Abstract
Migraine is a common and debilitating headache disorder. Although its pathogenesis remains elusive, abnormal trigeminal and central nervous system activity is likely to play an important role. Transient receptor potential (TRP) channels, which transduce noxious stimuli into pain signals, are expressed in trigeminal ganglion neurons and brain regions closely associated with the pathophysiology of migraine. In the trigeminal ganglion, TRP channels co-localize with calcitonin gene-related peptide, a neuropeptide crucially implicated in migraine pathophysiology. Many preclinical and clinical data support the roles of TRP channels in migraine. In particular, activation of TRP cation channel V1 has been shown to regulate calcitonin gene-related peptide release from trigeminal nerves. Intriguingly, several effective anti-migraine therapies, including botulinum neurotoxin type A, affect the functions of TRP cation channels. Here, we discuss currently available data regarding the roles of major TRP cation channels in the pathophysiology of migraine and the therapeutic applicability thereof.
Keywords: Migraine, TRPV1, TRPM8, TRPA1, TRPV4, Calcitonin gene-related peptide, Trigeminal ganglion, Neurogenic inflammation
Introduction
Migraine is one of the most debilitating neurological disorders, characterized by recurrent headache attacks [1, 2]. The concept that migraine is a channelopathy has been substantiated by the fact that familial hemiplegic migraine types 1 and 3 are caused by mutations in the genes encoding the α1 subunit of the CaV2.1 P/Q-type voltage-gated Ca2+ channel (CACNA1A) [3] and the α1 subunit of the neuronal NaV1.1 voltage-gated Na+ channel (SCN1A) [4], respectively. Furthermore, genetic abnormalities of the two-pore-domain K+ channel, TRESK, have been shown to cause a familial form of migraine with aura. Unlike the CaV2.1 and NaV1.1 channels, the TRESK channel is strongly expressed in trigeminal ganglion (TG) neurons, pointing to the importance of peripheral trigeminal nociception in migraine pathophysiology. Transient receptor potential (TRP) channels are non-selective cation channels that transduce various noxious stimuli into pain signals [5]. They are expressed in TG neurons, and some are associated with the functions of calcitonin gene-related peptide (CGRP) [6, 7], a key target molecule of migraine therapy [8]. Considerable data support the role of TRP channels in migraine, so TRP channel modulation may be a promising therapeutic strategy for its treatment. In addition, migraine attacks are known to be provoked and worsened by environmental factors [1, 2]. TRP channels may also be involved in the trigger mechanism of attacks, because they sense changes in ambient temperature [9] and environmental pollutants [10]. Here, we discuss the roles of TRP channels in the pathophysiology of migraine and the potential of TRP-based approaches to migraine therapy.
Migraine Pathophysiology
Migraine, a chronic neurological disorder that affects > 10% of the general population [1, 2], is clinically characterized by recurrent attacks of moderate to severe headache lasting 4–72 h without treatment. Attacks are usually accompanied by nausea, vomiting, and heightened sensitivity to light and sound. The Global Burden of Disease study has recently identified migraine as the most disabling neurological disorder and the second leading cause of years lived with disability worldwide [11]. Its pathophysiological mechanisms involve both the central and peripheral nervous systems. In 25%–30% of patients, some attacks are accompanied by an aura phase, which manifests with transient visual, sensory, and language or brainstem disturbances [12]. The aura is now believed to be caused by cortical spreading depolarization/depression (CSD), a slowly propagating wave of rapid, near-complete depolarization of brain cells that lasts for about 1 min and silences electrical activity for several minutes [13]. Moreover, many patients experience prodromes such as fatigue and changes in appetite hours before an attack. Neuroimaging data show abnormal activation of the hypothalamus during prodromes [14, 15]. On the other hand, intravenous administration of CGRP has been shown to induce attacks specifically in migraine patients [16, 17]. CGRP does not readily permeate the blood-brain barrier, thus making it likely that the neuropeptide acts at peripheral sites, such as the TG, the dura mater, and meningeal vessels in this setting. This tenet is endorsed by the fact that monoclonal antibodies targeting CGRP or its receptor, which do not cross the blood-brain barrier either, are efficacious in the prophylaxis of attacks [8]. Hence, peripheral CGRP actions clearly play a crucial role in the development of migraine attacks.
It remains elusive how migraine headaches are generated. However, it has been postulated that the release of neuropeptides such as CGRP and substance P (SP) by trigeminal nerve fibers causes neurogenic inflammation and subsequent sensitization [18]. These alterations may be responsible for the relatively long and severe headaches associated with migraine. Furthermore, animal studies have demonstrated that CSD can generate a nociceptive stimulus capable of activating the trigeminal system [19–21], which would account for the temporal relation between the aura and the headache phases. Recent studies have clarified that CSD also induces dural macrophage activation, mast cell degranulation, and dilatation of the pial and dural vessels, all of which seem to be causes of headache [22, 23].
In particular, meningeal mast cells seem to be relevant in consideration of their proximity to the meningeal nociceptors and their ability to release a plethora of pro-inflammatory and pro-algesic substances [24]. Migraine symptoms are affected by environmental factors. In some patients, attacks are triggered by changes in ambient temperature or atmospheric pressure [9]. Moreover, migraine headaches are exacerbated by light [25], sound, and chemical irritants, such as cigarette smoke [10]. These observations highlight the role of the information detected by sensory neurons in migraine pathophysiology.
