Abstract
Overall, major burn wounds may require special care and long-term hospitalization as they not only bring complications from the wound itself, but may also compromise the immune system, or even other organs. Previous studies have indicated that topical insulin cream shortened wound closure time in second-degree burns in rats. Transplanted adipose-derived stem cells (AD-MSCs) have been developed as an alternative to treat burns and to accelerate the healing process. The aim of the present study is to investigate the effect of topical insulin gel, associated with AD-MSCs intradermal administration to heal second-degree burn wounds in rat models who were subjected to second-degree dorsal burns. The models were divided into four groups (n = 10 per group): placebo gel (C), topical insulin gel (TI), topical insulin gel and adipose-derived mesenchymal stem cells (TIMSCs) and placebo gel and adipose-derived mesenchymal stem cells (CMSCs). Wounds were assessed on a daily basis and histological evaluations were made on 5 animals from each group on the seventh and fourteenth day. There was a significant macroscopic decrease in burn wound areas in the Control (P = 0.0083), TIMSCs and CMSCs (P = 0.042) groups between the seventh and fourteenth days. The TI treatment did not show any significant change (P > 0.05) throughout this same period. The histological analysis showed significant granulation tissue formation in CMSCs and TIMSCs (P = 0.02235) treatments during the experimental period. According to the results, intradermal administration of allogenic AD-MSCs in experimental second-degree burns for short periods of time in the rat model has contributed to reducing the inflammatory phase duration, improving wound re-epithelialization, tissue granulation and wound contraction, as well as increasing collagen deposition.
Keywords: Adipose-derived stem cells, burn wounds, rat model, topical insulin
Introduction
Wound healing process includes important phases, namely: coagulation, inflammation, angiogenesis (new blood vessels), granulation (re-epithelialization) and fibroblast proliferation (remodeling) [1]. This process depends on the development of granulation tissue in the wound bed due to the presence of connective tissue and the formation of new blood vessels that derive from pre-existing blood vessels in the injured area. These new blood vessels provide oxygen and nutrients to the injured area, since these elements are necessary for normal wound healing [2]. Severe burns can induce stress hyperglycemia, which has negative influence on the healing process and decreases the quality of the wounds [3]. High glucose levels can trigger complications that delay the healing process, as well as primary neuropathy and generalized degenerative changes in the blood vessels, which lead to vascular insufficiency. Infections are more frequent in severely burned or diabetic patients [4]. Hyperglycemia can cause the formation of advanced glycation end products (AGEs) that act to the detriment of protein function, mainly of collagen, by greatly delaying wound healing [5]. Burn patients encounter exacerbated risk of experiencing failure during the healing process, as well as decreased quality of life and higher health care costs. These complicating events were the trigger for researchers to develop several techniques, such as laser therapy and split-thickness skin grafts [6,7], topical insulin application [8-12] and mesenchymal stem cells [13-18] to improve and accelerate the healing process.
Many growth factors, such as the epidermal growth factor (EGF), the transforming growth factor beta (TGF-β) and the platelet derived growth factor (PRP), have been used to accelerate wound healing in animals, but at higher costs [19,20]. Benefits, such as inflammation reduction, increased collagen deposition, wound re-epithelialization acceleration and granulation tissue growth improvements, which were observed in previous human and animal burn wound healing assessment studies on diabetic or non-diabetic subjects, highlight the important role played by insulin in wound healing [21-23]. Studies have shown that application of low doses of topical insulin stimulates the migration of keratinocytes and the vascular endothelial growth factor (VEGF), thus accelerating re-epithelialization and angiogenesis [8,24,25]. Current studies into Mesenchymal stem cells (MSC) immunomodulation and wound support in other diseases provide additional insights into the beneficial properties of MSCs. Numerous experimental animal studies have shown the therapeutic potential of MSCs and their safety and efficacy in vivo, which motivated the interest in developing the present study [26]. The ability to distinguish several cell types leads to angiogenesis development, to the secretion of the extracellular matrix and, consequently, to healing process acceleration [16,27]. The potential of these cells to secrete cytokines, such as TGF-β, IL-10, IL-6, chemokines (CCL2, CCL5, CXCL12) and growth factors (VEGF, IGF, bFGF, SDF, HGF) that help the healing process is another key feature determining their use in diabetic ulcers and burn wounds [28,29]. Adipose-derived stem cells (ASCs) can accelerate wound-healing in acid burn skin injury [30], as well as the treatment of pressure ulcers [31], thermal burns [32] and full-thickness skin wounds [33]. As skin wounds happen on its surface, one must take care to avoid transplanted cell loss due to misinterpretation of therapeutic effects. Therefore, stem cells injections should be standardized so that the cells could remain longer on subcutaneous wounds to allow better interpreting of the collected data.
The aims of the present study were to evaluate the effect of local gel insulin administration associated, or not, with adipose-derived mesenchymal stem cells (AD-MSCs) to regenerate burn wounds in rat models and to establish the potential of topical insulin gel associated with AD-MSCs to accelerate the wound healing process in these models, as well as to determine whether the AD-MSCs transplantation method is effective to treat superficial cutaneous wounds.
