Dear Editor,
The brain experiences ongoing changes across different ages to support brain development and functional reorganization. During the span of adulthood, although the brain has matured from a neurobiological perspective, it is still continuously shaped by external factors such as habits, the family setting, socioeconomic status, and the work environment [1]. In contrast to chronological age (CA), brain (or biological) age (BA) is conceptualized as an important index for characterizing the aging process and neuropsychological state, as well as individual cognitive performance. Growing evidence indicates that BA can be assessed by neuroimaging techniques, including MRI [2]. Due to their short collection time, stable image features, and (usual) availability during clinical diagnosis, T1-weighted MRIs are considered the first choice for estimating BA, with structural features including local/global volumes of gray matter (GM) and white matter (WM), geometrical characteristics of the cerebral cortex, and distinctions between GM and WM at the boundary [3].
There are two elusive questions in T1-weighted MRI-based BA prediction. The first is how to improve prediction accuracy. At present, research is either limited by relatively poor accuracy or by incomplete age span datasets. Currently, the mean average error (MAE) for a full adult age span dataset for a single site is >4 years. As expected, reports based on multi-site data are usually much less accurate. For example, Valizadeh and colleagues [4] comprehensively tested a variety of feature extractions based on multi-site T1 images and prediction algorithms, and demonstrated varying prediction accuracies in different age classes, but generally ~5.5 years. The second question is how to understand the neurobiological principles in age prediction. Although both structural and functional differences between male and female brains are increasingly reported and are manifest in the aging trajectory [5], as characterized by MRI T1 images, whether there are brain region gender- and age-effects on age prediction remains less examined. These limitations have hindered the popularization of MRI-based age prediction and the associated assessment of cognitive capabilities.
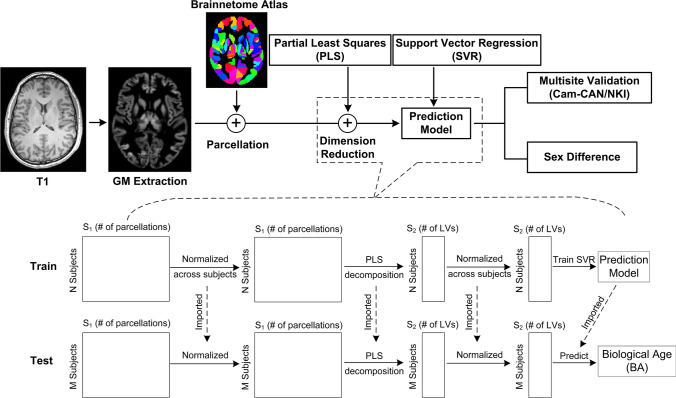
In this study, we propose an intuitive and effective age-prediction model using GM volume and the Brainnetome atlas [6] (Fig. 1). Briefly, the partial least squares (PLS) method was used to project GM volume features (classified by the Brainnetome atlas, which fully covers both cortical and subcortical GM) into orthogonal space, with only the important features that explained the greatest proportion of variance in regressing observers retained [7]. Unlike traditional principal component analysis (PCA), PLS extracts the latent variables by modeling the covariance structures in both predicted and observed variable space. Thus, this supervised dimension reduction method is more efficient than PCA. The PLS algorithm is therefore suitable for exploring the relationship between functional activation and external stimulus patterns, namely, the functional activation detection in fMRI analysis, and the relationship between functional patterns and behavioral measurements [7]. Therefore, it is also reasonable to transfer to projected GM volume features. The prediction was then modeled by support vector regression (SVR) with radial basis function kernels.
Fig. 1.
Diagram showing the construction and validation of the prediction model. N and M are the number of input subjects in the training and testing processes, respectively, and S1 and S2 are the number of variables before and after (then selected) PLS decomposition, respectively. When the 5-fold cross-validation method was used, the training and testing processes were independently repeated.
The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) dataset [8] was used to evaluate the proposed algorithm. Release #1 consists of 655 participants and includes T1-weighted MRI, resting-state fMRI, and diffusion MRI images. After removing the unqualified data, including degraded images and excessive head motion, 620 participants remained, with a male:female ratio of 305:315 and an age range of 18 years to 88 years. The prediction error (discrepancy between BA and CA) was estimated to determine its relationship to behavioral performance, including fluid intelligence (gF). In the Cam-CAN dataset, gF was assessed by the Cattell test, which contains four subtests of nonverbal puzzles involving series completion, classification, matrices, and conditions. Those participants scoring <12 were designated as non-engaging and were thus removed (as suggested in the Cam-CAN data package). Therefore, 603 out of the 620 participants had valid gF records, where age = 52.72 ± 18.19 years and gF score = 32.35 ± 6.59. Overview of Cam-CAN samples used in this study was shown in Fig. S1. The independent dataset, Enhanced Nathan Krow Institute-Rockland Sample (NKI) dataset was used for extended study to evaluate the prediction model.