Involvement of TRP Channels in Migraine
The trp gene was originally discovered in a Drosophila mutant with defective vision [26]. Subsequently, this gene was found to encode a protein that plays an important role in phototransduction. Light-activated rhodopsin induces phospholipase C to hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP2) which leads to increased Ca2+ permeability of the TRP channel causing the depolarization of photoreceptor cells [27]. The ancestral TRP channel, which possesses six transmembrane domains, is regarded as an ion channel prototype that transduces environmental stimuli into Ca2+ signaling.
In 1997, Caterina et al. [28] isolated a cDNA clone encoding a capsaicin receptor with non-selective cation channel activity. This receptor was initially referred to as vanilloid receptor 1 (VR1) because a vanilloid moiety of capsaicin was an essential component responsible for the activation of this novel receptor. Concomitantly, it was revealed that VR1 was structurally related to the TRP channel family. Hence, VR1 was later renamed TRP cation channel V1 (TRPV1). From the functional viewpoint, the gating of TRPV1 is driven by noxious heat (> 42°C) which helps us understand why the sensation induced by capsaicin ingestion is perceived as “hot” and “burning”. With the subsequent increase in the number of mammalian TRP family members, they are now classified into six subfamilies – TRPC, TRPV, TRPM, TRPML, TRPP, and TRPA [29, 30]. Some of them were found to be activated by specific temperature ranges [31]. These thermosensitive TRP channels are expressed in primary sensory neurons, which include TG neurons, to confer the ability to detect changes in ambient temperature. Besides, most TRP channels serve in nociceptors as a transducer of noxious stimuli other than non-physiological temperature changes into pain signals [5]. TRPV1 is activated by protons [32, 33] and TRPA1 by reactive oxygen species (ROS) [34]. It should be pointed out that both of these substances have been implicated in the pathogenesis of various pain disorders including migraine [35]. Furthermore, TRP channels are known to be sensitized under pathological conditions. For example, the sensitization of TRPV1 to heat is responsible for the thermal hyperalgesia associated with carrageenan-induced inflammation [36].
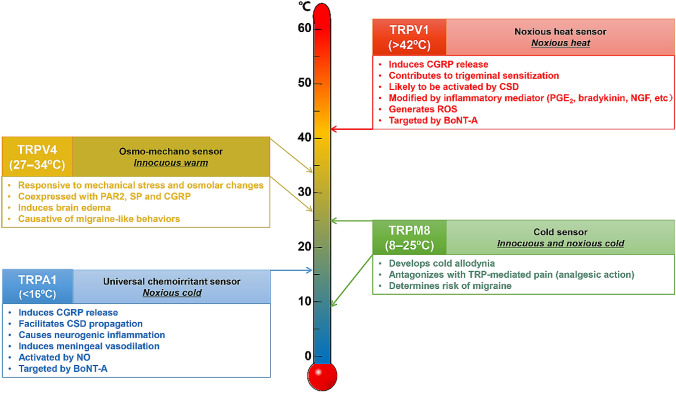
Taken together, TRP channels are involved in the detection of environmental changes and trigeminal nociception. In migraine pathophysiology, where neurogenic inflammation is considered to play an important role [37], TRP channel activity is likely to be upregulated, so blocking such sensitization would be a potential therapeutic strategy. For these reasons, the relationship between TRP channels and migraine has attracted attention [30, 38, 39]. Tremendous amounts of data on mammalian TRP channels have been accumulated since the discovery of VR1/TRPV1. In this article, we focus on the roles of TRPV1, TRPA1, TRPM8, and TRPV4 in the pathophysiology of migraine, because relatively sufficient data relevant to migraine are available for these four channels (Fig. 1).
Fig. 1.
Major functions of TRPV1, TRPM8, TRPA1, and TRPV4 channels relevant to migraine pathophysiology. BoNT-A, botulinum neurotoxin type A; CSD, cortical spreading depression; NGF, nerve growth factor; PGE2, prostaglandin E2; ROS, reactive oxygen species; TRP, transient receptor potential.
TRPV1
TRPV1 Localization and Function in the Trigeminal System
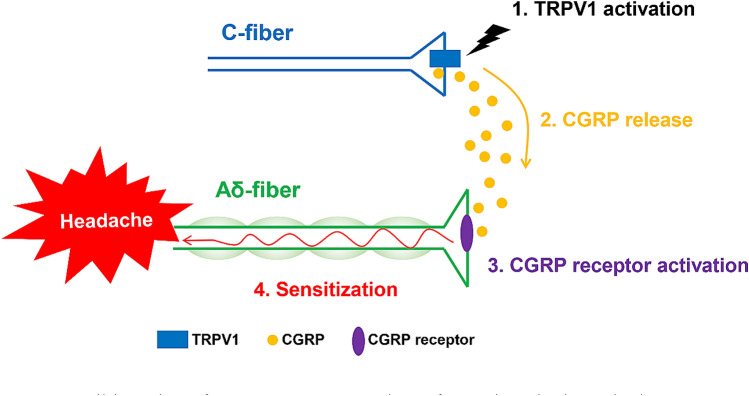
TRPV1 is expressed mainly in small- and medium-sized TG neurons [6, 40, 41]. Approximately 10%–20% of TG neurons are reported to be positive for TRPV1 with slight species differences [6, 40–42]. Moreover, a subset of TRPV1-positive neurons is known to contain CGRP [6, 41], and TRPV1 stimulation induces CGRP release [43–45]. The coexistence of TRPV1 and CGRP has also been confirmed in dural trigeminal fibers [6, 7]. The dura mater is considered to be an important disease locus of migraine [46]. Clinical features of attacks, such as a throbbing headache exacerbated by physical activity, nausea, and photophobia, have also been reported in patients with meningitis [46]. Sumatriptan, which is widely used in acute migraine therapy, was developed with success in animal studies using plasma protein extravasation and vasodilation in the dura mater as surrogate markers [37]. Although the exact role of TRPV1 in migraine pathogenesis remains