Materials and methods
Animals and study design
The study was approved by the Ethics Committee on the Use of Animals (CEUA) at the Federal University of Mato Grosso do Sul (UFMS). The experiment was conducted in compliance with the ethical principles adopted by the Brazilian College of Animal Experimentation (COBEA). Forty-two ten-week-old female and male Wistar rats weighing 250 gm ± 40 gm were provided by the Animal Laboratories of the Biology Institute at UFMS. Rats remained in clean and appropriate numbered individual cages throughout the experiment and were stored under controlled room temperature (22°C) at 12-hour night/day cycles. Animals had free access to standard rodent chow and water ad libitum for 2 weeks before the experiments. Two rats were maintained for AD-MSC isolation and culture. Forty rats were subjected to skin burning and divided into four groups (n = 10 per group): burned rats treated with placebo gel (C), burned rats treated with topical insulin gel (TI); burned rats treated with topical insulin gel and adipose-derived mesenchymal stem cells (TIMSCs) and burned rats treated with placebo gel and adipose-derived mesenchymal stem cells (CMSCs). Wounds were assessed on a daily basis between the 7th and 14th days after the burning procedure. Five (5) animals from each group were subjected to histological evaluations on the 7th day after burning and 5 animals underwent the same evaluations on the 14th day.
Experimental burn model
Wistar rats were anesthetized with intraperitoneal injection of 1 μg/g solution at a concentration of 1:1 (v/v) 10% ketamine (Ketalex, Rhobifarma, Hortolândia, Brazil) and 20 mg/ml xylazine (Xilazin, Rhobifarma, Hortolândia, Brazil) before the burn injuries were induced. Analgesics such as tramadol (10 mg/kg) and dipyrone (100 mg/kg) were subcutaneously administered to the rat models twice a day for 3 days after the burning procedure. Rats were taken back to their respective cages after the analgesic was administered and each group of models was further divided into 2 subgroups for histological purposes (Day 7 and Day 14, n = 5 each). All animals were housed in individual cages after the burning procedure in order to avoid new wounds resulting from injuries caused by other rats.
The burn model was performed based on pre-established rat models with some modifications. A metal plate was developed to create an experimental model for the burning procedure and kept in a dry water bath device (Marconi, Dry Block, MA 4004, Piracicaba, Brazil) to standardize the burn wounds. Hair in the dorsal region of the rats was shaved before applying the burning plate, which was sanitized with 10% povidone iodine and 70% alcohol. Rats were subjected to second-degree skin burning through moderate pressure of the metal plate (1.3 × 1.3 cm), which was heated to 140°C, against the animal’s dorsal area for 20 s. The placebo or the insulin gel were administered to the burned area right after inflicting the wound (Day 0) and administered daily until the end of the experiment. Rats treated with mesenchymal stem cells (approximately 4 × 106 cells administered through intradermal injection to the burn wound) only received this therapy once before gel application. Wound beds in animals in all groups were topically treated with 0.2 ml of topical insulin gel or placebo on a daily basis. A macroscopic evaluation of the wounds detected no signs of infection, a moist appearance and evidence of secondary intention healing.
Insulin and placebo gel
The used gel was prepared in a private pharmaceutical laboratory. It was composed of purified water (100 ml qsp), carbomer 940 (1.5%), propylene glycol (3%) and sodium hydroxide solution qsp (10%, pH 5); this formula was used as control, and the insulin gel was developed with the addition of 0.5 IU/100 g of insulin.
Adipose tissue harvesting and MSC isolation and culture
Two rats were euthanized with an overdose of isoflurane. Visceral adipose tissue was collected and washed three to four times with PBS and suspended in collagenase solution (2 mg collagenase/mL HEPES medium) at 2 mL solution/g adipose tissue - this solution was cultured overnight at 37°C with 5% CO2. Enzymatic activity was neutralized with DMEM low glucose (Dulbecco’s modified Eagle’s medium-Gibco Life Technologies) with 10% fetal bovine serum (Gibco Life Technologies). The infranatant was centrifuged at 1200 rpm for 10 min at room temperature in order to pellet the cells. Cells were filtered to remove debris and seeded on tissue culture plates for further expansion. Cell cultures were kept in DMEM low glucose supplemented with 10% FBS and antibiotic-antimycotic solution at 37°C with 5% CO2. The culture medium was changed every 2 days. The cells were trypsinized (0.025% trypsin) and plated at density of 5000 cells/cm2 after reaching approximately 80% confluence. This procedure was repeated every time cell confluence reached 80%. The cells were ready to be administered after two passages and injected right after trypsinization. Three flasks of cells were divided into two sterile tubes for adipogenic, osteogenic and chondrogenic differentiation.
Adipogenic, osteogenic and chondrogenic differentiation assays
Specific osteogenic and chondrogenic media, STEMPRO Osteogenesis and STEMPRO Condrogenesis Differentiation Kits (Invitrogen) were used for differentiation assay, whereas DMEM low glucose supplemented with fetal bovine serum, insulin, indomethacin, dimethyl sulfoxide, rosiglitazone, and dexamethasone was used for adipogenic differentiation. Cells selected for adipogenic and osteogenic differentiation were stored for 14 days on four plates at density of 2 × 105 cells/cm2; the medium was changed three times a week. The chondrogenic differentiation was performed as three-dimensional pellet culture in two sterile 15-ml conical tubes at 1 × 106 cells/cm2 and stored for 21 days. Cell differentiations were confirmed through staining with Oil Red O for adipogenic differentiation, through Alizarin Red staining for osteogenic differentiation and through hematoxylin, eosin and toluidine blue for chondrogenesis differentiation.
