Neurotransmitter receptors are concentrated in a morphological specialization of synapses known as the postsynaptic density (PSD). A prominent PSD beneath the postsynaptic membrane in electron micrographs is a defining feature of excitatory synapses. Typically, the excitatory PSD (ePSD) has a disc-like shape 200–800 nm in diameter and 20–50 nm in thickness [1]. In contrast, the inhibitory synapse only has thickened postsynaptic membranes in classical EM studies [2, 3], thus whether inhibitory synapses contain PSD-like structures has been debated for decades. A few years ago, Bi and colleagues characterized the ultrastructure of excitatory and inhibitory synapses in cultured neurons by cryo-electron tomography (cryo-ET) and their high-resolution tomograms clearly showed electron-dense assemblies in both excitatory and inhibitory synapses [4].
The formation and maintenance of PSDs and the dynamic clustering of neurotransmitter receptors by PSD scaffold proteins are crucial for efficient and accurate synaptic transmission. However, elucidating the organizational principles of PSD scaffold proteins and receptors at individual molecule resolution in situ have not been possible, due to both the extreme complexities of PSDs (ePSD in particular) and the lack of suitable imaging methods. In a recently published paper in Nature Neuroscience, Bi and colleagues have been able to obtain the organization principles of GABAA receptors (GABAARs) in the inhibitory PSD (iPSD) in situ, with the structure of GABAARs resolved at 19-Å resolution, using the state-of-the-art cryo-ET method [5]. In this study, the authors developed a template-free classification method by oversampling high-resolution cryo-ET sub-tomograms to automatically identify GABAARs on inhibitory postsynaptic membranes and obtain the structure of these receptors in situ. By analyzing the relative positions of GABAARs, they found that neighboring GABAARs show a characteristic distance of 11 nm with variable angles, such that the receptors self-organize into dense two-dimensional networks. Interestingly, the receptor networks contain sharp boundaries enclosing clustered receptors in iPSDs, indicating that the clustered receptors form a distinct “semi-ordered” state of organization similar to what is known as mesophase in soft-matter physics (Fig. 1A, B). These clustered GABAARs are also aligned with presynaptic vesicle release sites, which may maximize the efficiency of synaptic transmission.
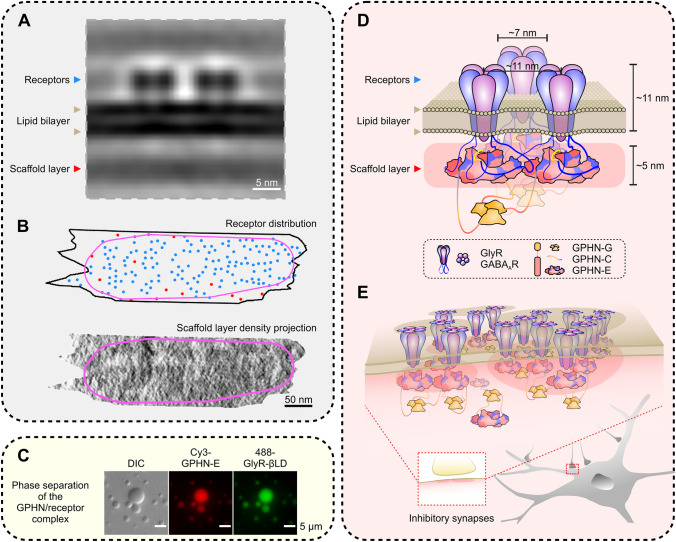
Fig. 1.
Gephyrin-mediated receptor clustering in inhibitory synapses via phase separation. A An example of the sub-tomogram average of GABAAR pairs in inhibitory synapses. B An example of GABAAR distribution on the postsynaptic membrane (upper panel) and the corresponding density projection of the scaffold layer (lower panel) (blue dots, clustered receptors; red dots, solitary receptors; magenta lines, boundary of the mesophase formed by the iPSD receptor and scaffold layers). C Representative differential interference contrast (DIC) and fluorescence images showing the phase separation of the gephyrin (GPHN)/GlyR complexes. D Model of interactions between gephyrin and GABAAR/GlyR pairs on the synaptic plasma membrane. The relative molecular scales are comparable to those in A. Note that the width of the gephyrin E-domain dimer is ~11 nm, and the distance between two GABAARs in iPSDs is also ~11 nm. E Diagram showing the formation of the iPSD sheet via phase separation.