obscure, the simplistic view that the headaches are caused by nociceptive stimuli to TRPV1-expressing nociceptors is not tenable, because the TRPV1 antagonist SB-705498 was not effective as an acute therapy in a clinical study [30, 47]. In the trigeminal nervous system, the CGRP receptor components calcitonin receptor-like receptor and receptor activity-modifying protein 1 are expressed in thinly-myelinated Aδ-fibers, whereas CGRP is present in unmyelinated C-fibers [48, 49]. This implies that C-fiber-derived CGRP would act on CGRP receptors located on Aδ-fibers. CGRP itself does not directly cause migraine; rather it induces migraine attacks in a delayed manner [16, 17]. Several lines of evidence show that CGRP is involved in sensitization [50–53]. CGRP-induced sensitization is known to play a role in the potentiation of N-methyl-D-aspartic acid receptor functions through protein kinase A-mediated phosphorylation [54–57]. Both CGRP and glutamate are increased in the cerebrospinal fluid from patients with chronic migraine [58]. Thus, it has speculated that TRPV1 contributes to trigeminal sensitization by promoting the release of CGRP from C-fibers (Fig. 2). This paradigm would be compatible with the failure of TRPV1 blockade to abort migraine attacks because TRPV1 activation is positioned as an upstream event in the development of attacks. Consequently, TRPV1 inhibition after an attack had begun would be too late to relieve the headache.
Fig. 2.
Possible action of TRPV1 on CGRP release from trigeminal terminals. TRPV1 activation (1) leads to CGRP release from C-fibers (2). Subsequently, CGRP acts on the CGRP receptor expressed on the surface of Aδ-fibers (3), resulting in the development of sensitization (4). Such sensitization of the trigeminal system may be responsible for the generation of the headache. CGRP, calcitonin gene-related peptide.
CSD and TRPV1 Functions
CSD is known to activate the MAP kinase extracellular signal-regulated kinase (ERK) in TG neurons [21]. Since this activation is disrupted by the TRPV1 inhibitor capsazepine, TRPV1 is likely to be activated by CSD [21]. TRPV1 is known to be expressed in those central nervous system regions relevant to migraine pathophysiology, such as the hippocampus, basal ganglia, thalamus, and hypothalamus [59]. Mechanically-induced CSD is not inhibited by TRPV1 blockade with A-993610, implying that TRPV1 activity does not play a significant role in the process of eliciting CSD [60]. However, repetitive capsaicin stimulation of the trigeminal region has been shown to lower the threshold of CSD induction by suppressing GABAergic activity [61]. It is inferred that repeated trigeminal nociceptive stimulation renders the cerebral cortex susceptible to CSD induction. An important clinical implication of this finding is that clustering of migraine aura attacks may increase the likelihood of recurrence.
Therapeutic Strategies in Migraine with Regard to TRPV1 Functions
TRPV1 functions are known to be modified by inflammatory mediators. Prostaglandin E2 sensitizes TRPV1 channels through protein kinase A-induced phosphorylation of the scaffold protein named A-kinase anchoring protein 150 [62]. Bradykinin enhances TRPV1-mediated currents in a protein kinase C-dependent manner [63]. complete Freund’s adjuvant promotes the translocation of TRPV1 to the cell surface via cyclin-dependent kinase 5-mediated TRPV1 phosphorylation at threonine 407 [64] and increases TRPV1 channel activity via small ubiquitin-like modifier (SUMO)ylation at lysine 822 [65]. Although nerve growth factor (NGF) promotes neuronal growth during development, it also serves as an inflammatory mediator with pro-algesic actions [66]. Of particular relevance, NGF is increased in the plasma and cerebrospinal fluid of patients experiencing chronic daily headaches [67]. NGF increases TRPV1 expression by activating p38 MAP kinase [68], which is mediated by the ubiquitin ligase MYCBP2 [69]. Furthermore, ligation of NGF to the TrkA receptor leads to TRPV1 phosphorylation at tyrosine 200 via Src kinase, causing increased insertion of TRPV1 into the plasma membrane [70]. Toll-like receptors (TLRs) are involved in innate immunity [71, 72], and TLR4 coexists with TRPV1 in TG neurons [73]. Recent evidence has shown that TLR4 inhibits the endocytosis of TRPV1, thus increasing its cell-surface expression level [74]. Collectively, in migraine management, anti-inflammatory measures would be favorable for restricting TRPV1 activity.