Burn wound measurement
Clinical assessments were carried out to evaluate wound size in all rats. Wound appearance was assessed through digital images taken with digital camera Sony Cyber-Shot (Model DSC-Rx100 20.2 MP Vario-Sonnar F1.8), which was kept at a fixed distance (30 cm) from the injured area. The images had the same resolution and were taken under the same lighting conditions on a daily basis. Wound measurements were taken in the CorelDrawX8 software. Initially, the images were positioned according to real measurements by using the first wound measurements, which were compatible to the iron branding as parameter (1.3 cm × 1.3 cm). Measurement alternation of 1.4 cm × 1.3 cm, on average must be taken into account because burn wound size increases depending on the move of used instruments, although it does not interfere with healing comparatives. Molds of the body structure of the rat models were made based on the image taken at day 0 and applied to images of the same group taken on the following days. It is essential to emphasize that some artifact generators were inevitable, namely: rats’ posture, weight and angulation. The renderization of rats’ body traces on day 0 was applied to images that were visually different to minimize this artifact; therefore, standardizing the body features made it possible to compare the healing processes. Animals were euthanized with an isoflurane overdose before skin sample collection at the previously mentioned intervals (Day 7 and Day 14). A wound sample from each animal in each group on each tested day was collected for histological analysis. A skin layer at full thickness (standard edge, healthy skin and burn wound 1.0 cm × 1.0 cm) was removed with a scalpel. The pathologist processed each specimen without any information about the evaluated groups, rat models or time in order to avoid possible biases.
Histological procedures
Two scales were used for the quantitative and qualitative evaluation of the healing process presented by the burn wounds. The first scale was based on inflammation degree and measured the wound sizes, whereas the second one was a subjective inflammation scale. Wounded skins were sampled and fixed in 10% neutral-buffered formalin, as well as routinely processed and embedded in paraffin for histological analyses. Three-micrometer paraffin sections were stained with hematoxylin and eosin (H&E) and used to assess wound structure. A qualitative method was used to assess the wound structure. Re-epithelialization, polymorph nuclear leucocytes (PMNL, inflammatory cells), granulation tissue (thickness of granulation layer) and collagen (filamentary and acellular quantification were graded from 0 to 3 (Table 1). The histological and macroscopic scores were calculated by the Kruskal-Wallis test followed by Dunn post-test, which were adopted as statistical analyses to compare means within the C, CMSCs, TICMSs and TI groups over time (on days 0, 1, 5, 7, 14) and to evaluate the histological parameters (on days 7 and 14). The “C group” was considered as baseline for the groups’ means analyses, “day 0” for time analyses and “day 7” for histological parameters. Differences were significant at P < 0.05. Statistical analysis was performed using Past 3.20 software.
Table 1.
Histological parameters and scale for the assessment of cutaneous wound healing
| Scale | Parameters | |||
|---|---|---|---|---|
|
| ||||
| Re-epithelialization | PMNL* | Granulation tissue | Collagen | |
| 0 | Thickness of cut edges | Absent | Absent (< 1%) | Absent |
| 1 | Migration of cells (< 50%) | Mild | Mild (< 25%) | Mild |
| 2 | Migration of cells (≥ 50%) | Moderate | Moderate (< 50%) | Moderate |
| 3 | Closure of epithelial gap | Marked | Marked (> 50%) | Marked |
Polymorphonuclear leucocytes.
Results
Superficial second-degree burns were generated on the back of all rats subjected to the four assessed treatments. Overall, the surface extension of the lesion was similar in the control and treatment groups shortly after the superficial burn (Day 0). Daily analysis allowed for detailed evaluation of the rat burn wound characteristics throughout the healing process. Although the microbiological analysis was not carried out, the clinical assessment showed that the wounds were clean and without evident signs of infection.
Differentiation of AD-MSCs cells
AD-MSCs successfully differentiated the adipocytes, osteocytes and chondrocytes and the efficiency of the protocols was confirmed by morphological changes observed in these cells. Alizarin Red stained the calcium deposit in the extracellular matrix, and this outcome confirmed the osteogenic differentiation shown in Figure 1B. The adipogenic differentiation shown in Figure 1D was confirmed by cells exhibiting typical intracellular and red-stained lipid vacuoles, which was validated through Oil Red O staining. Controlled cells (Figure 1A, 1C). The chondrogenic differentiation (Figure 1F) was confirmed through the intense extracellular matrix production in the differentiated pellets. Only isolated cells were observed in the control pellets (Figure 1E).
Figure 1.