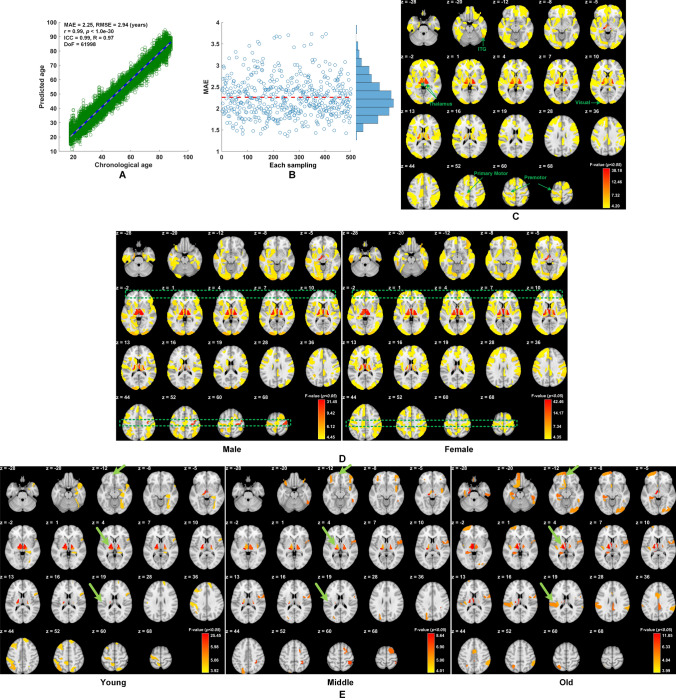
After reviewing the influence of the numbers of latent variables on PLS decomposition, only 13 were chosen for the subsequent SVR training and prediction as there was little difference in prediction accuracy when the number of latent variables varied from 12 to 18 (Table S1). Results based on the Cam-CAN dataset showed that the MAE for age prediction was ~2.25 years through 5-fold cross-validation and the MAE was stable across 500 bootstraps (Fig. 2A, B). Validation of the prediction model based on different combinations of two independent datasets was shown in Table S2. Further validation results were shown in Tables S3, S4. To evaluate the contributions from each brain region in age prediction, significance multivariate correlation (sMC) criteria were used to assess the variable importance selection in PLS [9]. Thus, the F-value for each region (246 regions in total in the Brainnetome atlas [6]) was used for selection importance. The results showed that those regions contributing most to age prediction were concentrated in the bilateral thalamus and primary cortex, including the visual and motor cortices. The non-colored regions in Fig. 2C indicate that sMC failed to reach a significant level (by default α = 0.01). It is well accepted that the thalamus is a pivotal hub of the brain, and connects to most parts of the cerebral cortex to form a variety of functional networks, including the thalamo-frontal, thalamo-parietal, and thalamo-limbic networks [10]. These functional networks consolidate the capabilities of the brain in attention, information processing, episodic memory, working memory, and other higher cognitive functions. Over the lifetime aging process, the thalamus and putamen are the top two subcortical structures whose variance is explained by age. Therefore, it is reasonable to conclude that the thalamus is a critical contributor in age prediction. In addition, interacting with the external environment by frequently accessing and calling, the primary cortex, including the visual and motor cortices, also intensively co-functions with the higher cortex and the thalamus, and reflects the aging process [11]. This partially addresses why the primary cortex is also an important contributor in age prediction. Other engaged regions include the frontal and parietal lobes as evidenced by Lin and colleagues [12].
Fig. 2.
A, B Mean average error (MAE) for prediction accuracy is ~2.25 years (5-fold cross-validation in the Cam-CAN dataset). C Regions contributing most include the posterior inferior temporal gyrus (ITG), thalamus, primary cortex (including visual and motor cortices), prefrontal cortex, lateral occipital cortex, and insular cortex (z in mm). D Different brain region contributions in male and female groups, particularly in prefrontal regions and motor cortex (primary and premotor cortices) (green boxes; z in mm). E Regional contributions in age prediction (similar to D) in the three subgroups, Young (163 subjects aged 17–39 years), Middle (172 subjects aged 44–61 years), and Old (175 subjects aged 69–88 years), where the green arrows in each row highlight the difference of contributions in the same anatomical regions between the three groups. MAE, mean average error; BA, biological age; CA, chronological age; ICC, intraclass correlation coefficient; RMSE, root mean squared error; DoF, degrees of freedom.
