Dear Editor,
Coronavirus disease 2019 (COVID-19) is a global public health emergency and new knowledge about it and its etiological agent, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is deemed necessary in order to reduce the death burden around the world [1]. In this regard, a 67-year-old Italian male patient, dyslipidemic, hypertensive, active smoker, and a 49-year-old Italian male patient, affected by hypertrophic cardiomyopathy, were admitted to the intensive care unit (ICU) in feverish state (> 38.1 °C) for SARS-CoV-2 pneumonia complicated with acute respiratory distress syndrome, characterized by diffuse alveolar damage (DAD) and diffuse alveolar hemorrhage (DAH) (Fig. 1a). Upon ICU admission, IgG and IgM serum immunoassay against herpes simplex virus 1 (HSV1) turned out negative (IgG: 3 U/mL and 1 U/mL; IgM: 5 U/mL and 2 U/mL; negativity ranges: IgG < 7.5 U/mL and IgM < 16 U/mL). During the hospitalization period, both the patients received ab initio oxygen and antipyretic and steroid therapy (methylprednisolone: 80 mg/day for 1 day; then 40 mg/day for 3 days; and, at last, 20 mg/day for 1 day), in addition to hydroxychloroquine and low-molecular-weight heparin; on the second day and on the thirteenth day of hospitalization, respectively, an interleukin-6 (IL-6) immunoassay revealed high values (587.86 ρg/mL and 813.34 ρg/mL) and tocilizumab was started in both cases. Persistently positive at molecular diagnostics for SARS-CoV-2 on nasopharyngeal swab or bronchoalveolar lavage, the patients died after 17 days and 39 days from the admission and minimally invasive autopsies in a negative pressure room with 6 air changes per hour were performed, in accordance with the guidelines from the Centers for Disease Control and Prevention of Atlanta (GA, USA) [2]. The quantitative polymerase chain reactions applied to plasma samples had detected in both patients more than 14,000,000 copies/mL of HSV1, respectively, 3 and 7 days before death, occurred despite acyclovir administration. Serum tests performed on the death days had revealed very high value of alanine transaminase (2.888 U/L and 3.102 U/L), aspartate transaminase (4.482 U/L and 2.889 U/L), lactate dehydrogenase (22.102 U/L and 12.427 U/L), total bilirubin (5.57 mg/dL and 7.35 mg/dL), and international normalized ratio (3.55 and 2.10), all laboratory data consistent with fulminant hepatitides. Moreover, 76 h prior to death, the oldest patient had undergone a computed tomography (CT) showing signs of acute hepatitis (Fig. S1). Therefore, the ultimate cause of death is presumably to be traced back to acute liver failure in both patients. Since caused by a novel pathogen, the ongoing pandemic surely requires innovative pharmacological approaches in the attempt to save critically ill patients [3]. Among these, corticosteroids and tocilizumab (atlizumab), a humanized monoclonal antibody against IL-6 receptor, have gain consensus for their ability to mitigate at some extent the cytokine storm in COVID-19 [4, 5]. In 2017, the USA Food and Drug Administration has approved tocilizumab use for the treatment of cytokine release syndrome, whose IL-6 is the key mediator, in course of chimeric antigen receptor T cell therapy [6]. However, superimposed infections are the most common adverse effects in oncohematological or rheumatic patients treated with tocilizumab due to induced immunosuppression [7]. In COVID-19 critical patients, lymphocytopenia worsens this clinical scenario [8, 9], as ascertained also in our two patients during the disease course, with absolute lymphocyte counts of 0.36 × 103/μL and 0.23 × 103/μL (normal range 1.00–4.50 × 103/μL), favoring opportunistic infections, such as invasive pulmonary aspergillosis (Fig. 1b, c) or fulminant herpetic hepatitis (Fig. 1d, e). They are burden by a high mortality rate and their incidence is rising because of an increased number of immunocompromised patients (e.g., bone marrow transplant, chemotherapy, AIDS) [10, 11]. Opportunistic sequelae should be also considered in serious COVID-19 patients submitted to anti-cytokine storm drug regimens.
Fig. 1.