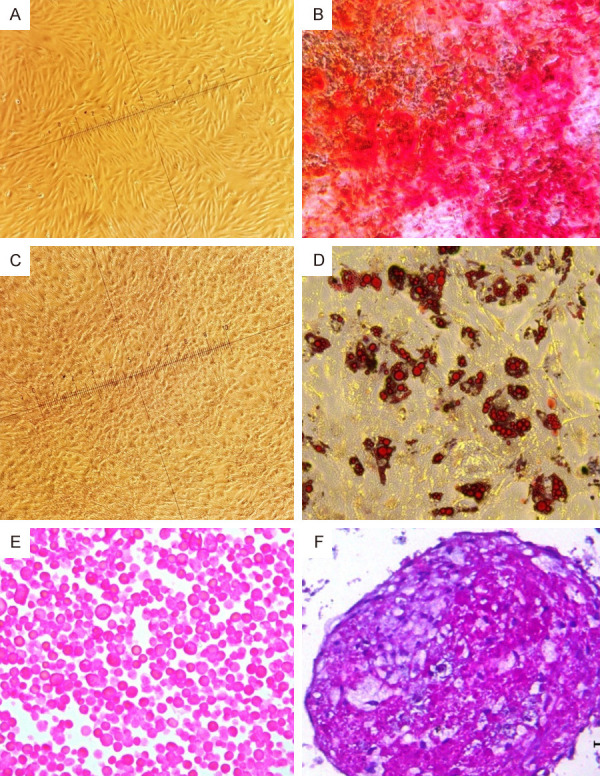
Differentiation assay. Differentiated MSCs, obtained in accordance with osteogenic, adipogenic, and chondrogenesis differentiation protocols. Morphological changes induced through differentiation of mesenchymal stem cells. (A and C) Control cells, maintained in DMEM low glucose: Cultures of adherent mesenchymal stem cells, not differentiated, plastic-adherent and typical spindle-shaped, fibroblast-like morphology. (B) MSCs differentiated into osteocytes: red-stained calcium deposits validated by Alizarin Red dye, confirming osteogenic differentiation. Adipogenic differentiation (D) cells with the typical intracellular, red-stained lipid vacuoles, which, in this case, were validated by Oil Red O dye. (20X magnification). Chondrogenic differentiation pellets: (E) control pellet and (F) the differentiated pellet showed typical and abundant extracellular matrix. cells (100X magnification).
Macroscopic images
The images of the wounds made it possible to analyze the evolution of the healing process. The burned areas increased in all treatment groups until the 7th evaluation day, and it was confirmed by the necrotic tissue progression at the edges of the wounds. There was a significant burn wound area decrease in animals in groups C (P = 0.0083), TIMSCs and CMSCs (P = 0.042) between the 7th and 14th days. There was no significant change (P > 0.05) due to the TI treatment throughout this period (Figures 2, 3).
Figure 2.
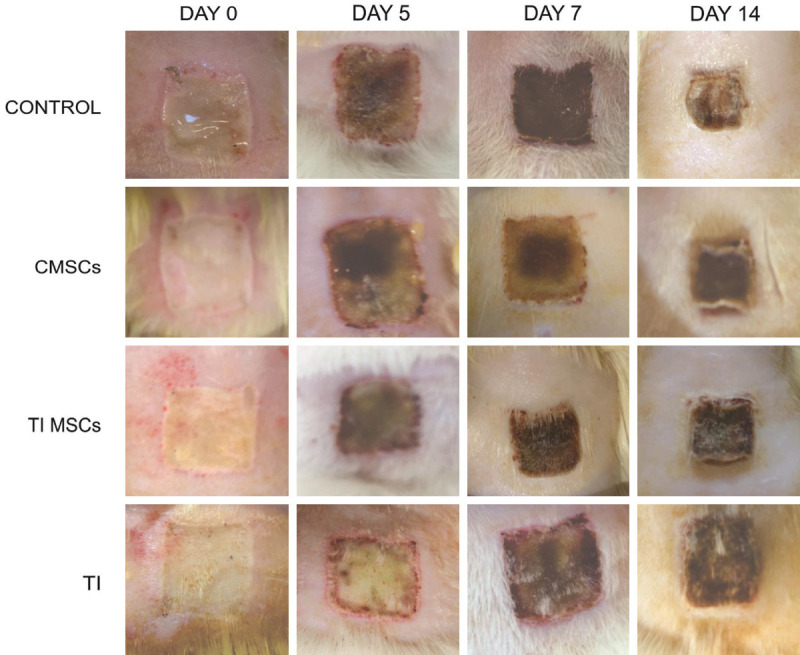
Representative images of wound healing at different points in time after second-degree skin burns were generated on the back of the model rats. Wound size significantly reduced in groups C (P = 0.0083), TIMSCs and CMSCs (P = 0.042). There was no significant change (P > 0.05) during this period in the TI treatment. Area measurements taken with a Sony Cyber-Shot (Model DSC-Rx100 20.2 MP _Vario-Sonnar F1.8).
Figure 3.
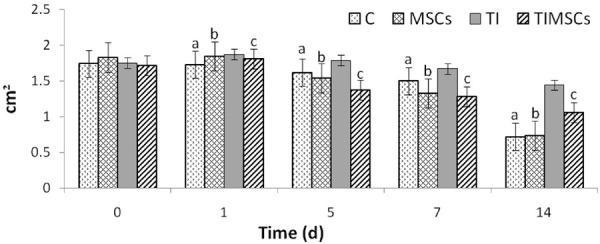
Wound healing percentage recorded for C- (n = 10), CMSCs- (n = 10), TI- (n = 10) and TIMSCs-treated (n = 10) groups on days 0, 1, 5, 7, and 14 after burning. Non-parametric tests (Kruskal-Wallis followed by Dunn’s tests) were used to evaluate the statistical significance of the results (P < 0.05). Different letters (a, b or c) above the columns indicate significant difference between the groups.