Furthermore, the prediction error (CA – BA) was significantly correlated with gF: r = −0.22, P < 1.0e−30, DoF (degrees of freedom) = 60,483. A further question was raised regarding whether gender influenced prediction accuracy. We first checked whether the male and female samples had intrinsically different distributions, and found no significant differences in age distribution [P = 0.59 (t-test), P = 0.63 (Kolmogorov-Smirnov (KS) test), DoF = 618 for the 620 subjects and P = 0.68 (t-test), P = 0.81 (KS test), DoF = 601 for the 603 subjects]. Furthermore, we found no significant differences in age or gF distributions in the groups containing intact gF records both for mean values and distributions of male and female samples, where the distributions were tested by the two-sample KS test in MatLab using the kstest2 function. Thus, it was statistically reasonable to examine the differences in prediction errors between the male and female groups. The results demonstrated that the prediction error in the male group was significantly higher than that in the female group (Δ = 0.24 years, P = 2.2e−16, DoF = 61998). Thus, males generally showed a higher estimated BA compared to their CA than that exhibited in females. Moreover, we further examined whether the brain region contributions to age-prediction showed different patterns between the male and female groups. Rather than predicting age across the entire group, we separately evaluated the regional sMC for the male and female groups and found differences in frontal and motor regions (highlighted by dashed green squares in Fig. 2D). Corresponding results of NKI were shown in Figs. S4-S6.
Intriguingly, the old group demonstrated more contributions from the frontal lobe (including frontal pole and medial frontal cortex) and left hemisphere (including the opercular cortex and thalamus) in contrast to their counterparts in the young group (Fig. 2E). The increased contributions from the frontal lobe are in agreement with the hypothesis of a posterior-anterior shift with aging [13]. Hemispheric asymmetry declines in the aging process; however, hemispheric asymmetry is a critical factor enabling modular efficiency in information processing. Our GM-based study further confirmed that hemispheric asymmetry and a left hemisphere-shift still exists in the healthy aging brain, in accordance with reports on aging [14].
We further investigated whether the gender-moderated effects on the relationship between age residuals and gF are significant. For experimental condition X, we have a dependent variable Y. In the case of significant correlations between X, Y, and an implicit factor M, the mediation analysis examines whether M indicates the underlying mechanism of the relationship between X and Y. However, the moderation analysis focuses on testing the influence of the moderator (gender in our study) on the relationship between X and Y [15]. The gender-moderated analysis (Fig. S3) revealed that gender showed a significant interaction effect on the relationship between the age residuals and intelligence (b = −0.14, standard error = 0.02, t = −7.76, P < 2.2e−16, DoF =60,483), where females showed a stronger negative correlation (b = −0.57, standard error = 0.01, t = −42.7, P < 2.2e−16, DoF = 30,319) between CA – BA and gF than males (b = −0.43, standard error = 0.01, t = −35.23, P < 2.2e−16, DoF = 30,177).
In summary, we propose an age-prediction method based on GM volumes parcellated by the Brainnetome atlas. This method is based only on GM extracted from T1-weighted MRI images, yet yields the highest accuracy reported to date for a large age-span dataset (>70 years). Furthermore, we determined that a higher estimated BA reflects a higher gF, and many regions across the brain contribute to age prediction, particularly the thalamus. There were significant gender and age differences in brain regions contributing to age prediction between males and females and between young and old. Specifically, we identified greater contributions from the prefrontal cortex and lower contributions from the primary cortex in females compared with males, and greater contributions from the frontal lobe and left hemisphere in the old group compared with the young group.
