Abstract
Background: Autism spectrum disorders [ASD] is a lifelong disability mainly affecting the development, communication, social interaction and behavior of an individual. Cell transplantation is emerging as a potential therapeutic strategy for ASD. Our previously published proof of concept study showed beneficial effects of cell transplantation in ASD. This study shows effect of cell transplantation in a larger sample size of ASD patients. Methods: 254 patients diagnosed with ASD on DSM V criteria were enrolled in this open label non-randomized study. The intervention included intrathecal transplantation of autologous bone marrow mononuclear cells and neurorehabilitation. On mean follow up of 7.50 months, percentage analysis was performed on all symptomatic changes. Changes in outcome measures, Indian Scale for Assessment of Autism [ISAA] and Childhood Autism Rating Scale [CARS], were analyzed statistically using Wilcoxon Signed-Rank Test. Comparative analysis of Positron Emission Tomography [PET CT] scan brain, performed before and 6 months after intervention, was done in 86 patients to monitor the outcome at cellular level. Change in the standardized uptake values was statistically evaluated using T-Test [P≤0.05]. Results: Improvements were observed in eye contact, attention and concentration, hyperactivity, sitting tolerance, social interaction, stereotypical behavior, aggressiveness, communication, speech, command following and self-stimulatory behavior. Statistically significant improvement was observed in scores of ISAA and CARS after intervention. A significantly better outcome of the intervention was found in patients at younger age and with shorter duration of disease [<5 years from time of diagnosis]. 86 patients who underwent a repeat PET CT scan showed improved brain metabolism after intervention in areas which correlated to the symptomatic changes. No major procedure related adverse events were recorded. However, 5 patients, with history of seizure and abnormal EEG, had an episode of seizure which was managed using medications. Outcome of intervention in these patients was not affected by seizures as improvements were observed in them. Conclusion: The results of this study indicate that autologous bone marrow mononuclear cells in combination with neurorehabilitation are a safe and effective treatment modality for ASD. It improves the quality of life of patients and helps them to integrate in mainstream lifestyle.
Keywords: Autologous, bone marrow mononuclear cells, autism spectrum disorders, PET CT scan, cell transplantation
Introduction
Autism spectrum disorder [ASD] is a group of neurodevelopmental disorders which begin at early childhood affecting the development, communication, social interaction and behavior and last lifelong. The exact cause of ASD is unknown, however, it involves a complex association of genetic, environmental and biological risk factors [1]. Common symptoms include poor eye-contact, difficulty reading non-verbal cues, deficits in speech and cognition, repetitive behavior and dependency for day to day activities. The occurrence of ASD has increased manifold over last few years. It puts a substantial socioeconomic burden and affects the quality of life of the affected individual along with the caretakers/family. Until now, no cure has been found for ASD. However, individual symptoms can be managed by medications with rehabilitation therapies which include Applied Behavioral Analysis [ABA], occupational therapy, speech therapy, etc. [2].
Extensive research has been carried out in the past decade to establish a standard therapeutic care which not only manages the symptoms but also addresses the underlying pathology of ASD. The pathology includes defect in neural connectivity, neural migration, excitatory-inhibitory networks, abnormal dendritic morphology, neuroimmune disturbances, oxidative stress, calcium signaling, etc. [3]. Cell transplantation has shown great potential in addressing these pathologies via various paracrine mechanisms or direct cell replacement of damaged/lost cells [4]. Ichim et al. were amongst the first to suggest use of stem cells in autism based on the ability of these cells to immunomodulate and reverse hypoperfusion [5]. Also, in an experimental study conducted on BTBR mice, it was observed that cellular transplantation led to increased hippocampal neurogenesis which must have resulted in behavioral improvements such as reduced stereotypical behaviors, decreased cognitive rigidity and improved social behavior [6]. Our previously published open label proof of concept study showed the beneficial effects of intrathecal administration of autologous bone marrow mononuclear cells [BMMNCs] in 32 patients with ASD. Therefore, to study the effect of autologous BMMNCs on a larger sample size, we conducted this study on 254 diagnosed cases of ASD. This intervention was combined with standard rehabilitation. This study also evaluates various factors influencing the outcome of cell transplantation. Objective measurements i.e. ISAA and CARS were used to assess the therapeutic efficacy while, PET CT scan brain was used as a monitoring tool to study the effect of intervention on brain metabolism. Bone marrow cells were selected as they are easily obtainable and do not involve ethical or moral controversies or tumorigenic risks. Also, autologous cells are safe as they bypass the possibility of any immune rejection.
Materials and method
Study design
This study is an open label non-randomized longitudinal study conducted on 254 patients diagnosed with ASD based on DSM V criteria. The primary aim of this study was to evaluate the efficacy of cell transplantation in combination with neurorehabilitation as a treatment strategy for ASD. The study was conducted at a single center from May 2012 to December 2017. The intervention included cell transplantation and neurorehabilitation. Cell transplantation comprised of intrathecal administration of autologous bone marrow mononuclear cells. Neurorehabilitation included behavior therapy, psychological intervention, occupational therapy, sensory integration therapy, activities of daily living [ADL] training, speech therapy, special education and dietary recommendations.
Patient selection
Patient selection was based on World Medical Association Helsinki Declaration for Ethical Principles for medical research involving human subjects [7]. The protocol of the study was reviewed and approved by the Central Drugs Standard Control Organisation [CDSCO] registered Institutional Ethics Committee. A written informed consent was obtained from the parents of all patients. The intervention was explained to them in detail along with possible adverse events. All the patients included in the study had confirmed diagnosis of ASD according to the DSM-V diagnostic criteria for ASD. The exclusion criteria were presence of severe anemia [hemoglobin <8], acute infections, human immunodeficiency virus [HIV]/hepatitis B virus [HBV]/hepatitis C virus [HCV], malignancies, bone marrow disorders, bleeding tendencies, pneumonia, renal failure, severe liver dysfunction and other acute medical conditions such as respiratory infection and pyrexia.