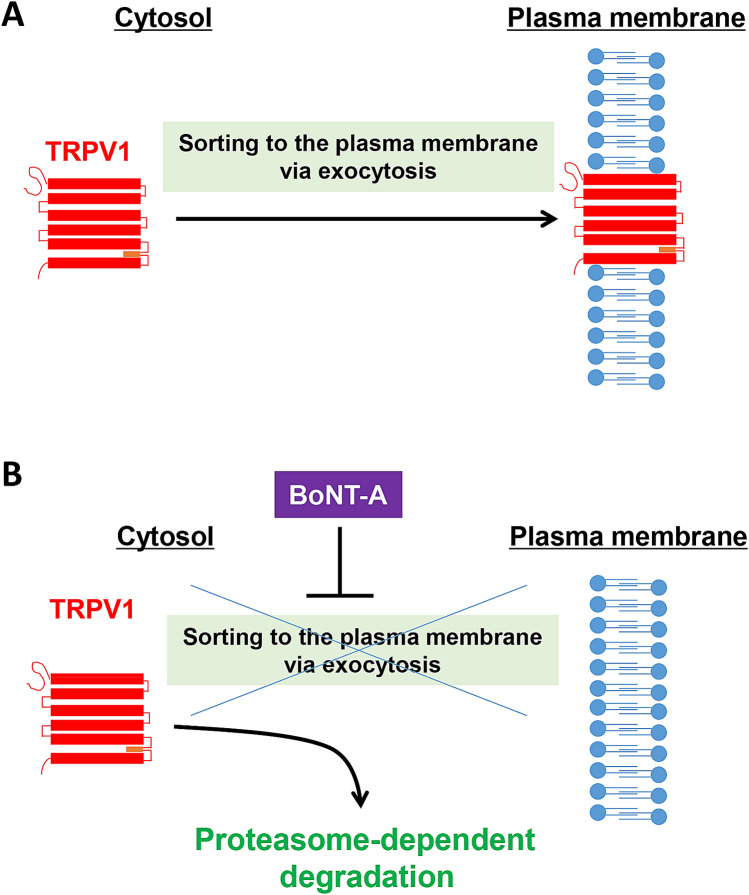
Botulinum neurotoxin type A (BoNT-A) is used to treat chronic migraine. Electrophysiological analyses have revealed that BoNT-A selectively inhibits the C-fibers of meningeal nociceptors [75]. There is anatomical evidence for meningeal nociceptors that send collaterals to the scalp, which provides a rationale for the ability of subcutaneously injected BoNT-A to affect dural trigeminal functions [76–78]. Furthermore, TRPV1 and TRPA1 functions are blocked by BoNT-A [79]. In agreement with these findings, BoNT-A decreases TRPV1 expression in the TG and trigeminal nerve fibers, while P2X3 expression is unaffected [80]. BoNT-A-treated mice are less responsive to capsaicin [80] and in primary cultures of TG neurons, TRPV1 cell-surface expression levels are reduced by BoNT-A treatment. Site-directed mutagenesis of TRPV1 at tyrosine 200 leads to a remarkable decrease in its expression, and this effect is reversed by proteasome inhibition. The last finding raises the possibility that TRPV1 that cannot be normally inserted into the plasma membrane is degraded by the cytoplasmic proteasome system (Fig. 3). The TRPV1 antagonist SB-705498 was not only abandoned as an acute anti-migraine therapy, but hyperthermia was found to be its unfavorable side-effect [30, 47]. By contrast, BoNT-A-mediated TRPV1 inhibition has the major advantage of not causing hyperthermia.
Fig. 3.
Impaired sorting of TRPV1 to the plasma membrane reduces the TRPV1 expression level. A Normally, TRPV1 is translocated to the plasma membrane via exocytosis. B BoNT-A inhibits the exocytosis-mediated sorting of TRPV1 to the plasma membrane. TRPV1 proteins that reside in the cytoplasm are subjected to proteolysis by the proteasome system, leading to reduced expression of TRPV1. BoNT-A, botulinum neurotoxin type A.
TRPM8
TRPM8 as a Cold Sensor
TRPM8 was discovered as a non-selective cation channel responsive to cold (8°C–25°C) and menthol [81, 82]. Regarding its activation, there is an interaction between these stimuli, such that exposure to menthol elevates the threshold temperature for cold stimulation [81, 82]. Intriguingly, PIP2, which negatively regulates TRPV1 functions, conversely enhances TRPM8 activity. Hence, phospholipase C activation following TRPM8 stimulation downregulates TRPM8 functions, thus causing rapid desensitization [83–85]. Genetic ablation studies have corroborated that TRPM8 plays a crucial role in cold sensation [86, 87]. Unlike TRPV1, TRPM8 expression is restricted to primary sensory neurons in the nervous system [82]. In the TG, TRPM8 is mainly expressed in small neurons [78, 88, 89].
Functional Roles of TRPM8 in Pain Disorders and Migraine
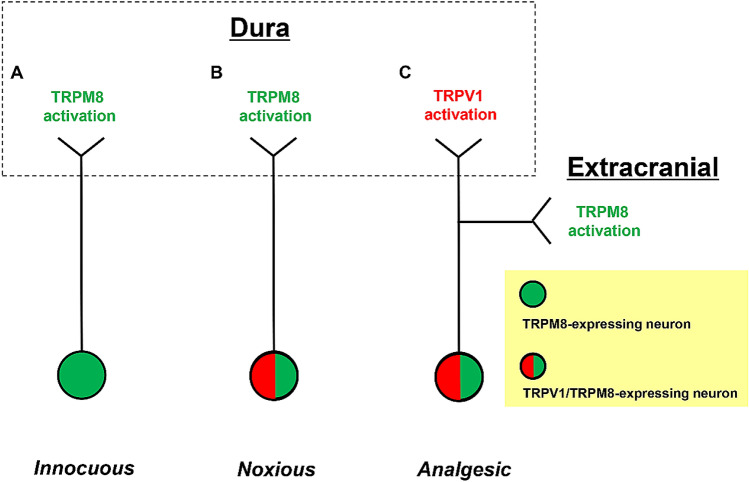
TRPM8 seems to have dual implications for pain; it is involved in the development of cold allodynia, whereas cold stimulation in the temperature range that activates TRPM8 provides innocuous and soothing sensations. Nerve injury-associated and complete Freund’s adjuvant-induced cold allodynia is attenuated in TRPM8-knockout mice [87]. In accord with this, there is a movement for therapeutic application of TRPM8 antagonists to pain disorders [90]. On the other hand, TRPM8 has been found to mediate the analgesic effects of moderate cooling against the painful stimulus induced by formalin administration [86]. From the clinical viewpoint, menthol application can relieve migraine headaches [91, 92]. It has been pointed out that menthol has differing effects on capsaicin-induced pain depending on the time between exposure to capsaicin and menthol [93]. Hence, it is inferred that TRPM8 is involved in either sensing unpleasant cold stimuli or mediating the effects of cold analgesia in a context-dependent manner. Since its discovery, TRPM8 has been found to be co-expressed with TRPV1 in a subset of TG neurons [81]. Although the significance of this coexistence remains to be fully elucidated, TG neurons co-expressing TRPM8 and TRPV1 may be involved in eliciting noxious pain [94]. The co-expression frequency of TRPV1 and TRPM8 in TG neurons is increased in inflammatory soup-induced meningeal inflammation [78]. While this may favor the occurrence of cold allodynia, the increased coexistence concomitantly provides a greater chance for the ability of TRPM8 stimulation to antagonize TRPV1 functions (Fig. 4). In support of the latter, TRPM8 activation with icilin alleviates thermal allodynia in inflammatory soup-induced meningeal inflammation [78], reminiscent of the efficacy of menthol in acute migraine attacks as noted above [91, 92].