The T1-weighted image is usually the first choice for MRI scans in clinical diagnosis, so T1-based studies for clinical prediction receive more attention, and multimodal MRI only provides limited enhancement of prediction accuracy according to a comprehensive comparative study [3]. The primary anatomical characteristics of brain tissue are extensively used, including the volumes and distinctions between GM and WM. In order to introduce an intuitive model with less of a computing burden for clinical application, we did not use the more advanced geometrical characteristics of cortex, such as cortical gyrification and complexity, and our results demonstrated better prediction accuracy than the existing reports based on single-site datasets. Although we evaluated the reproducibility of the proposed method across two datasets, practical MRI datasets usually have different features, including populations with different ages and different MRI vendors and parameters. Thus, more extensive evaluations are required to test the effectiveness of the method such as by using datasets from many MRI sites. Methodologically, the statistical test-retest strategy should be applied to evaluate the inter- and intra-class robustness. The proposed PLS+SVR prediction model structure is heuristic and can be readily extended to larger or multiple-site datasets with a low computational burden for retuning the parameters. It should be noted that the current findings are based on a cross-sectional dataset and should be further validated using multi-site and longitudinal datasets. The source code in MatLab and R (evaluating gender-moderated effects) for this study is available at https://gitee.com/nmzuo/AgePrediction.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (61971420), the Beijing Brain Initiative of the Beijing Municipal Science and Technology Commission (Z181100001518003), Special Projects of Brain Science of the Beijing Municipal Science and Technology Commission (Z161100000216139 and Z171100000117002), and the International Cooperation and Exchange of the National Natural Science Foundation of China (31620103905). Data were provided by the Cambridge Centre for Ageing and Neuroscience (http://www.mrc-cbu.cam.ac.uk/datasets/camcan).
Conflict of interest
The authors declare that they have no conflict of interest in this work.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s12264-022-00945-3
Nianming Zuo and Tianyu Hu have contributed equally to this work.
Change history
9/13/2022
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s12264-022-00945-3
References
- 1.Zuo N, Salami A, Liu H, Yang Z, Jiang T. Functional maintenance in the multiple demand network characterizes superior fluid intelligence in aging. Neurobiol Aging. 2020;85:145–153. doi: 10.1016/j.neurobiolaging.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Cole JH, Ritchie SJ, Bastin ME, Valdes Hernandez MC, Munoz Maniega S, Royle N, et al. Brain age predicts mortality. Mol Psychiatry. 2018;23:1385–1392. doi: 10.1038/mp.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liem F, Varoquaux G, Kynast J, Beyer F, Kharabian Masouleh S, Huntenburg JM, et al. Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage. 2017;148:179–188. doi: 10.1016/j.neuroimage.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Valizadeh SA, Hanggi J, Merillat S, Jancke L. Age prediction on the basis of brain anatomical measures. Hum Brain Mapp. 2017;38:997–1008. doi: 10.1002/hbm.23434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goyal MS, Blazey TM, Su Y, Couture LE, Durbin TJ, Bateman RJ, et al. Persistent metabolic youth in the aging female brain. Proc Natl Acad Sci U S A. 2019;116:3251–3255. doi: 10.1073/pnas.1815917116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The human brainnetome atlas: A new brain atlas based on connectional architecture. Cereb Cortex. 2016;26:3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56:455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 8.Taylor JR, Williams N, Cusack R, Auer T, Shafto MA, Dixon M, et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: Structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. Neuroimage. 2017;144:262–269. doi: 10.1016/j.neuroimage.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran TN, Afanador NL, Buydens LMC, Blanchet L. Interpretation of variable importance in Partial Least Squares with Significance Multivariate Correlation (sMC) Chemom. Intell. Lab. Syst. 2014;138:153–160. doi: 10.1016/j.chemolab.2014.08.005. [DOI] [Google Scholar]
- 10.Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 11.West KL, Zuppichini MD, Turner MP, Sivakolundu DK, Zhao Y, Abdelkarim D, et al. BOLD hemodynamic response function changes significantly with healthy aging. Neuroimage. 2019;188:198–207. doi: 10.1016/j.neuroimage.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Y, Li M, Zhou Y, Deng W, Ma X, Wang Q, et al. Age-Related Reduction in Cortical Thickness in First-Episode Treatment-Naive Patients with Schizophrenia. Neurosci Bull. 2019;35:688–696. doi: 10.1007/s12264-019-00348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sala-Llonch R, Bartres-Faz D, Junque C. Reorganization of brain networks in aging: a review of functional connectivity studies. Front Psychol. 2015;6:663. doi: 10.3389/fpsyg.2015.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esteves M, Magalhaes R, Marques P, Castanho TC, Portugal-Nunes C, Soares JM, et al. Functional Hemispheric (A)symmetries in the Aged Brain-Relevance for Working Memory. Front Aging Neurosci. 2018;10:58. doi: 10.3389/fnagi.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nie Y, Lau S, Liau AK. Role of academic self-efficacy in moderating the relation between task importance and test anxiety. Learn Individ Differ. 2011;21:736–741. doi: 10.1016/j.lindif.2011.09.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.