Intervention protocol
Pre-intervention evaluation
All the patients were evaluated thoroughly before the intervention. A detailed history of every patient was recorded along with serological, biochemical, and hematological tests. All the patients underwent Magnetic resonance imaging [MRI] of the brain, electroencephalography [EEG] and 18F-fluoro-2-deoxyglucose Positron emission tomography-computed tomography [FDG PET-CT] scan brain.
BMMNC aspiration
All the patients were administered Granulocyte Colony Stimulating Factor [GCSF] injections, 72 hours and 24 hours before procuring BMMNCs. On the day of transplantation, bone marrow was aspirated under general anesthesia with sedation in the operation theatre with aseptic precautions. 80-100 ml of bone marrow [depending on the age and body weight of the patient] was aspirated from the anterior superior iliac spine using bone marrow aspiration needle and collected in heparinized tubes.
Isolation of BMMNCs
The bone marrow samples were qualitatively and quantitatively analyzed using Leishman’s stains to rule out preexisting malignancy if any and to ensure that the sample was representative of normal bone marrow. The bone marrow was further subjected to MNC separation using differential centrifugation. It was diluted in the ratio of 1:1 with normal saline. The diluted bone marrow was subjected to density gradient separation by centrifuging it at 440×g rpm for 35 minutes in a swinging bucket rotar without brake at 20°C. MNCs were obtained as a buffy coat. The MNCs were washed thrice with normal saline by centrifuging at 300×g for 15 minutes in a swinging bucket rotar without brake at 20°C and finally resuspended in 1 ml of normal saline. Viable count of the isolated MNCs was performed using trypan blue vital dye which was mixed in 1:1 proportion and loaded on to the haemocytometer for the total cell count and viable count. This was further confirmed by TALI cell counter. CD34+ analysis was done using Fluorescence activated cell sorting [FACS] using CD34 PE antibody.
Administration of BMMNCs
The isolated BMMNCs were injected immediately using a 25G spinal needle between fourth and fifth lumbar vertebrae under general anesthesia with sedation. Simultaneously 20 mg/kg body weight methyl prednisolone in 500 mL Ringer Lactate was given intravenously to reduce inflammation and help in survival of the transplanted cells. Patients were monitored for adverse events.
Neurorehabilitation
Every patient underwent extensive neurorehabilitation for 4 days after cell transplantation. The rehabilitation protocol was personalized for individual patients. It mainly included applied behavioral analysis [ABA], psychological intervention, occupational therapy, sensory integration therapy, activities of daily living [ADL] training, speech therapy, special education and dietary recommendations. Patients were given home program to continue rehabilitation after discharge.
Follow up
The mean physical follow up duration for this study was 7.5 months. A detailed neuro-evaluation was repeated at follow up for every patient. Objective scales; ISAA and CARS were performed again at follow up.
Adverse event monitoring
All the patients were monitored for minor or major adverse events [AE] occurring during their stay in the hospital as well as in the period of follow up. Long term AE were also monitored.
Outcome measures
The outcome measures used to record the effect of intervention at follow up were Indian Scale for Assessment of Autism [ISAA] [8] and Childhood Autism Rating Scale [CARS] [9]. Symptomatic improvements were also recorded. PET CT scan brain was repeated and compared to the pre-intervention scan to find the effect of intervention on brain metabolism in ASD. Parents of 86 patients gave their consent to repeat the PET CT scan.
Methodology of analysis
A detailed analysis was carried out to study the outcome of our intervention. This included percentage analysis of improvement in functional symptoms at a mean follow up of 7.5 months. Sub group analysis was performed to study the effect of age, gender, severity and time from diagnosis to intervention.
Statistical analysis
The outcome of intervention on ISAA and CARS was analyzed statistically using Wilcoxon Signed-Rank Test with level of significance being P value ≤0.05. Change in the standardized uptake values [SUVs] in the PET CT scan performed before and after intervention was statistically evaluated using T-Test [P<0.05]. All statistical tests were performed using SPSS statistic software version 9.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
A total of 254 patients underwent the intervention, out of which 223 were males and 31 were females [ratio 7.2:1] with an age range of 2 to 34 years [Table 1].
Table 1.
Demographic data
| Total | 254 | |
|---|---|---|
| Gender | Male | 223 |
| Female | 31 | |
| Age | 0-5 years | 90 |
| 5-10 years | 90 | |
| 10-15 years | 50 | |
| 15 years and above | 24 | |
| Duration of illness | 0-1 years | 43 |
| 1-5 years | 120 | |
| 5 years and above | 91 | |
| Severity on ISAA | Mild Autism [70-106] | 116 |
| Moderate Autism [107-153] | 136 | |
| Severe Autism [>153] | 2 |
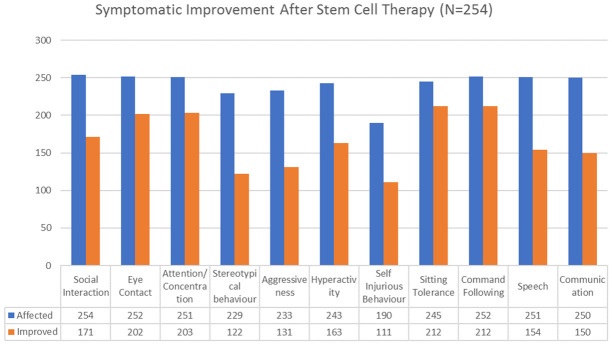
On performing percentage analysis of the symptomatic changes at a mean follow up of 7.5 months, it was found that 86.53% showed improved sitting tolerance, 84.12% showed improved command following, 80.87% showed improved attention and concentration, 80.15% showed improvement in eye contact, 67.3% improved in social interaction, 67.07% showed reduced hyperactivity, 61.35% improved in speech while 60% showed improvement in communication, 58.42% showed reduced self-injurious behavior, 56.22% showed reduced aggressiveness and 53.27% showed reduced stereotypical behavior [Figure 1].