Fig. 4.
Altered actions of TRPM8 in different situations. A TRPM8 activation in TG neurons exclusively expressing TRPM8 channels is believed to generate only innocuous sensations. B TRPM8 activation of TG neurons expressing both TRPV1 and TRPM8 causes noxious sensations. C When TRPV1/TRPM8-expressing TG neurons are subjected to TRPV1 activation in the dura, TRPM8 activation in their extracranial collateral axons can exert an analgesic effect, thus assuaging pain.
TRPM8 Gene Polymorphism Determines the Susceptibility to Migraine Development
The relationship between TRPM8 and migraine has been attracting particular attention because genome-wide association studies have reproducibly shown that single nucleotide polymorphisms of the TRPM8 gene (rs10166942[C/T] and rs17862920[T/C]) determine an increased risk of migraine [95–97]. TRPM8 mRNA expression from the chromosome harboring rs10166942[C] was found to be lower than that derived from the chromosome harboring rs10166942[T] in dorsal root ganglion samples, and rs10166942[C] carriers are significantly less sensitive to cold pain than non-carriers [98]. Although the exact mechanism of this allelic expression imbalance is unclear, impaired transcription and/or transcript instability might be involved. Intriguingly, TRPM8 channels are subject to a variety of post-transcriptional modifications [99]. Epidemiologically, rs10166942[T] carriers are associated with reduced migraine risk compared to rs10166942[C] carriers. Hence, increased TRPM8 activity seems to favor the risk of developing migraine. This is consistent with preclinical findings in rats showing that icilin applied to the dura results in cutaneous allodynia [100]. Furthermore, migraine patients carrying rs10166942[T] are more likely to have chronic migraine and allodynic symptoms [101] indicating that TRPM8-related single nucleotide polymorphisms can affect the clinical phenotypes of migraine as well.
TRPA1
TRPA1 as a Polymodal TRP Cation Channel
TRPA1 (also known as ANKTM1), the sole member of the TRPA subfamily, was cloned from cultured human fetal lung fibroblasts [102]. TRPA1 is co-expressed with TRPV1 in a subpopulation of non-myelinated or thinly myelinated C- or Aδ-fiber neurons in the dorsal root ganglion, TG, and vagus ganglion [103]. TRPA1 expression has been identified in both peptidergic sensory neurons (enriched in CGRP, SP, and neurokinin A) and non-peptidergic, IB4-binding neurons [104]. There is direct evidence for the involvement of TRPA1 in pain disorders. A gain-of-function mutation in TRPA1 (p.Asp855Cys) has been identified as the cause of familial episodic pain syndrome characterized by recurrent episodes of debilitating upper body pain, triggered or exacerbated by fatigue, cold exposure, fasting, and weather changes [105]. TRPA1 was originally identified as a noxious cold-sensitive cation channel activated by temperatures below 16°C [106]. In addition, TRPA1 is sensitive to pungent food ingredients (e.g., allyl isothiocyanate [mustard oil], cinnamaldehyde [cinnamon], allicin [garlic], and eugenol [clove bud oil compounds]), environmental irritants, and industrial pollutants, as well as endogenous substances (e.g., bradykinin, ROS, nitric oxide [NO], and lipid oxidation products) [107, 108], some of which are known to trigger migraine attacks.
Is TRPA1 a Crucial Molecule for Migraine Headache Generation?