Histological analysis
The histological analysis showed a trend of burn wound evolution improvement in animals subjected to CMSCs and TIMSCs treatments during the experimental period. There were no significant differences (P > 0.05) between the records of re-epithelialization (P = 0.5291), PMNL (P = 0.7185) and collagen (P = 0.8707) between treatments on the analyzed days in C, TI, TIMSCs and CMSCs treatments. Granulation tissue formation was significant (P < 0.05) in CMSCs and TIMSCs treatments (P = 0.02235) during the experimental period (Figures 4, 5).
Figure 4.
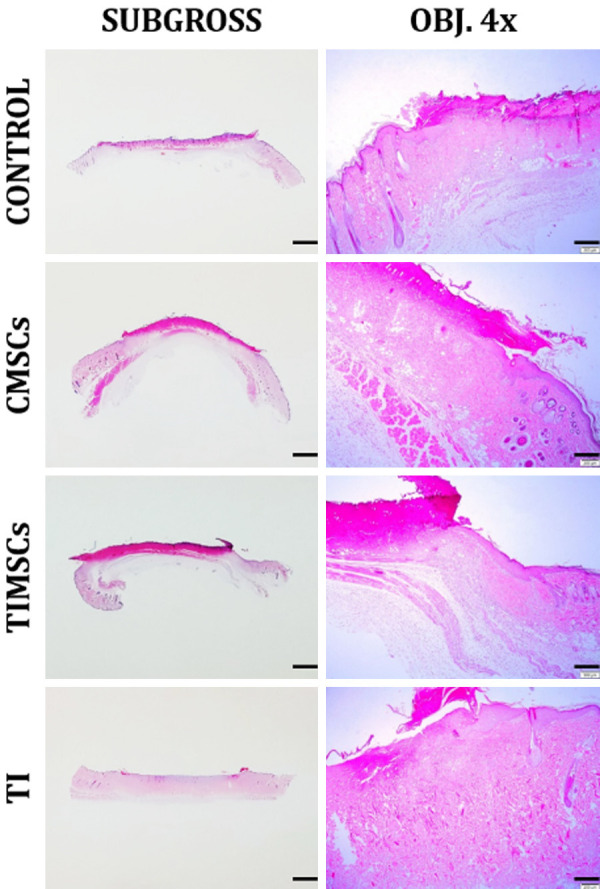
Post-wounding skin repair on Day 7. Mild difference between groups on Day 7. The wound is flat and the skin looks relaxed. Re-epithelialization, PMNL and collagen infiltration were at grade 1 in the herein pictured groups. Granulation tissue was at grade 1 in Groups C and TIMSCs, at grade 2 in CMSCs and at grade 0 in TI. H&E. Bar in the left-hand column, 2000 µm. Bar in the right-hand column, 250 µm.
Figure 5.
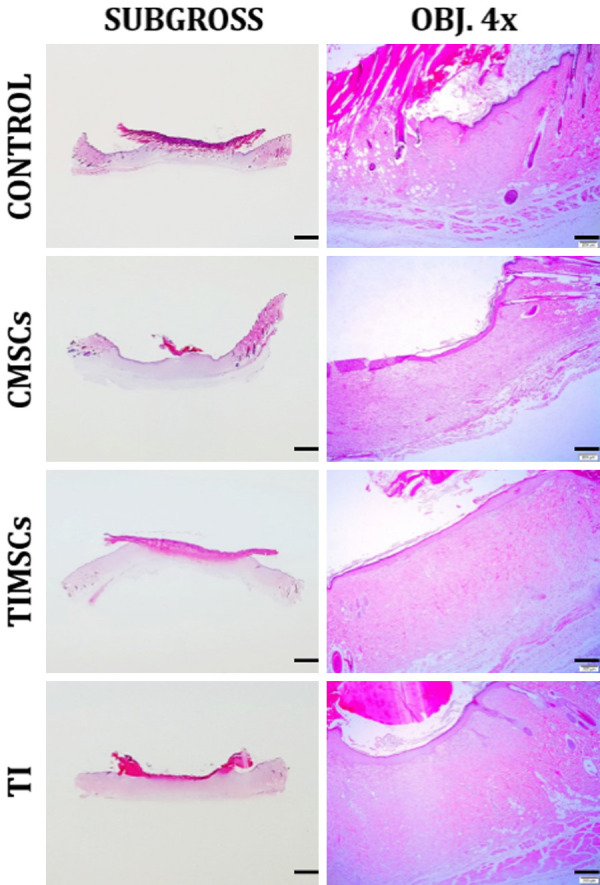
Post-wounding skin repair; Day 14. Groups C and CMSCs exhibited greater wound contraction than TIMSCs and TI. Wound bed in these groups is clearly pale to lightly basophilic due to the large amount of granulation tissue (grade 3 and 2, respectively) and to fibroblast abundance. H&E. Bar in the left-hand column, 2000 µm. Bar in the right-hand column, 250 µm.