Figure 1.
Graph representing improvements in symptoms after intrathecal transplantation of autologous BMMNCs.
On analyzing the scores on ISAA and CARS scale at follow up after cell transplantation, it was found that 94.48% patients showed a positive change on ISAA while 95.27% patients showed an improved score on CARS.
Statistical results
On statistically analyzing the data using Wilcoxon Signed Rank test with level of significance as P≤0.05, it was observed that the improvements on ISAA and CARS were statistically significant [Table 2].
Table 2.
Wilcoxon signed-rank test [level of significance P≤0.05]
| Scale | Pre Mean Score | Post Mean Score | Z value | P value |
|---|---|---|---|---|
| ISAA | 108.79 | 92.65 | -13.36 | <0.0001 |
| CARS | 34.01 | 29.68 | -13.12 | <0.0001 |
Factors influencing the outcome of intervention
Severity based on ISAA
We carried out a subgroup analysis based on the severity of disorder on ISAA [Figure 2]. Cases were divided into ISAA scores: 70-106 [mild], ISAA scores: 106-153 [moderate] and ISAA scores >153 [severe]. There were only 2 patients in the group with severe autism who also showed improvement.
Figure 2.
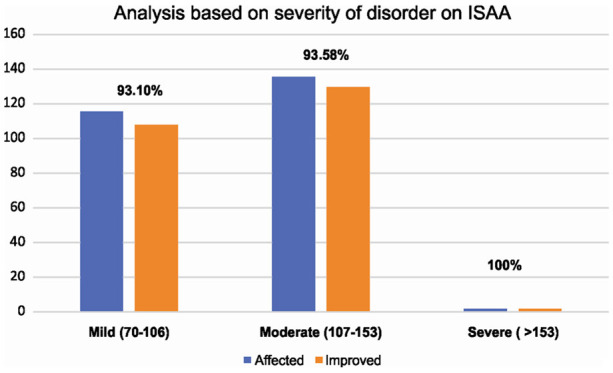
Graph representing results based on severity of disorder on ISAA.
Age
On performing age-wise analysis of ISAA and CARS, all showed more than 90 % improvement. It was seen that younger the age better was the outcome. [Age less than 10 years showed more than 95% improvement] [Figure 3].
Figure 3.
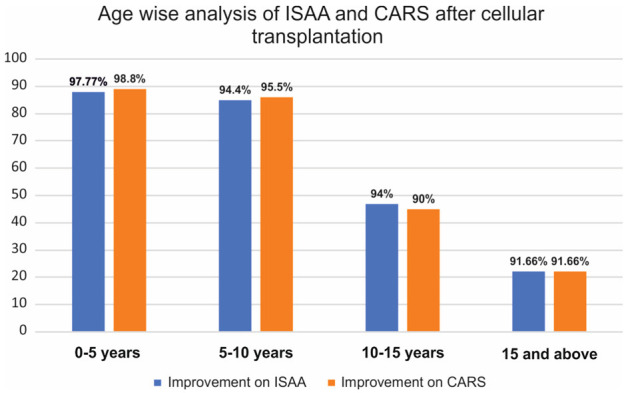
Graph representing post cell transplantation results of ISAA and CARS based on age of patients.
Gender
On analyzing the outcome on ISAA and CARS based on the gender of the patients, it was found that number of female patients was less than the male patients. Both groups showed similar improvements [Figure 4]. Thus, gender did not influence the outcome after intervention.
Figure 4.
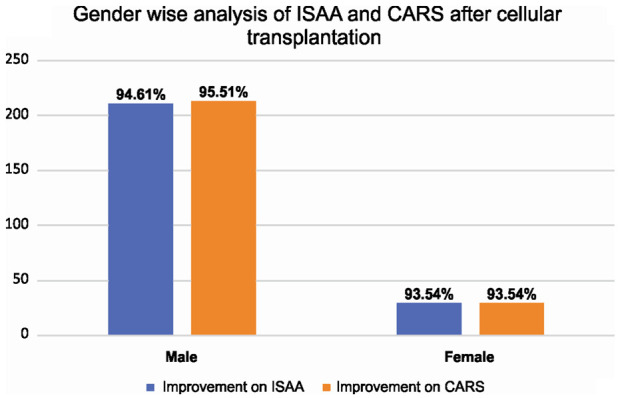
Graph representing post cell transplantation results of ISAA and CARS based on gender.
Duration from diagnosis to intervention
We analyzed the scores of ISAA and CARS based on the duration from diagnosis to intervention. It was observed that the patients who were administered intervention in less than 5 years from diagnosis showed more improvement as compared to those who underwent intervention after 5 years from diagnosis [Figure 5].
Figure 5.
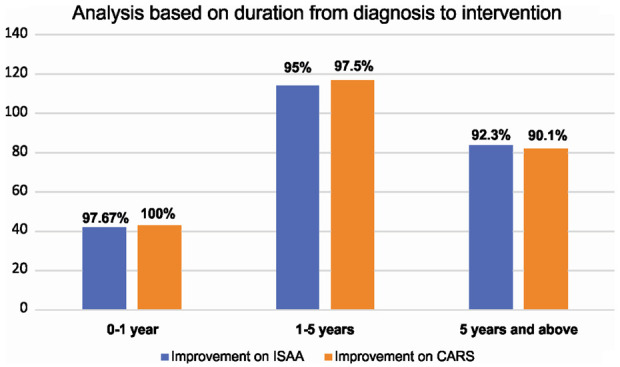
Graph representing post cell transplantation results of ISAA and CARS based on duration from diagnosis to intervention.
