay
3-(4,5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT; Sigma) assay was used to assess the metabolic activity of mNSPCs at 4 hours after paraquat treatment. For the assay, mNSPCs (control, PQ2+, HspB5-PQ2+) were incubated with MTT (0.5 mg/mL) and incubated for 3 hours at 37°C. Following incubation, the MTT solution was removed, and 100 µL of DMSO was added to dissolve formazan crystals. Absorbance was measured at 560 nm using a microplate reader (Synergy H1 Hybrid multi-mode plate reader; Gen5 software).
Immunocytochemistry
The cells were fixed with 4% paraformaldehyde in 0.1 M PBS, pH 7.4, for 30 minutes. After the PBS wash, the cells were permeabilized with 0.5% Triton X-100 in PBS for 10 minutes. Non-specific sites were blocked with 2% bovine serum albumin (SRL) for 1 hour at room temperature. Appropriate primary antibodies were added and incubated overnight at 4°C. After PBS washes, the secondary antibodies were added and incubated for 2 hours at room temperature. After washing with PBS, the cells were counterstained with DAPI and visualized using a fluorescence microscope. Primary antibodies used were Caspase-3; 1:250; Cell Signaling Technologies - 9915S, HspB5; 1:200; Enzo life sciences - ADI-SPA-223. Secondary antibodies used were anti-rabbit/mouse; 1:500; conjugated with Alexa Flour 594; Life Technologies - A11037/A11005.
Western blotting
The cells were lysed with RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl, pH 8.0, Protease inhibitor cocktail), and the concentration of proteins was determined by Bradford method. Equal amounts of protein (50 µg) from all the samples were loaded onto a 12% polyacrylamide gel and were electrophoresed. Further, the proteins on the SDS-PAGE gel were blotted onto a PVDF membrane using an ECL Semi-dry blotter (TE 77 Semi-dry transfer unit, Amersham Biosciences). The membrane was blocked with 5% BSA solution for 1 hour at room temperature. The membrane was then incubated with primary antibodies overnight at 4°C. After the PBS wash, it was further incubated with HRP-conjugated secondary antibodies at room temperature for 3 hours and was followed by a PBS wash. The blot was developed using a chemiluminescent substrate (Super Signal West Pico, Thermo Scientific - 34080). The intensity of the bands was quantified using the Image J software. Primary antibodies used were HspB5: 1:1500, Enzo life sciences - ADI-SPA-223; Procaspase-3: 1:1500, Cell Signaling Technology - 9915S: β-actin: 1:1500, Abcam - ab8226. Secondary antibodies - anti-rabbit/mouse: 1:3000, Invitrogen /626520.
Statistical analysis
The densitometric measurements of all the detected proteins were performed by Scion NIH Image Analysis Software (Image J) (version 3.5). All western blots were represented as a mean of at least 3 independent experiments. Data are expressed as mean ± SD. Paired 2 -tailed Student’s t-test was used for the analysis of the significance of the variations. p<0.001 was considered as statistically significant. GraphPad Prism 3 (GraphPad Software, Inc.) was used for statistical analysis.
Results
Protein purification
The recombinant human HspB5 was expressed in E.coli and purified to its homogeneity (Figure 1A).
Figure 1.