Discussion
Burn treatments are problematic because of several complications such as protective barrier losses, delayed healing, oxidative stress and exuberant inflammation. Protective barrier losses cause greater loss of fluids and, in most cases, may lead to bacterial sepsis.
Nowadays, MSC has been used in numerous clinical studies due to its features, which include hematopoiesis support, the ability to distinguish several cell lineages, pro-growth secretion and survival factors, and its immunomodulatory and anti-inflammatory capacity [34-36]. Burn wound healing is hampered by the wound itself, which creates a barrier to prevent cytokine migration and growth factors that are essential for good wound healing processes [37]. It is known that an anti-inflammatory response is developed after the injury to induce systemic immunosuppression, which impairs the healing process of burn wounds [38]. A previous study has shown that MSCs have the ability to distinguish several skin cell types such as keratocytes, endothelial cells, pericytes and monocytes [27]. A study has evaluated extensive burns in rats and evidenced that wounds treated with MSCs for 45 days presented better healing, because it led to better vessel formation, to more granulation tissue and lesser inflammation than wounds in the control group [39]. The macroscopic analysis of the burn wounds carried out in the current study evidenced that C, CMSCs and TIMSCs treatments significantly decreased the dry crust area of the wounds and accelerated the healing process. However, this decrease was not observed in animals subjected to the TI treatment at the herein adopted studied times. One study showed that topical insulin accelerated the healing process in diabetic rats by modulating the inflammatory response and repairing cellular function [40]. It is well-known that the appropriate inflammatory response is the essential feature to achieve the correct wound healing response. Abnormal inflammatory responses have negative influence on wound healing, and these responses are observed in burn wounds or in diabetic patients [41]. Many studies have shown the beneficial effects of topical insulin on wound healing [21-25]. The current results assumingly derived from the inappropriate formulation and manipulation of the insulin gel used in the present study. Another supposition lies on the fact that the herein adopted experimental model counted on non-diabetic rats, and many previous studies have shown the benefits of the treatments on diabetic rats. It can be stated that topical insulin may have more effect on diabetogenic wounds. Azevedo et al. [42] achieved excellent results due to topical insulin administration in burned areas of diabetic rats, but they did not inform the adopted gel formulation for patent reasons; therefore, it may have influenced the action of insulin as a suitable vehicle.
A preliminary meta-analysis with randomized controlled trials compared topical insulin administration to wounds with normal saline, but studies conducted in this field were preliminary; therefore, it was difficult to draw any conclusions about the topical use of insulin to treat wounds [43]. Burn wounds in the test rats have shown granulation tissue formation and re-epithelialization on the fourteenth evaluation day in CMSCs and TIMSCs treatments. MSCs present several beneficial factors for wound healing, such as their ability to migrate to the injury or inflammation sites, to participate in damaged tissue regeneration, to stimulate the proliferation and differentiation of resident progenitor cells, to recover injured cells through the secretion of growth factor and matrix remodeling and to exert immunomodulatory and anti-inflammation effects [44]. Early granulation tissue formation and re-epithelialization are fundamental factors for cutaneous wound healing because they cover the surface of the wound, protect and nourish the wounds, as well as are basically composed of fibroblasts, new capillaries and infiltrated inflammatory cells. The analysis conducted on Day 7 detected burn wounds with granulation tissue with fibroblasts and on Day 14 contraction in wounds with large amounts of granulation tissue and abundant fibroblasts, both for the CMSC treatment. Based on these results, AD-MSC administration increased cell proliferation and led to enhanced burn wound regeneration. Previous studies have shown that AD-MSCs can accelerate wound healing in acid burn skin injuries, in thermal burns and in full-thickness skin wounds [30,32,33] associated with increased angiogenesis, reepithelialization and decreased inflammation [27]. The present research showed clinically relevant local inflammation 5-7 days after MSC injection, and this outcome may suggest the permanence of these cells at least during this period. After these 5-7 days, the inflammatory reaction decreased and dry crust formation started. Burn wounds cause significant damage to the capillaries due to consequent thrombotic/inflammatory disorders that cause homeostasis maintenance losses. However, MSCs remained in the wounds for 25 days after topical application and such response may have contributed to wound healing [41].
Based on the present results, the effect of AD-MSCs injections was beneficial for tissue formation in second-degree burns in rats and, assumingly, this process was induced by granulation tissue acceleration and increased fibroblast proliferation, which probably increased the deposition of collagen fibers. The current study had several limitations. Firstly, an immunohistochemical analysis was not carried out to evaluate neovascular formation and fibroblast differentiation in burn wound beds. Secondly, only short-term results were evaluated and it impaired the forecast of long-term results; finally, the use of an animal model in a relatively small sample.
Conclusion
According to the results, AD-MSCs are beneficial to heal early stage burn wounds in rats. Intradermal administration of allogenic AD-MSCs in experimental second-degree burns for short periods of time in rat model has contributed to reducing the inflammatory phase duration, improving wound re-epithelialization, tissue granulation and wound contraction, as well as to increasing collagen deposition. Further studies with larger samples and longer observation periods are necessary to test the herein assessed treatments as regenerative therapy to patients affected by deep burns.