Comparative analysis of FDG-PET CT scan brain
Parents of 86 patients gave consent to repeat the FDG-PET CT scan for comparative analysis after 6 months of intervention. Before intervention, FDG-PET/CT scan of these patients showed reduced metabolic activity [hypometabolism] in the bilateral medial temporal cortex, thalamus and cerebellum. Moreover, they also showed increased metabolic activity [hypermetabolism] in the caudate head, putamen, orbital frontal cortex and prefrontal cortex. Six months after cell transplantation, all the patients showed improved brain activity. Previously, hypometabolic regions [medial temporal cortex, thalamus and cerebellum] showed increased FDG uptake while hypermetabolic areas [caudate head, putamen, orbital frontal cortex and prefrontal cortex] showed decreased FDG uptake [Figure 6]. On performing the T-Test, a statistically significant change [P<0.05] was found in the mean SUVs of these areas obtained before and after intervention.
Figure 6.
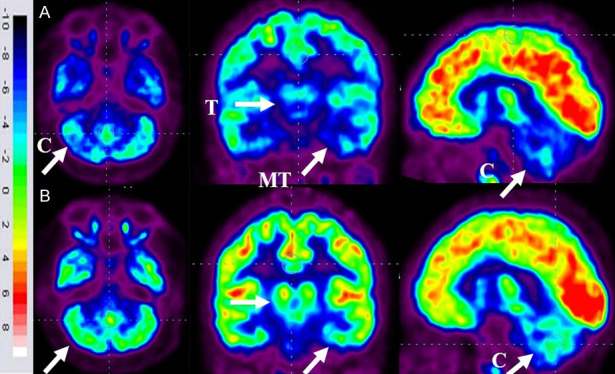
Representative images of FDG-PET/CT scan brain of ASD patients performed before and 6 months after cell therapy. A. Top Row: blue areas marked with arrows demonstrate hypometabolism. B. Below Row: green areas marked with arrows demonstrate improved metabolism following cell transplantation. Thalamus [T], Medial temporal cortex [MT] and Cerebellum [C].
Discussion
Cell transplantation has recently gained a lot of attention as a therapeutic modality for neurological disorders such as autism [10-18]. Though autism is a multifactorial disorder, multiple etiologies are associated with it all converging towards defects in neurodevelopmental pathways [19]. Cell transplantation can address the core underlying neuropathology of autism.
Neuropathophysiology of autism
Defect in cortical connectivity and neural migration to cerebral cortex have been potentially associated with autism [20]. Functional MRI [fMRI] studies have demonstrated lower functional connectivity between frontal and posterior brain regions in autism which could further result in behavioral abnormalities. Altered brain connectivity also affects the neural communication and excitatory-inhibitory balance [21]. Minor change in the excitation and inhibition balance may have a major impact on the neurological functions such as cognition, language and communication, sensory aspects and spatial reasoning [22]. Immune dysfunction has also been proposed as one of the major pathophysiology of autism. Individuals with autism have shown marked inflammation, microglial activation, increased levels of pro-inflammatory cytokines and nitric oxide [NO] [23-25]. These abnormal neuroimmune changes lead to defects in the white matter and neural connectivity which may further cause behavioral abnormalities that are persistent in autism [26]. Cytokines and chemokines are known to regulate brain functions. Activated microglia, along with increasing pro-inflammatory cytokines, enhances phagocytosis which may lead to phagocytosis of normal neurons in autism [27]. Elevated levels of NO increase permeability of the blood brain barrier in autism which consequently leads to leukocyte migration and neuroinflammation in the brain [28,29]. In autism, oxidative stress is also recorded due to decreased levels of antioxidant enzymes. This stimulates inflammatory response and affects the brain function and plasticity. Glutamate excitotoxicity is also one of the major neurobiological mechanisms associated with autism. Increased levels of glutamate levels along with over expression of glutamate receptors leads to increased calcium influx and oxidative stress and progressive cellular degeneration and cell death [30]. GI tract disturbances, sleep disorders and behavioral issues in autism could be linked to this immune dysfunction [31]. Hypoperfusion is also associated with autism, which causes hypoxia which may be responsible for the inflammation, oxidative stress, excitotoxicity, increased BBB permeability and apoptosis [32]. Hypoperfusion is also consistent with behavioral, emotional and language impairments which are typically seen in autism [33].
Supporting evidence
In 2007, Ichim et al. were one of the initial groups to propose use of cell transplantation for treatment of autism. In their review, they proposed the use of stem cell therapy addressing the two pathologies broadly known to be associated with autism-immune dysregulation and neural hypoperfusion [5]. Limited clinical data is presently available on the use of cell transplantation in autism. Our previously published, open label proof of concept study was the first study demonstrating the effect of autologous BMMNCs in 32 patients with autism [34]. 29 [91%] patients improved on ISAA scores and 20 patients [62%] showed decreased severity on CGI-I. On CGI-II 96% of patients showed global improvement. Subsequently there are 4 other studies conducted by Lv et al., Bradstreet et al., Dawson et al. and Chez et al. showing the efficacy of cell transplantation in autism [35-38]. Lv et al. studied the safety and efficacy of combined transplantation of human cord blood mononuclear cells [CBMNCs] and umbilical cord-derived mesenchymal stem cells [UCMSCs] in 37 patients with autism. They reported the intervention to be safe with no severe adverse effects along with statistically significant improvements on CARS, ABC scores and CGI evaluation in the two treatment groups compared to the control at 24 weeks post-treatment [P<0.05] [35]. Bradstreet et al. investigated the safety and efficacy of Fetal Stem Cell [FSC] transplantation in autism. Subjects were monitored at pre, and then 6 and 12 months following the transplantations, which consisted of two doses of intravenously and subcutaneously administered FSCs. On follow up at 6 months and 12 months, no adverse events were observed. Statistically significant changes were shown on the Autism Treatment Evaluation Checklist [ATEC] test and Aberrant Behavior Checklist [ABC] scores [36]. In 2017 and 2018, Dawson et al. and Chez et al. from USA, respectively studied the safety and efficacy of autologous cord blood stem cells in autism. Dawson et al., reported significant improvements in overall symptoms including behavior, social communication skills and attention [37]. Whereas, Chez et al. established the safety of intervention but the improvements observed were not statistically significant [38].