A growing number of studies have implicated TRPA1 in the development of migraine headache [109], and this is supported by the finding from the “headache tree”. It has long been known that exposure to Umbellularia californica, a tree native to southwestern Oregon and northern California, causes headache crises. Umbellulone, the major volatile component of its leaves, was found to increase the intracellular Ca2+ concentration in TRPA1-transfected HEK293 cells in a concentration-dependent manner, and umbellulone-evoked currents in mouse TG neurons were abrogated by TRPA1 knockout [110]. These findings indicate that umbellulone is a TRPA1 agonist. Furthermore, umbellulone administration evokes CGRP release from TG neurons and dural trigeminal nerves, increases meningeal blood flow [110], and facilitates CSD propagation [111]. Regarding the involvement of TRPA1 in CSD induction, a recent study using brain slices demonstrated that local ROS application (H2O2) promotes cortical responsiveness to CSD in a way that involves TRPA1 and CGRP [112]. Consistent with this, cortical neurons have been shown to express TRPA1 [112, 113] and CGRP [114]. However, in this paradigm, it is unclear how ROS is generated within the cerebral cortex at the initial step. Another possible scenario for the involvement of these three key players in determining CSD susceptibility might be that CSD stimulates meningeal nociceptors [20], thereby causing TRPA1/TRPV1 activation [21], CGRP release, and neurogenic inflammation in the dura mater [22, 23], thus lowering the threshold for CSD induction [61]. In this model, it is envisioned that ROS production in meningeal nociceptors is induced by CSD [115] and/or TRPV1 activation [116], where TRPV1-generated ROS might be able to activate TRPA1 in an autocrine and/or paracrine manner. The involvement of ROS-induced TRPA1 activation has also been reported in a trigeminal neuropathic pain model, where chemokine (C-C motif) ligand 2-medated mobilization of macrophages/monocytes plays a pivotal role [117]. Moreover, it has been shown that ROS production downstream of TRPA1 activation in Schwann cells may contribute to the development of neuropathic pain [118].
TRPA1 and Environmental Migraine Triggers
In addition, some substances cause headaches in susceptible individuals. Acrolein is known to be a major irritant in cigarette smoke and an established migraine trigger [10]. The intranasal application of acrolein also evokes CGRP-dependent meningeal vasodilation via TRPA1 activation [119]. Furthermore, acrolein exposure produces chronic migraine phenotypes, such as peri-orbital allodynia, c-Fos induction in the trigeminal nucleus caudalis, and altered behavior in rats [120]. Therapeutically, the acrolein-induced increase in meningeal blood flow is attenuated by sumatriptan and valproic acid [120], the latter of which is known to be a prophylactic drug for migraine [121]. Hence, these anti-migraine drugs seem to exert an inhibitory action on TRPA1 itself or its downstream events.
Migraine attacks are provoked by NO donors, such as nitroglycerin and glyceryl trinitrate [122]. Glyceryl trinitrate has been reported to cause facial allodynia by inducing the TRPA1-mediated generation of reactive oxygen and carbonyl species within the TG [123]. Furthermore, endogenous NO and hydrogen sulfide contribute to CGRP release by activating TRPA1 in sensory nerves [108], which promotes nociceptive firing in the primary afferents underlying migraine pain under neuroinflammatory conditions [124].
TRPA1 as a Therapeutic Target of Migraine
Intriguingly, a number of anti-migraine drugs have been shown to desensitize or inhibit TRPA1 activity [108]. In particular, isopetasin (a major constituent of butterbur extracts) is reported to desensitize TRPA1, which may account for the anti-migraine action of butterburs [125]. Caffeine has been demonstrated to suppress human TRPA1 channels by an unknown mechanism [126]. Paracetamol (acetaminophen) has been demonstrated to exert an anti-nociceptive effect by desensitizing TRPA1 [127, 128]. Lastly, extracranial administration of BoNT-A inhibits meningeal nociceptors by reducing the expression of TRPA1 as well as TRPV1 [79]. However, it should be noted that it takes 7 days for BoNT-A to reduce the cell-surface expression of TRPV1 and TRPA1 in the dural nerve endings of meningeal nociceptors [79, 80]. Hence, the development of BoNT-like drugs with a more rapid onset of action is awaited.
TRPV4
TRPV4 is a Unique Polymodal TRP Cation Channel Discovered as an Osmotic Sensor
TRPV4 (also called VR-OAC [vanilloid receptor-related osmotically activated ion channel], VRL-2 [vanilloid receptor-like channel 2], TRPL2 [transient receptor-like channel 2], and OTRPC4 [osmosensory protein 9-like TRP channel, member 4]) was originally cloned as the vanilloid receptor-related channel activated by osmotic changes; it is strongly expressed in the kidney, liver, and heart [129, 130]. TRPV4 is a mammalian homolog of OSM-9 in Caenorhabditis elegans [130–133], and TRPV4 expression has been found to restore the osmotic avoidance response in OSM-9-deficient worms [134]. TRPV4 is now known to play an evolutionarily-conserved role in the transduction of osmotic and mechanical stimuli [29, 135, 136].
TRPV4 is a polymodal receptor with pleiotropic functions and widespread expression in various cell types/tissues throughout the body [103, 137]. It can be activated by various stimuli including physical factors (altered osmolarity, moderate heat [27°C–34°C] and mechanical stimuli such as membrane stretch and shear stress), chemical factors (endocannabinoids, arachidonic acid and its metabolites, and 4α-phorbol esters), and protons [103, 138–141].
Function and Localization of TRPV4 in Relation to Pain Disorders
TRPV4 is also involved in a plethora of pain conditions [29], encompassing mechanically-evoked [132, 142], inflammatory [143], neuropathic [144], visceral [145], and trigeminal pain conditions [146, 147]. Moreover, ultraviolet B-induced TRPV4 activation in the epidermis may be responsible for the development of sunburn pain [148]. A recent study has disclosed that the Piezo1–TRPV4 axis is involved in the exacerbation of pancreatitis via sustained elevation of intracellular Ca2+, thus highlighting the role of TRPV4 in visceral pain [149]. TRPV4 activation is known to potentiate the tetrodotoxin-sensitive Na+ current [150] and TRPV1 function [151] in TG neurons. Thus, TRPV4 also seems to serve as a pain enhancer in TG neurons.