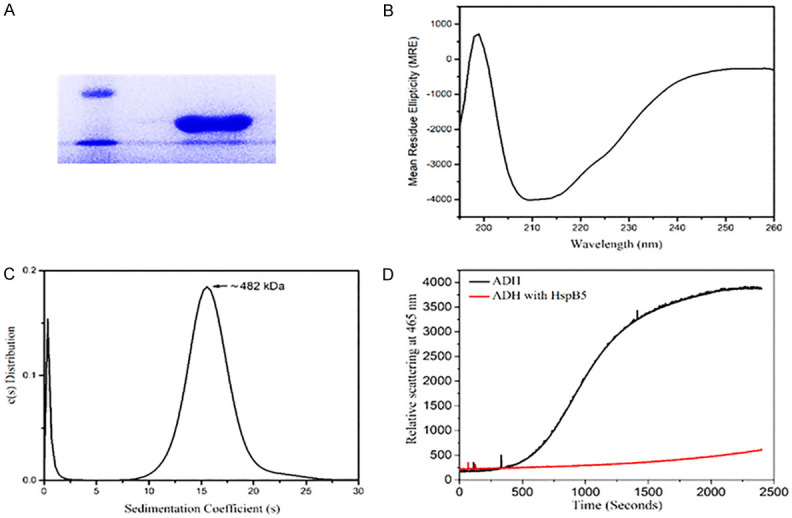
Characterization of the HspB5 protein. A. A 12 % SDS-PAGE shows the purified human recombinant HspB5 at ~20 kDa (lane to the right) against a Low Mol.Wt. protein marker (lane to the left); B. Far-UV CD Spectrum indicates a predominantly beta-sheet structure (minima ~215 nm) along with a slight alpha-helical component, typical of HspB5; C. Sedimentation profile of HspB5 shows a sedimentation coefficient of 16.6 S and a molecular mass of 482 kDa, indicating a 24-mer oligomeric structure; D. HspB5 (red line) shows protection against the temperature-induced aggregation of Alcohol dehydrogenase (black line).
Biophysical characterization
Circular dichroism
The far-UV CD spectral data obtained for the recombinant human HspB5 (Figure 1B) was analyzed using the CDNN software (Table 1). The protein was found to be predominantly beta-sheet in nature, as reported in earlier studies [31,32] (Figure 1B).
Table 1.
Secondary structural studies using Far-UV CD: CDNN analysis of the data suggests that the recombinant HspB5 has beta-sheet structures predominantly
| Wave length | Helix | Antiparallel | Parallel | Beta-turn | Random coil | Total |
|---|---|---|---|---|---|---|
| 195 nm-260 nm | 17.6% | 25.0% | 14.3% | 20.5% | 44.1% | 121.5% |
Sedimentation-velocity measurements
The sedimentation coefficient profile of HspB5 showed a peak at 16.6 S (Figure 1C). Using the ‘SEDFIT’ software, the molecular weight of HspB5 was calculated to be 481820 Da or ~ 482 kDa, indicating that the protein exists as a 24-mer, as reported in the literature [33] (Figure 1C).
Chaperone assay
Even at an exceptionally low concentration, 1:0.0625 (ADH: HspB5), HspB5 showed significant protection by preventing the thermal aggregation of Alcohol dehydrogenase (Figure 1D).
Isolation and maintenance of mNSPCs
mNSPCs isolated from the embryonic day 14 (E14) embryos were cultured and maintained in neural stem cell maintenance media. Immunostaining results showed specific neural stem cell markers (SOX2 and Nestin) (Figure 2A). Upon incubation with varying concentrations of FITC-tagged HspB5, mNSPCs showed the localization of HspB5 in the cytoplasm and perinuclear region of the cell, showing the capability of protein to cross the cell membrane (Figure 2B). Western blot was performed to determine the localization of exogenously added HspB5 in the mNSPCs. The presence of HspB5 in the cell lysate for the protein-treated-group, compared to the untreated group, suggests that the externally added protein crossed the cell membrane and localized in the cytoplasm of mNSPCs (Figure 2C).
Figure 2.