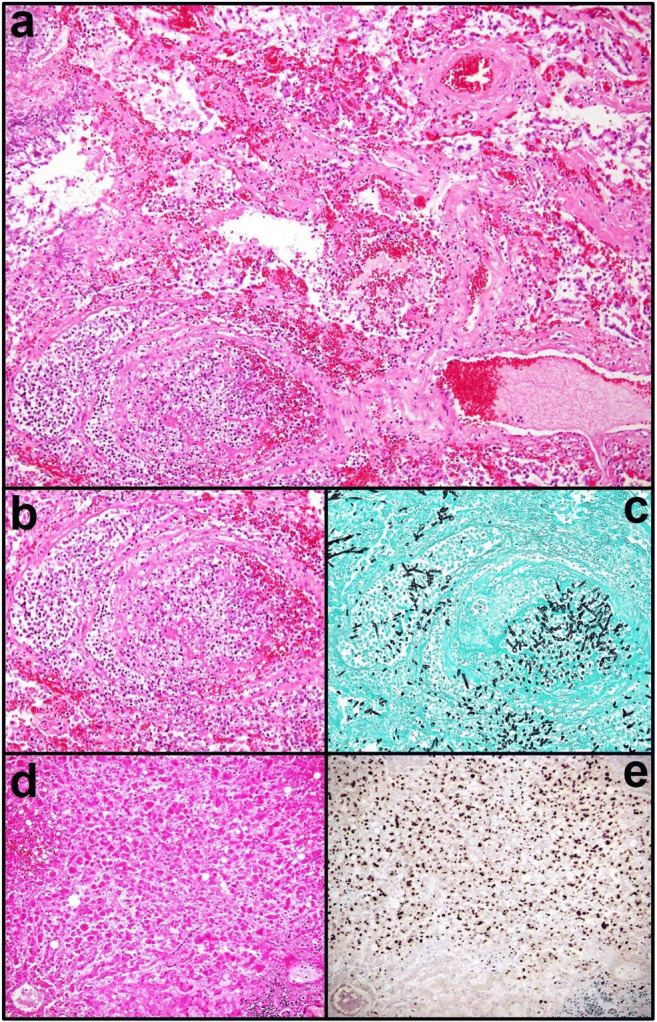
Invasive pulmonary aspergillosis arisen on DAD and DAH from SARS-CoV-2 infection (a H&E, × 10 objective); at higher magnification (b H&E, × 20 objective), fungal angioinvasion is noticeable. Spores and septate hyphae at acute angles of around 45° are well highlighted in black by Grocott’s methenamine silver stain (c × 20 objective). The liver is massively involved by an acute necrotizing inflammation due to herpetic infection (d H&E, × 20 objective): almost all the hepatocytes are in fact positive for HSV1, as shown in brownish staining by specific immunohistochemical assay (e 10A3 clone, DAB chromogen kit, × 20 objective)
Supplementary Information
(DOCX 157 kb).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Not applicable since the patients died.
Footnotes
Summary
COVID-19 is the most dramatic pandemic of the new millenium without socio-economic precedents and measures to counter the death trend are globally needed. SARS-CoV-2, its etiological agent, is able to cause simultaneously a reversible state of cell-mediated immunocompromisation and an acute picture of cytokine storm in the most serious patients. To suppress this life-threatening overlap, anti-cytokine storm drugs are required, with the risk to aggravate the virus-induced immunodeficiency, so favoring fatal opportunistic infections, even fulminant, towards which clinicians should be alerted.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roncati L, Gallo G, Manenti A, Palmieri B. Renin-angiotensin system: the unexpected flaw inside the human immune system revealed by SARS-CoV-2. Med Hypotheses. 2020;140:109686. doi: 10.1016/j.mehy.2020.109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC (2020) Collection and submission of postmortem specimens from deceased persons with known or suspected COVID-19, March 2020 (interim guidance). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-postmortem-specimens.html. Accessed 15 June 2020
- 3.Roncati L, Palmieri B. What about the original antigenic sin of the humans versus SARS-CoV-2? Med Hypotheses. 2020;142:109824. doi: 10.1016/j.mehy.2020.109824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye Z, Wang Y, Colunga-Lozano LE, Prasad M, Tangamornsuksan W, Rochwerg B, Yao L, Motaghi S, Couban RJ, Ghadimi M, Bala MM, Gomaa H, Fang F, Xiao Y, Guyatt GH. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2020;192(27):756–767. doi: 10.1503/cmaj.200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA (2017) FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-b-cell-all-and-tocilizumab-cytokine-release-syndrome?platform=hootsuite. Accessed 30 August 2017
- 7.Sheppard M, Laskou F, Stapleton PP, Hadavi S, Dasgupta B. Tocilizumab (Actemra) Hum Vaccin Immunother. 2017;13(9):1972–1988. doi: 10.1080/21645515.2017.1316909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roncati L, Ligabue G, Fabbiani L, Malagoli C, Gallo G, Lusenti B, Nasillo V, Manenti A, Maiorana A. Type 3 hypersensitivity in COVID-19 vasculitis. Clin Immunol. 2020;217:108487. doi: 10.1016/j.clim.2020.108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roncati L, Nasillo V, Lusenti B, Riva G. Signals of Th2 immune response from COVID-19 patients requiring intensive care. Ann Hematol. 2020;99(6):1419–1420. doi: 10.1007/s00277-020-04066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naaraayan A, Kavian R, Lederman J, Basak P, Jesmajian S. Invasive pulmonary aspergillosis - case report and review of literature. J Community Hosp Intern Med Perspect. 2015;5(1):26322. doi: 10.3402/jchimp.v5.26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Then EO, Gayam V, Are VS, Sunkara T, Gaduputi V. Herpes simplex virus hepatitis: a brief review of an oft-overlooked pathology. Cureus. 2019;11(3):e4313. doi: 10.7759/cureus.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 157 kb).