TRPV4 expression has been reported not only in primary sensory neurons [147, 152] but also in satellite glial cells [153]. TRPV4 expression has also been recognized in the central nervous system [154]. TRPV4 mRNA has been found in neurons [132, 155], astrocytes [156], and microglia [157]. Astroglial TRPV4 has been shown to mediate brain edema after traumatic injury [158], a condition frequently encountered in familial hemiplegic migraine [159].
Emerging Evidence for the Importance of TRPV4 as a Novel Therapeutic Target for Migraine
Evidence for the role of TRPV4 in migraine pathophysiology is still scarce. However, its activation in response to mechanical stress and osmolarity changes fits into several aspects of migraine. For example, TRPV4 sensitization may be responsible for the worsening of migraine headaches by routine physical activity [160]. Also, trigeminal afferents are known to be sensitized by dural application of solutions with either increased or decreased osmolarity [161, 162]. In agreement with this, in vivo electrophysiological patch-clamp recordings demonstrated TRPV4-like currents in dural afferents in response to the application of hypotonic solutions and the TRPV4 activator 4α-PDD [163]. Furthermore, activation of TRPV4 within the dura of freely-moving animals induces migraine-like behaviors (cephalic and extracephalic allodynia) that are inhibited by a TRPV4 antagonist [163]. Formalin injection into the whisker pad has been found to induce trigeminal nocifensive behavior by activating Ca2+ entry through TRPV4 [147]. Mechanistically, concomitant exposure to formalin and high humidity seems to activate the TRPV4–p38 MAP kinase pathway [164].
In rat sensory neurons, immunoreactive TRPV4 is co-expressed with protease-activated receptor 2 (PAR2), SP, and CGRP, all of which are associated with migraine pathophysiology [165]. In particular, PAR2 activation in the meninges has been found to cause migraine-like pain behaviors [166]. Since PAR2 is known to underpin sustained activation of TRPV4 [167], a vicious cycle can be formed between these two molecules. Hence, the involvement of the PAR2–TRPV4 pathway in migraine pathophysiology may warrant further investigations.
Collectively, these findings raise the possibility that TRPV4 blockade can be a promising novel therapeutic strategy against migraines. A novel small molecule dual-channel inhibitor of TRPV4 and TRPA1 has been developed for attenuation of inflammation and pain including trigeminal irritant pain [143, 168].
Concluding Remarks
We reviewed the possible roles of four thermosensitive TRP channels in the pathophysiology of migraine. It is apparent that each of these channels operates as a detector of specific noxious stimuli. As discussed in this article, numerous preclinical and clinical data are available that support their various roles in migraine. The efficacy of BoNT-A in the management of chronic migraine implies that TRPV1 and TRPA1 are bona fide therapeutic targets of migraine. Notwithstanding, there is no definite proof that TRP channels mediate migraine headaches because TRP channel blockade has never been successful as a migraine therapy. Considering that migraine is a paroxysmal disorder, it is necessary to develop TRP antagonists with a rapid onset of action. Concomitantly, they should not have any adverse effect on body temperature in terms of clinical application. A better understanding of the relationship between TRP channels and CGRP, as well as adequate control of inflammatory conditions, may be key to maximizing the effectiveness of TRP channel-based anti-migraine therapies. Furthermore, TRP channel overactivity can profoundly affect cellular functions, for example, via mitochondrial toxicity [169, 170]. Hence, proper management of TRP channel activity would be protective against neuropathic changes.
Acknowledgements
This review was supported by the Japan Society for the Promotion of Science KAKENHI (26460706 and 19K07849), a Japan-China Sasakawa Medical Fellowship (2017816), and a State Scholarship Fund of the China Scholarship Council (201908500072).
Conflict of interest
The authors claim that there are no conflicts of interest.
Footnotes
Mamoru Shibata and Chunhua Tang have contributed equally to this review.
References
- 1.Charles A. Migraine. N Engl J Med. 2017;377:553–561. doi: 10.1056/NEJMcp1605502. [DOI] [PubMed] [Google Scholar]
- 2.Dodick DW. Migraine. Lancet. 2018;391:1315–1330. doi: 10.1016/S0140-6736(18)30478-1. [DOI] [PubMed] [Google Scholar]
- 3.Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 4.Dichgans M, Freilinger T, Eckstein G, Babini E, Lorenz-Depiereux B, Biskup S, et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet. 2005;366:371–377. doi: 10.1016/S0140-6736(05)66786-4. [DOI] [PubMed] [Google Scholar]
- 5.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu T, Toriumi H, Sato H, Shibata M, Nagata E, Gotoh K, et al. Distribution and origin of TRPV1 receptor-containing nerve fibers in the dura mater of rat. Brain Res. 2007;1173:84–91. doi: 10.1016/j.brainres.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 7.Huang D, Li S, Dhaka A, Story GM, Cao YQ. Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Mol Pain. 2012;8:66. doi: 10.1186/1744-8069-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodick DW. CGRP ligand and receptor monoclonal antibodies for migraine prevention: Evidence review and clinical implications. Cephalalgia. 2019;39:445–458. doi: 10.1177/0333102418821662. [DOI] [PubMed] [Google Scholar]
- 9.Prince PB, Rapoport AM, Sheftell FD, Tepper SJ, Bigal ME. The effect of weather on headache. Headache. 2004;44:596–602. doi: 10.1111/j.1526-4610.2004.446008.x. [DOI] [PubMed] [Google Scholar]