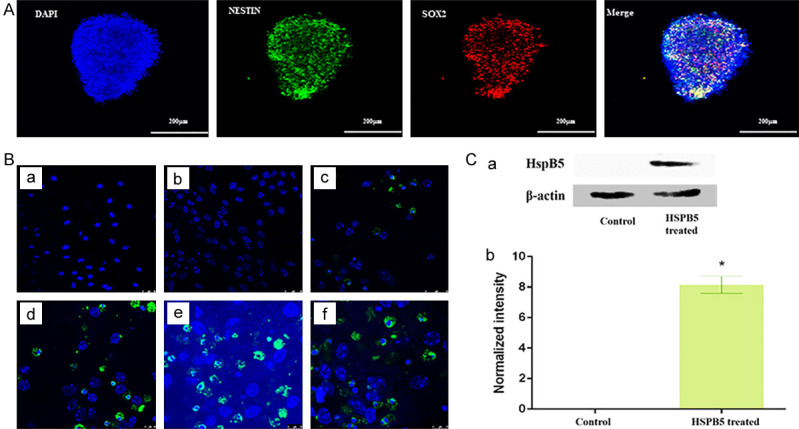
A. Stem cells markers, suggesting the stemness in the cultured Neurospheres; Blue represents Nucleus (DAPI); Green represents Nestin; Red represents SOX2. B. Localization of exogenously added HspB5 (varying concentrations) in mNSPCs; (a) Media only (b) 10 µg/mL (c) 50 µg/mL (d) 100 µg/mL (e) 150 µg/mL (f) 200 µg/mL. C. (a) HspB5 Immunoblot. Photograph showing HspB5 expression in treated and untreated groups. (b) The densitometric measurements of all the detected proteins were performed by Scion NIH Image Analysis Software (Image J) (version 3.5). Each measurement represents the mean ± SD of the intensity of the bands normalized to their respective control (β-actin).
Anti-apoptotic activity of HspB5 against paraquat-induced oxidative stress
Hoechst-PI staining
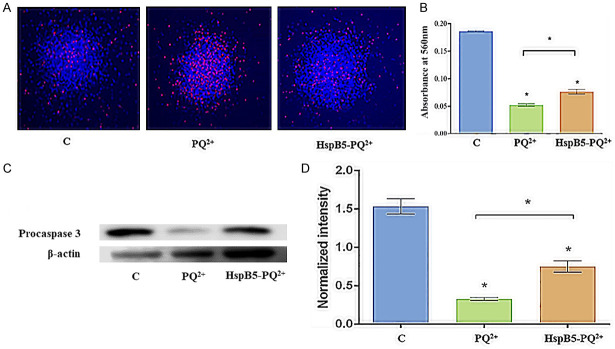
Hoechst-PI staining was used to distinguish the apoptotic cells from viable cells; a greater proportion of PI-positive cells (red) that represent dead cells were seen in the control mNSPCs treated with paraquat. However, the HspB5-treated mNSPCs were significantly protected from cell death caused due to paraquat, as evidenced by a decrease in PI-positive cells (Figure 3A).
Figure 3.
Anti-apoptotic activity of HspB5 against paraquat-induced oxidative stress. A. mNSPCs were exposed to 3 mM paraquat for 4 h, stained with Hoechst (10 µg/mL) - PI (50 µg/mL) and observed under the fluorescence microscope. Here, [C] represents the control mNSPCs without any paraquat stress, [PQ2+] represents the mNSPCs treated with paraquat, and [HspB5-PQ2+] represents the mNSPCs pre-treated with HspB5 (100 µg/mL). Scale bar = 100 µm; B. Quantification of cell viability using the MTT assay; * represents statistical significance between the different treatment groups at p<0.001. C. Immunoblotting of apoptotic procaspase-3 in mNSPCs after paraquat stress. D. Western blot showing the expression of procaspase-3 expression in control, PQ2+ treated, and HspB5-PQ2+ treated. The densitometric measurements of all the detected proteins were performed by Scion NIH Image Analysis Software (Image J) (version 3.5). Each measurement represents the mean ± SD of the intensity of the bands normalized to their respective control (β-actin). A significant difference (p<0.001) was found between the different groups by paired 2-tailed Student’s t-test.
MTT assay
The metabolic activity of mNSPCs after 4 hours of paraquat treatment was assessed by MTT assay. The cell viability between the control and the experimental groups (PQ2+, HspB5-PQ2+) and between the experimental groups showed significant variation (p<0.001), indicating that HspB5 pre-treatment of mNSPCs confers protection against paraquat-induced-oxidative stress (Figure 3B).