Acknowledgements
The authors are grateful to the pharmacist Ana Paula Zandavalli for preparing the gels used in the study.
Disclosure of conflict of interest
None.
References
- 1.Medina A, Scott PG, Ghahary A, Tredget EE. Pathophysiology of chronic nonhealing wounds. J Burn Care Rehabil. 2005;26:306–319. doi: 10.1097/01.bcr.0000169887.04973.3a. [DOI] [PubMed] [Google Scholar]
- 2.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:7380–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 3.Finnerty CC, Ali A, McLean J, Benjamin N, Clayton RP, Andersen CR, Mlcak RP, Suman OE, Meyer W, Herndon DN. Impact of stress-induced diabetes on outcomes in severely burned children. J Am Coll Surg. 2014;218:783–795. doi: 10.1016/j.jamcollsurg.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Memmel H, Kowal-Vern A, Latenser BA. Infections in diabetic burn patients. Diabetes Care. 2004;27:229–333. doi: 10.2337/diacare.27.1.229. [DOI] [PubMed] [Google Scholar]
- 5.Stern DM, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation end products (RAGE) and the complications of diabetes. Ageing Res Rev. 2002;1:1–15. doi: 10.1016/s0047-6374(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 6.Llanos S, Danilla S, Barraza C, Armijo E, Piñeros JL, Quintas M, Searle S, Calderon W. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double-masked, controlled trial. Ann Surg. 2006;244:700–705. doi: 10.1097/01.sla.0000217745.56657.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahmardehei M, Kazemikhoo N, Vaghardoost R, Mokmeli S, Momeni M, Nilforoushzadeh MA, Ansari F, Amirkhani A. Effects of low level laser therapy on the prognosis of split-thickness skin graft in type 3 burn of diabetic patients: a case series. Lasers Med Sci. 2016;31:497–502. doi: 10.1007/s10103-016-1896-9. [DOI] [PubMed] [Google Scholar]
- 8.Lima MH, Caricilli AM, de Abreu LL, Araújo EP, Pelegrinelli FF, Thirone AC, Tsukumo DM, Pessoa AF, dos Santos MF, de Moraes MA, Carvalheira JB, Velloso LA, Saad MJ. Topical insulin accelerates wound healing in diabetes by enhancing the AKT and ERK pathways: a double-blind placebo-controlled clinical trial. PLoS One. 2012;7:e3697. doi: 10.1371/journal.pone.0036974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Liu Y, Zhang X. Topical insulin application improves healing by regulating the wound inflammatory response. Wound Rep Reg. 2012;20:425–434. doi: 10.1111/j.1524-475X.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- 10.Emanuelli T, Burgeiro A, Carvalho E. Effects of insulin on the skin: possible healing benefits for diabetic foot ulcers. Arch Dermatol Res. 2016;308:677–694. doi: 10.1007/s00403-016-1686-z. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Wang J, Feng J. Local application of low-dose insulin in improving wound healing after deep burn surgery. Exp Ther Med. 2016;12:2527–2530. doi: 10.3892/etm.2016.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng M, Zhi Y, Liu W, Zhang W, Xu J. Clinical study on local application of low-dose insulin for promoting wound healing after operation for deep burns. Exp Ther Med. 2016;12:3221–3226. doi: 10.3892/etm.2016.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan J, Xia L, Liang W, Liu Y, Cai Q. Transplantation of bone marrow-derived mesenchymal stem cells promotes delayed wound healing in diabetic rats. J Diabetes Res. 2013;2013:647107. doi: 10.1155/2013/647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozturk S, Karagoz H. Experimental stem cell therapies on burn wound: do source, dose, timing and method matter? Burns. 2015;41:1133–1139. doi: 10.1016/j.burns.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Chae DS, Han S, Son M, Kim SW. Stromal vascular fraction shows robust wound healing through high chemotactic and epithelialization property. Citotherapy. 2017;19:543–554. doi: 10.1016/j.jcyt.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Motegi S, Ishikawa O. Mesenchymal stem cells: the roles and functions in cutaneous wound healing and tumor growth. J Dermatol Sci. 2017;86:83–89. doi: 10.1016/j.jdermsci.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Rodgers K, Jadhav SS. The application of mesenchymal cells to treat thermal and radiation burns. Adv Drug Deliv Rev. 2018;123:75–81. doi: 10.1016/j.addr.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Watt SM, Pleat JM. Stem cells niches and scaffolds: applications to burns and wound care. Adv Drug Deliv Rev. 2018;123:82–106. doi: 10.1016/j.addr.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Rep Reg. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 20.Maciel FB, DeRossi R, Módolo TJ, Pagliosa RC, Leal CR, Delben AA. Scanning electron microscopy and microbiological evaluation of equine burn wound repair after platelet-rich plasma gel treatment. Burns. 2012;38:1058–1065. doi: 10.1016/j.burns.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 21.Pierre EJ, Barrow RE, Hawkins HK, Nguyen TT, Sakurai Y, Desai M, Wolfe RR, Herndon DN. Effects of insulin on wound healing. J Trauma. 