The formation and maintenance of ePSDs have been proposed to be via phase separation, by which the scaffolding proteins form membraneless self-organized condensates and cluster glutamate receptors both in solution and on lipid membrane bilayers [6, 7]. The mesophasic organization of GABAARs at inhibitory synapses revealed by the cryo-ET study by Bi and colleagues suggests that iPSDs may also be formed via phase separation. The iPSD scaffold layer forms sheet-like condensates beneath the postsynaptic membrane (Fig. 1A) rather than the thick, disc-like condensates in ePSDs. Gephyrin is the most abundant scaffold protein and is thought to be the predominant organizer to cluster receptors in iPSDs. Gephyrin was initially identified as a glycine receptor (GlyR)-associated protein [8], containing a trimerized G-domain and a dimerized E-domain. Later, it was found that the E-domain is responsible for binding to both GABAARs and GlyRs [9]. The densities in the scaffold layer found in the work by Liu et al. [5] match well with the two-fold symmetric structure of the gephyrin E-domain. In addition, their simulation studies showed that GABAARs and gephyrin can form dense clusters via a self-organizing process. In a completely independent study from the authors of this Highlight (appeared on the same day as the paper by Liu et al. [5] in Cell Research [10]), it was demonstrated that the iPSD complexes formed between GABAARs (or GlyRs) and gephyrin undergo phase separation both in solution and on membrane bilayers (Fig. 1C). Combining the cryo-ET studies of inhibitory synapses in situ [5] and the biochemical reconstitution studies of iPSD assembly in vitro [10], a compelling model depicting the gephyrin-mediated receptor clustering at inhibitory synapses via phase separation readily emerges (Fig. 1D, E). In this model, the gephyrin E-domain binds to the cytoplasmic TM3-4 loops of selected subunits of GABAARs or GlyRs. The multivalent nature of GABAARs or GlyRs can polymerize the gephyrin E-domain dimer, forming a sheet-like assembly via phase separation. The width of a gephyrin E-domain dimer is ~11 nm, matching the distance of the nearest-neighbor distribution of GABAARs at inhibitory synapses measured by cryo-ET. The thickness of the iPSD sheet also roughly matches the height of the E-domain dimer (~5 nm). The trimerization G-domain of gephyrin, which is likely located beneath the iPSD sheet distal to the plasma membranes, can promote the phase separation of the gephyrin/receptor complex by increasing the valency of the assembly.
The concept of phase separation is gaining immense popularity in biology. However, though dense molecular organizations in neuronal synapses have been observed by electron microscopy for more than five decades, the study by Liu et al. [5], by taking advantage of the recent developments of cryo-ET technology, is the first direct evidence supporting the hypothesis that the mesophasic-scale organization of the iPSD is formed via phase separation. In addition to making a major advance in synaptic biology as demonstrated in the work by Liu et al. [5], cryo-ET technology is poised to make many more revelations in the phase separation-mediated formation of biological condensates in diverse cellular systems.
Acknowledgments
Work in our laboratory was supported by grants from the Ministry of Science and Technology of China (2019YFA0508402) and the Research Grant Council of Hong Kong (AoE-M09-12 and C6004-17G). MZ is a Kerry Holdings Professor of Science and a Senior Fellow of the Institute for Advanced Study at HKUST. We thank Drs Changlu Tao and Guoqiang Bi for providing the images used in Figure 1A, B.
References
- 1.Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters A, Palay SL. The morphology of synapses. J Neurocytol. 1996;25:687–700. doi: 10.1007/BF02284835. [DOI] [PubMed] [Google Scholar]
- 3.Colonnier M. Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain Res. 1968;9:268–287. doi: 10.1016/0006-8993(68)90234-5. [DOI] [PubMed] [Google Scholar]
- 4.Tao CL, Liu YT, Sun R, Zhang B, Qi L, Shivakoti S, et al. Differentiation and characterization of excitatory and inhibitory synapses by cryo-electron tomography and correlative microscopy. J Neurosci. 2018;38:1493–1510. doi: 10.1523/JNEUROSCI.1548-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu YT, Tao CL, Zhang X, Xia W, Shi DQ, Qi L, et al. Mesophasic organization of GABAA receptors in hippocampal inhibitory synapses. Nat Neurosci. 2020;23:1589–1596. doi: 10.1038/s41593-020-00729-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng M, Chen X, Guan D, Xu J, Wu H, Tong P, et al. Reconstituted postsynaptic density as a molecular platform for understanding synapse formation and plasticity. Cell. 2018;174(1172–1187):e1116. doi: 10.1016/j.cell.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Wu X, Wu H, Zhang M. Phase separation at the synapse. Nat Neurosci. 2020;23:301–310. doi: 10.1038/s41593-019-0579-9. [DOI] [PubMed] [Google Scholar]
- 8.Pfeiffer F, Graham D, Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982;257:9389–9393. doi: 10.1016/S0021-9258(18)34082-1. [DOI] [PubMed] [Google Scholar]
- 9.Kasaragod VB, Schindelin H. Structure-function relationships of glycine and GABAA receptors and their interplay with the scaffolding protein gephyrin. Front Mol Neurosci. 2018;11:317. doi: 10.3389/fnmol.2018.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai G, Wang Y, Zhang M. Gephyrin-mediated formation of inhibitory postsynaptic density sheet via phase separation. Cell Res. 2020 doi: 10.1038/s41422-020-00433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]