Western blotting
Caspase-3 is known to be produced in a cell as a low activity proenzyme that is cleaved under apoptotic conditions to produce the active enzyme caspase-3 that functions as an ‘executioner’ caspase. Once activated, it cleaves several cellular substrates committing the cell to apoptotic death [30,34]. Western blot was performed to determine the localization of exogenously added HspB5 in the mNSPCs. The abundance of HspB5 in the cell lysate for the protein-treated-group, compared to the untreated group, suggests that the externally added protein crossed the cell membrane and localized in the cytoplasm of mNSPCs. Western blot analysis was performed to assess the involvement of apoptotic proteins in paraquat-induced cell injury. Paraquat treatment decreased the expression of procaspase-3 in the mNSPCs, whereas procaspase-3 expression of the mNSPCs pre-treated with HspB5 was comparable to that of the control group. In untreated mNSPCs, the expression of apoptotic protein procaspase-3 decreased significantly (p<0.001), suggesting the role of HspB5 in preventing apoptotic cell death during paraquat injury (Figure 3C, 3D).
Discussion
The ability of HspB5 to prevent protein aggregation and its anti-apoptotic role can have therapeutic implications. For example, intravenously injected HspB5 promoted axonal regeneration of the optic nerve in rats after optic nerve crush [35]. Exogenously administered HspB5 rescued the optic nerve oligodendrocytes in an experimental model of anterior ischemic optic neuropathy in mice by inhibiting microglial activation [36]. Because of the advantages of HspB5 in cell-protection from apoptosis, attempts are being made to deliver HspB5 into the cells tagged with a cell penetration peptide (CPP) [26]. The cell-entry strategy of HspB5 into lens epithelial cells showed enhanced protection against various stresses such as heat and oxidative stress [27]. Our studies suggest that HspB5 can be delivered into mNSPCs by exogenously adding the protein to the medium (Figure 2B). The mechanism of uptake is still not clear, and further experiments need to be carried out to understand how the cells take up the exogenously added HspB5 in the medium.
Our studies confirm that 24 h pre-treatment with HspB5 protected mNSPCs from paraquat-induced apoptosis. The protective ability of HspB5 was confirmed with a decrease in cell death, as evidenced by Hoechst-PI staining and MTT data (Figure 3A, 3B). The mechanism of protection seems to involve the prevention of activation of procaspase-3 to the mature executioner caspase-3 (Figure 3C, 3D). In agreement with our findings, earlier studies showed that HspB5 interacts with procaspase-3, preventing their maturation to caspase-3 [37,38]. It is interesting to know that the overexpression of HspB5 in C6 astroglioma cells protects them from H2O2-induced apoptosis by inhibiting caspase-3 activation [39]. It is also known to regulate the anti-apoptotic function through caspase-3, Bcl-X, and Bax [40-42] and also known to inhibit the release of cytochrome C from mitochondria by downregulating Bcl-2 in H2O2 injured cells [43]. The anti-apoptotic activity of HspB5 has been associated with its translocation to mitochondria, binding with cytochrome c, caspase-3, caspase-12, and voltage-dependent anion channels [44]. The mNSPCs take up the FITC-labeled HspB5 upon 24 h treatment, and the protein was found to be localized in the cytoplasm, especially surrounding the nucleus (Figure 2B).
Neurological and neurodegenerative diseases are characterized by neuroinflammation and cell death. HspB5 is demonstrated to have anti-inflammatory activity in many pathological conditions through the inhibition of T-cell proliferation, reduced secretion of pro-inflammatory cytokines, and limiting apoptosis [10]; the anti-inflammatory activity of HspB5 thus reduces the burden of oxidative stress. The secretion of HspB5 via exosomes plays an essential role in suppressing neuroinflammation and can act through autocrine [45] and paracrine manner [46] in suppressing the inflammation in microglial cells.
In the present study, we have demonstrated that exogenously added HspB5 could cross the cell membrane and protect the mNSPCs from oxidative stress induced by paraquat. Pre-treatment of the cells with HspB5 decreased the proportion of apoptotic cells when challenged with paraquat. Our study provides compelling evidence that the baseline antioxidant capacity increases with the availability of HspB5, thus leading to stem-cell protection.