Counteracting mechanism of stem cells
Bone marrow derived cells have so far been an ideal source for cell transplantation in neurological disorders due to their capacity to differentiate into neural cells-neurons and glia both in vitro and in vivo [39,40]. On administration, these cells migrate and home towards the abnormally functioning brain areas [41]. Along with differentiation, they produce paracrine factors which stimulate and enhance the neuroprotective and restorative process of the endogenous latent cells [42]. They release growth factors such as vascular endothelial growth factor [VEGF], brain-derived neurotrophic factor [BDNF] and glia-derived neurotrophic factor [GDNF] which play a pivotal role in neural damage repair [43-45]. This was corroborated by an experimental study conducted to study the effect of mesenchymal stem cells in BTBR mice which exhibit similar behavioral deficits like autism. Post transplantation, BDNF levels were elevated in the hippocampus along with increased hippocampal neurogenesis [6]. These cells also exert immunomodulatory effects [46]. They inhibit the release of pro-inflammatory cytokines such as TNF-α, IFN-γ, and IL-1 and secrete anti-inflammatory cytokine IL-10 which results in reduction of inflammation in autism [47,48]. Evidence suggests, by producing large amounts of IL-6 and IL-10, bone marrow derived cells suppress the microglial activity and inhibit their activation and promotes their clearance [49]. These cells also possess antioxidant potential which reduces oxidative stress and neuronal death. This was demonstrated in a study which recorded decreased superoxide, apoptotic cells and lipid peroxidation associated with oxidative stress, after administration of bone marrow mesenchymal cells in rat model of stroke [50]. Experimental studies have shown that bone marrow derived cells release angiogenic factors and promote angiogenesis by differentiating into endothelial cells [49]. This reverses hypoxia and increases supply of blood and oxygen to the brain. These cells also secrete exosomes which may contribute to functional recovery [51].
In our current study, we administered autologous BMMNCs in 254 patients with ASD. These cells were selected based on their high differentiation potential accompanied with the above mentioned ability of bone marrow cells to alleviate the mechanisms associated with autism. These cells are a mixture of hematopoietic stem cells [HSCs], mesenchymal stem cells [MSCs], endothelial progenitor cells [EPCs], and very small embryonic-like stem cells [VSELs]. Hence, they provide cumulative benefit of all the cells which is more advantageous as compared to individual sub-fractions [53]. Their safety and efficacy has already been established in other incurable neurological disorders [54-56].
Route of administration
In this study, we administered the BMMNCs intrathecally as it is a relatively minimally invasive and an effective procedure as compared to other routes of administration. The altered permeability of the blood brain barrier in autism further allows the transplanted cells to migrate to the brain areas with inflammation and carry out the repair process [57]. In case of intravenous administration, pulmonary passage is a major hurdle. The administered cells get trapped in the lungs which affect the volume of cells reaching the target organ [58].
Clinical findings
ISAA and CARS were used to assess the therapeutic efficacy of cell transplantation in this study. ISAA was specially developed for the Indian population of autism patients with an aim to quantify the severity of symptoms and enable measurement of associated disability [6]. It has 40 items categorized under 6 domains specific to the difficulties experienced by individuals with autism. It grades the symptoms on a scale of 1 to 5 in ascending order of intensity. A score of <70 indicates no autism, 70-106-mild autism, 107-153-moderate autism and >153 is severe autism. Whereas, CARS is an assessment tool which evaluates behavior based on 14 domains which are generally affected in autism, plus one general category for impressions of features of autism [7]. A score of below 30 indicates no autism, 30-36.5 mild or moderately autism and above 36.5 is severe autism. The accuracy, reliability, and validity of both ISAA and CARS have been tested in the Indian population and are found to be suitable [8,59]. The findings of this study showed a statistically significant improvement on ISAA and CARS along with symptomatic improvements. It was observed that age of the patient affects the outcome of the intervention. Children below the age of 5 years showed more improvement as compared to children above 5 years of age indicating that the outcome of the intervention is better in the younger population of ASD. It was also observed that the patients who underwent cell transplantation within 5 years of their diagnosis demonstrated more improvements indicating the earlier they receive cell transplantation the better is the outcome. Hence, early intervention at a younger age can result in obtaining optimum benefits of autologous BMMNCs in ASD. In this study, severity based on CARS was not considered as few cognitive components such as variable attention, delays in response, unusual memory or savant ability are not included in CARS. On basis of ISAA, irrespective of the severity, patients showed improvements. Also, both genders showed significant improvements.
Objective neuroimaging
FDG PET CT scan brain was used as an additional outcome measure for the patients who underwent multiple doses of cell transplantation. It was used to monitor the effect of intervention at a cellular level. This functional neuroimaging technique utilizes 18-FDG, to study the metabolic activity of the brain [60]. In 86 patients, we performed a comparative study of FDG PET CT scan brain which demonstrated a balancing effect on metabolism. The hypometabolic areas showed increased metabolism while hypermetabolic areas showed reduced metabolism.