1998;44:342–345. doi: 10.1097/00005373-199802000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Zhang XJ, Chinkes DL, Sadagopa Ramanujam VM, Wolfe RR. Local injection of insulin zinc stimulates DNA synthesis in skin donor site wound. Wound Repair Regen. 2007;15:258–265. doi: 10.1111/j.1524-475X.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Lv L. Effect of local insulin injection on wound vascularization in patients with diabetic foot ulcer. Exp Ther Med. 2016;11:397–402. doi: 10.3892/etm.2015.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Petreaca M, Martins-Green M. Cell and molecular mechanisms of insulin-induced angiogenesis. J Cell Mol Med. 2009;13:4492–4504. doi: 10.1111/j.1582-4934.2008.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Petreaca M, Yao M, Martins-Green M. Cell and molecular mechanisms of keratinocyte function stimulated by insulin during wound healing. BMC Cell Biol. 2009;10:1. doi: 10.1186/1471-2121-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otero-Viñas M, Falanga V. Mesenchymal stem cells in chronic wounds: the spectrum from basic to advanced therapy. Adv Wound Care. 2016;5:149–163. doi: 10.1089/wound.2015.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 28.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:729–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 29.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Muhammad G, Xu J, Bulte JWM, Jablonska IA, Walczak P, Janowski M. Transplanted adipose-derived stem cells can be short-lived yet accelerate healing of acid-burn skin wounds: a multimodal imaging study. Sci Rep. 2017;7:4644. doi: 10.1038/s41598-017-04484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strong AL, Bowles AC, MacCrimmon CP, Frazier TP, Lee SJ, Wu X, Katz AJ, Gawronska-Kozak B, Bunnell BA, Gimble JM. Adipose stromal cells repair pressure ulcers in both young and elderly mice: potential role of adipogenesis in skin repair. Stem Cells Transl Med. 2015;4:632–642. doi: 10.5966/sctm.2014-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bliley JM, Argenta A, Satish L, McLaughlin MM, Dees A, Tompkins-Rhoades C, Marra KG, Rubin JP. Administration of adipose-derived stem cells enhances vascularity, induces collagen deposition, and dermal adipogenesis in burn wounds. Burns. 2016;42:1212–1222. doi: 10.1016/j.burns.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Shi R, Jin Y, Cao C, Han S, Shao X, Meng L, Cheng J, Zhang M, Zheng J, Xu J, Li M. Localization of human adipose-derived stem cells and their effect in repair of diabetic foot ulcers in rats. Stem Cell Res Ther. 2016;7:155. doi: 10.1186/s13287-016-0412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 35.Zhao S, Wehner R, Bornhäuser M, Wassmuth R, Bachman M, Schmitz M. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 2010;19:607–614. doi: 10.1089/scd.2009.0345. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Hisha H, Mizokami T, Cui W, Cui Y, Shi A, Song C, Okazaki S, Li Q, Feng W, Kato J, Ikehara S. Mouse mesenchymal stem cells can support human hematopoiesis both in vitro and in vivo: the crucial role of neural cell adhesion molecule. Haematologica. 2010;95:884–891. doi: 10.3324/haematol.2009.013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 38.Ward NR, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically patients. Clin Chest Med. 2008;29:1–12. doi: 10.1016/j.ccm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caliari-Oliveira C, Yaochite JN, Ramalho LN, Palma PV, Carlos D, Cunha Fde Q, De Souza DA, Frade MA, Covas DT, Malmegrim KC, Oliveira MC, Voltarelli JC. Xenogeneic mesenchymal stromal cells improve wound healing and modulate the immune response in an extensive burn model. Cell Transplant. 2016;25:201–215. doi: 10.3727/096368915X688128. [DOI] [PubMed] [Google Scholar]
- 40.Yu T, Gao M, Yang P, Pei Q, Liu D, Wang D, Zhang X, Liu Y. Topical insulin accelerates cutaneous wound healing in insulin-resistant diabetic rats. Am J Transl Res. 2017;9:4682–4693. [PMC free article] [PubMed] [Google Scholar]
- 41.Rasulov MF, Vasilenko VT, Zaidenov VA, Onishchenko NA. Cell transplantation inhibits inflammatory reaction and stimulates repair processes in burn wound. Bull Exp Biol Med. 2006;142:112–115. doi: 10.1007/s10517-006-0306-x. [DOI] [PubMed] [Google Scholar]
- 42.Azevedo F, Pessoa A, Moreira G, Dos Santos M, Liberti E, Araujo E, Carvalho C, Saad M, Lima MH. Effect of topical insulin on second-degree burns in diabetic rats. Biol Res Nurs. 2016;18:181–192. doi: 10.1177/1099800415592175. [DOI] [PubMed] [Google Scholar]
- 43.Sridharan K, Sivaramakrishnan G. Efficacy of topical insulin in wound healing: a preliminary systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2017;25:279–287. doi: 10.1111/wrr.12511. [DOI] [PubMed] [Google Scholar]
- 44.Hanson SE, Bentz ML, Hematti P. Mesenchymal stem cell therapy for nonhealing cutaneous wounds. Plast Reconstru Surg. 2010;125:510–516. doi: 10.1097/PRS.0b013e3181c722bb. [DOI] [PMC free article] [PubMed] [Google Scholar]