Acknowledgements
The authors acknowledge Dr. T Ramakrishna for critical reading of the manuscript and suggestions. Dr. Kiran Kumar Bokara acknowledges CSIR network project mIND (BSC-0115) for financial assistance. Dr. Ch Mohan Rao acknowledges the Department of Science and Technology, Government of India, for the Sir JC Bose National Fellowship.
Disclosure of conflict of interest
None.
References
- 1.Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nat Rev Neurosci. 2010;11:176–187. doi: 10.1038/nrn2761. [DOI] [PubMed] [Google Scholar]
- 2.Temple S. Division and differentiation of isolated CNS blast cells in microculture. Nature. 1989;340:471–473. doi: 10.1038/340471a0. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 4.Jensen JB, Parmar M. Strengths and limitations of the neurosphere culture system. Mol Neurobiol. 2006;34:153–161. doi: 10.1385/MN:34:3:153. [DOI] [PubMed] [Google Scholar]
- 5.Giachino C, Basak O, Taylor V. Isolation and manipulation of mammalian neural stem cells in vitro. Methods Mol Biol. 2009;482:143–158. doi: 10.1007/978-1-59745-060-7_9. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi Y, Lin HT, Lee CC, Tsai KJ. Effects of neural stem cell transplantation in Alzheimer’s disease models. J Biomed Sci. 2020;27:29. doi: 10.1186/s12929-020-0622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Ma W, Wang L, Gong H, Tian Y, Zhang J, Liu J, Lu H, Chen X, Liu Y. Activation of type 4 metabotropic glutamate receptor attenuates oxidative stress-induced death of neural stem cells with inhibition of JNK and p38 MAPK signaling. Stem Cells Dev. 2015;24:2709–2722. doi: 10.1089/scd.2015.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hachem LD, Mothe AJ, Tator CH. Effect of BDNF and other potential survival factors in models of in vitro oxidative stress on adult spinal cord-derived neural stem/progenitor cells. Biores Open Access. 2015;4:146–159. doi: 10.1089/biores.2014.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsopoulos P, Suntres ZE. Cytotoxicity and gene array analysis of alveolar epithelial A549 cells exposed to Paraquat. Chem Biol Interact. 2010;188:427–436. doi: 10.1016/j.cbi.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Budnar P, Singh NP, Rao CM. HSPB5 (αB-crystallin) confers protection against paraquat-induced oxidative stress at the organismal level in a tissue-dependent manner. Cell Stress Chaperones. 2020;26:229–239. doi: 10.1007/s12192-020-01171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donati YR, Slosman DO, Polla BS. Oxidative injury and the heat shock response. Biochem Pharmacol. 1990;40:2571–2577. doi: 10.1016/0006-2952(90)90573-4. [DOI] [PubMed] [Google Scholar]
- 12.Donaire V, Niso M, Moran JM, Garcia L, Gonzalez-Polo RA, Soler G, Fuentes JM. Heat shock proteins protect both MPP(+) and paraquat neurotoxicity. Brain Res Bull. 2005;67:509–514. doi: 10.1016/j.brainresbull.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Shende P, Bhandarkar S, Prabhakar B. Heat shock proteins and their protective roles in stem cell biology. Stem Cell Rev Rep. 2019;15:637–651. doi: 10.1007/s12015-019-09903-5. [DOI] [PubMed] [Google Scholar]
- 14.Bakthisaran R, Tangirala R, Rao ChM. Small heat shock proteins: role in cellular functions and pathology. Biochim Biophys Acta. 2015;1854:291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Iwaki T, Kume-Iwaki A, Goldman JE. Cellular distribution of alpha B-crystallin in non-lenticular tissues. J Histochem Cytochem. 1990;38:31–39. doi: 10.1177/38.1.2294148. [DOI] [PubMed] [Google Scholar]