Effect of neurorehabilitation on the outcome of intervention
Neurorehabilitation plays a vital role in the outcome of this study. Most of the patients included in this study attended special schools and were on rehabilitation since their diagnosis. Evidence suggests that neurorehabilitation upregulates neural plasticity [61]. However, it was observed that the improvements exhibited by administering the combination of cell transplantation and neurorehabilitation were more and were achieved faster. Hence, it can be assumed that the functional recovery of these patients was attained due to the synergistic effect of both the interventions.
Adverse event monitoring
Adverse events [AE] were categorized into procedure related AE and cell transplantation related AE.
Procedure related AE
Procedure related AE associated with bone marrow aspiration and injection via lumbar puncture included spinal headache, nausea, diarrhea, vomiting, pain or bleeding at the site of aspiration/injection, fever amongst others. These minor AEs were treated using medications. Patients were also checked for anesthetic complications and allergic reactions.
Cell transplantation related AE
AE occurring in greater than 10% patients are common AE and if the occurrence is 10% or less it is termed uncommon. In this study, the uncommon adverse events reported were hyperactivity [8.2%], aggressiveness [9.8%], self-injurious behavior [6.3%] and seizures [1.9%]. These adverse events were treated with medications. Seizure was considered as an adverse event only if there was an increase in frequency or duration of seizure or a new onset seizure. Our previously published study has demonstrated that antiepileptic prophylactic regimen decreases the incidence of seizures along with limiting the onset of new ones after cell transplantation. It also suggested that pre-existing epileptogenic focus in EEG predicts the occurrence of seizures [62]. In this study, 30 out of 254 patients had an abnormal EEG and history of seizures. These patients were administered prophylactic antiepileptics before they underwent cell transplantation. Only 5 patients had seizures after intervention which was managed using medication. Occurrence of seizures did not affect the outcome of the intervention in these patients.
Limitations
One of the limitations of this study was lack of a control group. Also, the rehabilitation after therapy was not under our control, hence could be varied as it was a long-term study. The number of females in the study group was less as compared to males. In this study, comparative analysis of PET scan could not be done for all the patients as consent was obtained only from parents of 86 patients.
Conclusion
In this study, intrathecal administration of autologous BMMNCs in combination with neurorehabilitation for ASD was found to be safe and effective. A significantly better outcome of the intervention was found in patients with younger age [<10 years] and shorter duration from diagnosis [<5 years]. This indicates earlier intervention at a younger age can improve the quality of life of these patients and help them to integrate in mainstream lifestyle. The clinical improvements seen in the patients also correlated with the objective metabolic improvements on PET CT scan post cell transplantation. Cell transplantation in combination with neurorehabilitation can be used as a therapeutic strategy for ASD. It addresses the core issue of the abnormal neural functions and results in significant symptomatic improvements. This study contributes to the advancement of research in the field of cell transplantation for ASD.
Disclosure of conflict of interest
None.
References
- 1.Kim KY, Jung YW, Sullivan GJ, Chung L, Park IH. Cellular reprogramming: a novel tool for investigating autism spectrum disorders. Trends Mol Med. 2012;18:463–471. doi: 10.1016/j.molmed.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blenner S, Reddy A, Augustyn M. Diagnosis and management of autism in childhood. BMJ. 2011;343:d6238. doi: 10.1136/bmj.d6238. [DOI] [PubMed] [Google Scholar]
- 3.Watts TJ. The pathogenesis of autism. Clin Med Pathol. 2008;1:99–103. doi: 10.4137/cpath.s1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho IM, Coelho PB, Costa PC, Marques CS, Oliveira RS, Ferreira DC. Current neurogenic and neuroprotective strategies to prevent and treat neurodegenerative and neuropsychiatric disorders. Neuromolecular Med. 2015;17:404–422. doi: 10.1007/s12017-015-8369-3. [DOI] [PubMed] [Google Scholar]
- 5.Ichim TE, Solano F, Glenn E, Morales F, Smith L, Zabrecky G, Riordan NH. Stem cell therapy for autism. J Transl Med. 2007;5:30. doi: 10.1186/1479-5876-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segal-Gavish H, Karvat G, Barak N, Barzilay R, Ganz J, Edry L, Aharony I, Offen D, Kimchi T. Mesenchymal stem cell transplantation promotes neurogenesis and ameliorates autism related behaviors in BTBR mice. Autism Res. 2016;9:17–32. doi: 10.1002/aur.1530. [DOI] [PubMed] [Google Scholar]
- 7.World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborty S, Thomas P, Bhatia T, Nimgaonkar VL, Deshpande SN. Assessment of severity of autism using the Indian scale for assessment of autism. Indian J Psychol Med. 2015;37:169–174. doi: 10.4103/0253-7176.155616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schopler E, Van Bourgondien ME, Wellman GJ, Love SR. The Childhood Autism Rating Scale. 2nd edition. United States of America: Western Psychological Services; 2010. [Google Scholar]