- 16.Bakthisaran R, Akula KK, Tangirala R, Rao Ch M. Phosphorylation of alphaB-crystallin: role in stress, aging and patho-physiological conditions. Biochim Biophys Acta. 2016;1860:167–182. doi: 10.1016/j.bbagen.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O’Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 18.Hagemann TL, Boelens WC, Wawrousek EF, Messing A. Suppression of GFAP toxicity by alphaB-crystallin in mouse models of Alexander disease. Hum Mol Genet. 2009;18:1190–1199. doi: 10.1093/hmg/ddp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bousette N, Chugh S, Fong V, Isserlin R, Kim KH, Volchuk A, Backx PH, Liu P, Kislinger T, MacLennan DH, Emili A, Gramolini AO. Constitutively active calcineurin induces cardiac endoplasmic reticulum stress and protects against apoptosis that is mediated by alpha-crystallin-B. Proc Natl Acad Sci U S A. 2010;107:18481–18486. doi: 10.1073/pnas.1013555107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L, Steinberg GK. Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc Natl Acad Sci U S A. 2011;108:13287–13292. doi: 10.1073/pnas.1107368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ojha J, Karmegam RV, Masilamoni JG, Terry AV, Cashikar AG. Behavioral defects in chaperone-deficient Alzheimer’s disease model mice. PLoS One. 2011;6:e16550. doi: 10.1371/journal.pone.0016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adhikari AS, Singh BN, Rao KS, Rao ChM. alphaB-crystallin, a small heat shock protein, modulates NF-kappaB activity in a phosphorylation-dependent manner and protects muscle myoblasts from TNF-alpha induced cytotoxicity. Biochim Biophys Acta. 2011;1813:1532–1542. doi: 10.1016/j.bbamcr.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Brownell SE, Becker RA, Steinman L. The protective and therapeutic function of small heat shock proteins in neurological diseases. Front Immunol. 2012;3:74. doi: 10.3389/fimmu.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christopher KL, Pedler MG, Shieh B, Ammar DA, Petrash JM, Mueller NH. Alpha-crystallin-mediated protection of lens cells against heat and oxidative stress-induced cell death. Biochim Biophys Acta. 2014;1843:309–315. doi: 10.1016/j.bbamcr.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klopstein A, Santos-Nogueira E, Francos-Quijorna I, Redensek A, David S, Navarro X, Lopez-Vales R. Beneficial effects of alphaB-crystallin in spinal cord contusion injury. J Neurosci. 2012;32:14478–14488. doi: 10.1523/JNEUROSCI.0923-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang YH, Wang DW, Wu N, Wang Y, Yin ZQ. alpha-Crystallin promotes rat axonal regeneration through regulation of RhoA/rock/cofilin/MLC signaling pathways. J Mol Neurosci. 2012;46:138–144. doi: 10.1007/s12031-011-9537-z. [DOI] [PubMed] [Google Scholar]
- 27.Pangratz-Fuehrer S, Kaur K, Ousman SS, Steinman L, Liao YJ. Functional rescue of experimental ischemic optic neuropathy with alphaB-crystallin. Eye (Lond) 2011;25:809–817. doi: 10.1038/eye.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu N, Yu J, Chen S, Xu J, Ying X, Ye M, Li Y, Wang Y. alpha-Crystallin protects RGC survival and inhibits microglial activation after optic nerve crush. Life Sci. 2014;94:17–23. doi: 10.1016/j.lfs.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad MF, Raman B, Ramakrishna T, Rao ChM. Effect of phosphorylation on alpha B-crystallin: differences in stability, subunit exchange and chaperone activity of homo and mixed oligomers of alpha B-crystallin and its phosphorylation-mimicking mutant. J Mol Biol. 2008;375:1040–1051. doi: 10.1016/j.jmb.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Schuck P. On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal Biochem. 2003;320:104–124. doi: 10.1016/s0003-2697(03)00289-6. [DOI] [PubMed] [Google Scholar]
- 31.Kumar LV, Ramakrishna T, Rao CM. Structural and functional consequences of the mutation of a conserved arginine residue in alphaA and alphaB crystallins. J Biol Chem. 1999;274:24137–24141. doi: 10.1074/jbc.274.34.24137. [DOI] [PubMed] [Google Scholar]
- 32.Perng MD, Muchowski PJ, van Den IP, Wu GJ, Hutcheson AM, Clark JI, Quinlan RA. The cardiomyopathy and lens cataract mutation in alphaB-crystallin alters its protein structure, chaperone activity, and interaction with intermediate filaments in vitro. J Biol Chem. 1999;274:33235–33243. doi: 10.1074/jbc.274.47.33235. [DOI] [PubMed] [Google Scholar]
- 33.Peschek J, Braun N, Rohrberg J, Back KC, Kriehuber T, Kastenmuller A, Weinkauf S, Buchner J. Regulated structural transitions unleash the chaperone activity of alphaB-crystallin. Proc Natl Acad Sci U S A. 2013;110:E3780–3789. doi: 10.1073/pnas.1308898110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin Q, Park HH, Chung JY, Lin SC, Lo YC, da Graca LS, Jiang X, Wu H. Caspase-9 holoenzyme is a specific and optimal procaspase-3 processing machine. Mol Cell. 2006;22:259–268. doi: 10.1016/j.molcel.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chis R, Sharma P, Bousette N, Miyake T, Wilson A, Backx PH, Gramolini AO. alpha-Crystallin B prevents apoptosis after H2O2 exposure in mouse neonatal cardiomyocytes. Am J Physiol Heart Circ Physiol. 2012;303:H967–978. doi: 10.1152/ajpheart.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dou G, Sreekumar PG, Spee C, He S, Ryan SJ, Kannan R, Hinton DR. Deficiency of alphaB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic Biol Med. 2012;53:1111–1122. doi: 10.1016/j.freeradbiomed.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin JK, Whittaker R, Glassy MS, Barlow SB, Gottlieb RA, Glembotski CC. Localization of phosphorylated alphaB-crystallin to heart mitochondria during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H337–344. doi: 10.1152/ajpheart.00881.2007. [DOI] [PubMed] [Google Scholar]
- 38.Whittaker R, Glassy MS, Gude N, Sussman MA, Gottlieb RA, Glembotski CC. Kinetics of the translocation and phosphorylation of alphaB-crystallin in mouse heart mitochondria during ex vivo ischemia. Am J Physiol Heart Circ Physiol. 2009;296:H1633–1642. doi: 10.1152/ajpheart.01227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin JH, Kim SW, Lim CM, Jeong JY, Piao CS, Lee JK. alphaB-crystallin suppresses oxidative stress-induced astrocyte apoptosis by inhibiting caspase-3 activation. Neurosci Res. 2009;64:355–361. doi: 10.1016/j.neures.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Hamann S, Metrailler S, Schorderet DF, Cottet S. Analysis of the cytoprotective role of alpha-crystallins in cell survival and implication of the alphaA-crystallin C-terminal extension domain in preventing Bax-induced apoptosis. PLoS One. 2013;8:e55372. doi: 10.1371/journal.pone.0055372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamradt MC, Lu M, Werner ME, Kwan T, Chen F, Strohecker A, Oshita S, Wilkinson JC, Yu C, Oliver PG, Duckett CS, Buchsbaum DJ, LoBuglio AF, Jordan VC, Cryns VL. The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280:11059–11066. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- 42.Kamradt MC, Chen F, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- 43.Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 44.Hu WF, Gong L, Cao Z, Ma H, Ji W, Deng M, Liu M, Hu XH, Chen P, Yan Q, Chen HG, Liu J, Sun S, Zhang L, Liu JP, Wawrousek E, Li DW. alphaA- and alphaB-crystallins interact with caspase-3 and Bax to guard mouse lens development. Curr Mol Med. 2012;12:177–187. doi: 10.2174/156652412798889036. [DOI] [PubMed] [Google Scholar]
- 45.Guo YS, Liang PZ, Lu SZ, Chen R, Yin YQ, Zhou JW. Extracellular alphaB-crystallin modulates the inflammatory responses. Biochem Biophys Res Commun. 2019;508:282–288. doi: 10.1016/j.bbrc.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 46.Reddy VS, Jamma T, Reddy GB. Small heat shock proteins in inflammatory diseases. Dordrecht: Springer Netherlands; pp. 1–29. [Google Scholar]