- 10.Sharma A, Gokulchandran N, Chopra G, Kulkarni P, Lohia M, Badhe P, Jacob VC. Administration of autologous bone marrow derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Cell Transplant. 2012;21(Suppl 1):S79–90. doi: 10.3727/096368912X633798. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, Gokulchandran N, Badhe P, Kulkarni P, Mishra P, Shetty A, Sane H. An improved case of autism as revealed by PET CT scan in patient transplanted with autologous bone marrow derived mononuclear cells. J Stem Cell Res Ther. 2013;3:2. [Google Scholar]
- 12.Sharma A, Gokulchandran N, Shetty A, Sane H, Kulkarni P, Badhe P. Autologous bone marrow mononuclear cells may be explored as a novel. Potential therapeutic option for autism. J Clin Case Rep. 2013;3:7. [Google Scholar]
- 13.Sharma A, Gokulchandran N, Sane H, Kulkarni P, Thomas N, Paranjape A, Badhe P. Intrathecal autologous bone marrow mononuclear cell transplantation in a case of adult autism. Autism Open Access. 2013;3:2. [Google Scholar]
- 14.Sharma A, Gokulchandran N, Sane H, Bhovad P, Biju H, Shetty A, Kali M, Badhe P. Cell therapy effects portrayed on positron emission tomography computerized tomography scan of the brain serve as a new dimension for autism: a case report. J Paediatr Neurol. 2014;12:3. [Google Scholar]
- 15.Sharma A, Gokulchandran N, Shetty A, Kulkarni P, Sane H, Badhe P. Neuropsychiatric disorder tackled by innovative cell therapy-a case report in autism. J Stem Cell Res Transplant. 2014;1:4. [Google Scholar]
- 16.Sharma A, Gokulchandran N, Sane H, Patil A, Shetty A, Biju H, Kulkarni P, Badhe P. Amelioration of autism by autologous bone marrow mononuclear cells and neurorehabilitation: a case report. Am J Med Case Rep. 2015;3:304–309. [Google Scholar]
- 17.Sharma A, Sane H, Gokulchandran N, Badhe P, Patil A, Kulkarni P, Paranjape A. PET-CT scan shows decreased severity of autism after autologous cellular therapy: a case report. Autism Open Access. 2016;6:169. [Google Scholar]
- 18.Sharma A, Gokulchandran N, Sane H, Kulkarni P, Nivins S, Maheshwari M, Badhe P. Therapeutic effects of cellular therapy in a case of adult autism spectrum of disorder. Int Biol Biomed J. 2018;4:98–103. [Google Scholar]
- 19.De Felice A, Ricceri L, Venerosi A, Chiarotti F, Calamandrei G. Multifactorial origin of neurodevelopmental disorders: approaches to understanding complex etiologies. Toxics. 2015;3:89–129. doi: 10.3390/toxics3010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zikopoulos B, Barbas H. Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front Hum Neurosci. 2013;7:609. doi: 10.3389/fnhum.2013.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottfried C, Bambini-Junior V, Francis F, Riesgo R, Savino W. The impact of neuroimmune alterations in autism spectrum disorder. Front Psychiatry. 2015;6:121. doi: 10.3389/fpsyt.2015.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goines PE, Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol Teratol. 2013;36:67–81. doi: 10.1016/j.ntt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 26.Patel AS, Zalcman SS. Interleukin-2 treatment induces an acquired behavioral response pattern [repetitive stereotyped movements] mediated by dopamine D1 and D2 receptors. Int Neuropsychiatr Dis J. 2014;2:175–85. [Google Scholar]
- 27.Neher JJ, Neniskyte U, Zhao JW, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011;186:4973–4983. doi: 10.4049/jimmunol.1003600. [DOI] [PubMed] [Google Scholar]
- 28.Verkhratsky A, Rodríguez JJ, Parpura V. Neuroglia in ageing and disease. Cell Tissue Res. 2014;357:493–503. doi: 10.1007/s00441-014-1814-z. [DOI] [PubMed] [Google Scholar]
- 29.Sweeten TL, Posey DJ, Shankar S, McDougle CJ. High nitric oxide production in autistic disorder: a possible role for interferon-gamma. Biol Psychiatry. 2004;55:434–7. doi: 10.1016/j.biopsych.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Essa MM, Braidy N, Vijayan KR, Subash S, Guillemin GJ. Excitotoxicity in the pathogenesis of autism. Neurotox Res. 2013;23:393–400. doi: 10.1007/s12640-012-9354-3. [DOI] [PubMed] [Google Scholar]
- 31.Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Dalla Bernardina B, Bonassi S. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic Biol Med. 2012;52:2128–2141. doi: 10.1016/j.freeradbiomed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Fumagalli S, Perego C, Pischiutta F, Zanier ER, De Simoni MG. The ischemic environment drives microglia and macrophage function. Front Neurol. 2015;6:81. doi: 10.3389/fneur.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, Sasaki M. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000;123:1838–44. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- 34.Sharma A, Gokulchandran N, Sane H, Nagrajan A, Paranjape A, Kulkarni P, Shetty A, Mishra P, Kali M, Biju H, Badhe P. Autologous bone marrow mononuclear cell therapy for autism-an open label proof of concept study. Stem Cells Int. 2013;2013:623875. doi: 10.1155/2013/623875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv YT, Zhang Y, Liu M, Qiuwaxi JN, Ashwood P, Cho SC, Huan Y, Ge RC, Chen XW, Wang ZJ, Kim BJ, Hu X. Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. J Transl Med. 2013;11:196. doi: 10.1186/1479-5876-11-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradstreet JJ, Sych N, Antonucci N, Klunnik M, Ivankova O, Matyashchuk I, Demchuk M, Siniscalco D. Efficacy of fetal stem cell transplantation in autism spectrum disorders: an open-labeled pilot study. Cell Transplant. 2014;23(Suppl 1):S105–112. doi: 10.3727/096368914X684916. [DOI] [PubMed] [Google Scholar]
- 37.Dawson G, Sun JM, Davlantis KS, Murias M, Franz L, Troy J, Simmons R, Sabatos-DeVito M, Durham R, Kurtzberg J. Autologous cord blood infusions are safe and feasible in young children with autism spectrum disorder: results of a single-center phase I open-label trial. Stem Cells Transl Med. 2017;6:1332–1339. doi: 10.1002/sctm.16-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chez M, Lepage C, Parise C, Dang-Chu A, Hankins A, Carroll M. Safety and observations from a placebo-controlled, crossover study to assess use of autologous umbilical cord blood stem cells to improve symptoms in children with autism. Stem Cells Transl Med. 2018;7:333–341. doi: 10.1002/sctm.17-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darabi S, Tiraihi T, Delshad A, Sadeghizadeh M. A new multistep induction protocol for the transdifferentiation of bone marrow stromal stem cells into GABAergic neuron-like cells. Iran Biomed J. 2013;17:8–14. doi: 10.6091/IBJ.1112.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song S, Song S, Zhang H, Cuevas J, Sanchez-Ramos J. Comparison of neuron-like cells derived from bone marrow stem cells to those differentiated from adult brain neural stem cells. Stem Cells Dev. 2007;16:747–56. doi: 10.1089/scd.2007.0027. [DOI] [PubMed] [Google Scholar]
- 41.Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. doi: 10.1155/2013/130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trueman RC, Klein A, Lindgren HS, Lelos MJ, Dunnett SB. Repair of the CNS using endogenous and transplanted neural stem cells. Curr Top Behav Neurosci. 2013;15:357–98. doi: 10.1007/7854_2012_223. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki M, Radtke C, Tan AM, Zhao P, Hamada H, Houkin K, Honmou O, Kocsis JD. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J Neurosci. 2009;29:14932–14941. doi: 10.1523/JNEUROSCI.2769-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3:63–70. doi: 10.1016/j.scr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Jeong SR, Kwon MJ, Lee HG, Joe EH, Lee JH, Kim SS, Suh-Kim H, Kim BG. Hepatocyte growth factor reduces astrocytic scar formation and promotes axonal growth beyond glial scars after spinal cord injury. Exp Neurol. 2012;233:312–322. doi: 10.1016/j.expneurol.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 46.Siniscalco D, Kannan S, Semprún-Hernández N, Eshraghi AA, Brigida AL, Antonucci N. Stem cell therapy in autism: recent insights. Stem Cells Cloning. 2018;11:55–67. doi: 10.2147/SCCAA.S155410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6:552–570. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siniscalco D, Bradstreet JJ, Sych N, Antonucci N. Perspectives on the use of stem cells for autism treatment. Stem Cells Int. 2013;2013:262438. doi: 10.1155/2013/262438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan K, Zhang R, Sun C, Chen L, Li P, Liu Y, Peng L, Sun H, Qin K, Chen F, Huang W, Chen Y, Lv B, Du M, Zou Y, Cai Y, Qin L, Tang Y, Jiang X. Bone marrow-derived mesenchymal stem cells maintain the resting phenotype of microglia and inhibit microglial activation. PLoS One. 2013;8:e84116. doi: 10.1371/journal.pone.0084116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calió ML, Marinho DS, Ko GM, Ribeiro RR, Carbonel AF, Oyama LM, Ormanji M, Guirao TP, Calió PL, Reis LA, Simões Mde J, Lisbôa-Nascimento T, Ferreira AT, Bertoncini CR. Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress, apoptosis, and hippocampal damage in brain of a spontaneous stroke model. Free Radic Biol Med. 2014;70:141–54. doi: 10.1016/j.freeradbiomed.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 51.Cairns K, Finklestein SP. Growth factors and stem cells as treatments for stroke recovery. Phys Med Rehabil Clin N Am. 2003;14(Suppl):S135–42. doi: 10.1016/s1047-9651(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–67. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pösel C, Möller K, Fröhlich W, Schulz I, Boltze J, Wagner DC. Density gradient centrifugation compromises bone marrow mononuclear cell yield. PLoS One. 2012;7:e50293–e50293. doi: 10.1371/journal.pone.0050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma A, Sane H, Gokulchandran N, Kulkarni P, Gandhi S, Sundaram J, Paranjape A, Shetty A, Bhagawanani K, Biju H, Badhe P. A clinical study of autologous bone marrow mononuclear cells for cerebral palsy patients: a new frontier. Stem Cells Int. 2015;2015:905874. doi: 10.1155/2015/905874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma A, Gokulchandran N, Sane H, Badhe P, Kulkarni P, Lohia M, Nagrajan A, Thomas N. Detailed analysis of the clinical effects of cell therapy for thoracolumbar spinal cord injury: an original study. J Neurorestoratol. 2013;1:13–22. [Google Scholar]
- 56.Sharma A, Sane H, Gokulchandran N, Khopkar D, Paranjape A, Sundaram J, Gandhi S, Badhe P. Autologous bone marrow mononuclear cells intrathecal transplantation in chronic stroke. Stroke Res Treat. 2014;2014:234095. doi: 10.1155/2014/234095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Theoharides TC, Zhang B. Neuro-inflammation, blood-brain barrier, seizures and autism. J Neuroinflammation. 2011;8:168. doi: 10.1186/1742-2094-8-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–92. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russell PS, Daniel A, Russell S, Mammen P, Abel JS, Raj LE, Shankar SR, Thomas N. Diagnostic accuracy, reliability and validity of childhood autism rating scale in India. World J Pediatr. 2010;6:141–147. doi: 10.1007/s12519-010-0029-y. [DOI] [PubMed] [Google Scholar]
- 60.Manglunia AS, Puranik AD. FDG PET/CT findings in a clinically diagnosed case of childhood autism. Indian J Nucl Med. 2016;31:138–140. doi: 10.4103/0972-3919.178302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kleim JA. Neural plasticity and neurorehabilitation: teaching the new brain old tricks. J Commun Disord. 2011;44:521–528. doi: 10.1016/j.jcomdis.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Sharma A, Sane H, Paranjape A, Gokulchandran N, Takle M, Badhe P. Seizures as an adverse event of cellular therapy in pediatric neurological disorders and its prevention. J Neurol Disord. 2014;2:164. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.