- 10.Kunkler PE, Zhang L, Pellman JJ, Oxford GS, Hurley JH. Sensitization of the trigeminovascular system following environmental irritant exposure. Cephalalgia. 2015;35:1192–1201. doi: 10.1177/0333102415574845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collaborators GBDH Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954–976. doi: 10.1016/S1474-4422(18)30322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev. 2017;97:553–622. doi: 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pietrobon D, Moskowitz MA. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat Rev Neurosci. 2014;15:379–393. doi: 10.1038/nrn3770. [DOI] [PubMed] [Google Scholar]
- 14.Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2014;137:232–241. doi: 10.1093/brain/awt320. [DOI] [PubMed] [Google Scholar]
- 15.Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain. 2016;139:1987–1993. doi: 10.1093/brain/aww097. [DOI] [PubMed] [Google Scholar]
- 16.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 17.Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30:1179–1186. doi: 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- 18.Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol. 1984;16:157–168. doi: 10.1002/ana.410160202. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. 2011;69:855–865. doi: 10.1002/ana.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci. 2010;30:8807–8814. doi: 10.1523/JNEUROSCI.0511-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwashita T, Shimizu T, Shibata M, Toriumi H, Ebine T, Funakubo M, et al. Activation of extracellular signal-regulated kinase in the trigeminal ganglion following both treatment of the dura mater with capsaicin and cortical spreading depression. Neurosci Res. 2013;77:110–119. doi: 10.1016/j.neures.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Kocak E, Sen ZD, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339:1092–1095. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- 23.Schain AJ, Melo-Carrillo A, Stratton J, Strassman AM, Burstein R. CSD-Induced Arterial Dilatation and Plasma Protein Extravasation Are Unaffected by Fremanezumab: Implications for CGRP’s Role in Migraine with Aura. J Neurosci. 2019;39:6001–6011. doi: 10.1523/JNEUROSCI.0232-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy D, Burstein R, Strassman AM. Mast cell involvement in the pathophysiology of migraine headache: A hypothesis. Headache. 2006;46(Suppl 1):S13–S18. doi: 10.1111/j.1526-4610.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- 25.Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 27.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 28.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 29.Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB. Regulation of Pain and Itch by TRP Channels. Neurosci Bull. 2018;34:120–142. doi: 10.1007/s12264-017-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benemei S, Dussor G. TRP channels and migraine: recent developments and new therapeutic opportunities. Pharmaceuticals (Basel) 2019;12:54. doi: 10.3390/ph12020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vay L, Gu C, McNaughton PA. The thermo-TRP ion channel family: properties and therapeutic implications. Br J Pharmacol. 2012;165:787–801. doi: 10.1111/j.1476-5381.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 33.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 34.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvemini D, Little JW, Doyle T, Neumann WL. Roles of reactive oxygen and nitrogen species in pain. Free Radic Biol Med. 2011;51:951–966. doi: 10.1016/j.freeradbiomed.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 37.Williamson DJ, Hargreaves RJ. Neurogenic inflammation in the context of migraine. Microsc Res Tech. 2001;53:167–178. doi: 10.1002/jemt.1081. [DOI] [PubMed] [Google Scholar]
- 38.Dussor G, Cao YQ. TRPM8 and Migraine. Headache. 2016;56:1406–1417. doi: 10.1111/head.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meents JE, Neeb L, Reuter U. TRPV1 in migraine pathophysiology. Trends Mol Med. 2010;16:153–159. doi: 10.1016/j.molmed.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Ichikawa H, Sugimoto T. VR1-immunoreactive primary sensory neurons in the rat trigeminal ganglion. Brain Res. 2001;890:184–188. doi: 10.1016/s0006-8993(00)03253-4. [DOI] [PubMed] [Google Scholar]
- 41.Hou M, Uddman R, Tajti J, Kanje M, Edvinsson L. Capsaicin receptor immunoreactivity in the human trigeminal ganglion. Neurosci Lett. 2002;330:223–226. doi: 10.1016/s0304-3940(02)00741-3. [DOI] [PubMed] [Google Scholar]
- 42.Quartu M, Serra MP, Boi M, Poddighe L, Picci C, Demontis R, et al. TRPV1 receptor in the human trigeminal ganglion and spinal nucleus: immunohistochemical localization and comparison with the neuropeptides CGRP and SP. J Anat. 2016;229:755–767. doi: 10.1111/joa.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng J, Ovsepian SV, Wang J, Pickering M, Sasse A, Aoki KR, et al. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. 2009;29:4981–4992. doi: 10.1523/JNEUROSCI.5490-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akerman S, Kaube H, Goadsby PJ. Vanilloid type 1 receptors (VR1) on trigeminal sensory nerve fibres play a minor role in neurogenic dural vasodilatation, and are involved in capsaicin-induced dural dilation. Br J Pharmacol. 2003;140:718–724. doi: 10.1038/sj.bjp.0705486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akerman S, Kaube H, Goadsby PJ. Anandamide acts as a vasodilator of dural blood vessels in vivo by activating TRPV1 receptors. Br J Pharmacol. 2004;142:1354–1360. doi: 10.1038/sj.bjp.0705896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 47.Gunthorpe MJ, Hannan SL, Smart D, Jerman JC, Arpino S, Smith GD, et al. Characterization of SB-705498, a potent and selective vanilloid receptor-1 (VR1/TRPV1) antagonist that inhibits the capsaicin-, acid-, and heat-mediated activation of the receptor. J Pharmacol Exp Ther. 2007;321:1183–1192. doi: 10.1124/jpet.106.116657. [DOI] [PubMed] [Google Scholar]
- 48.Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience. 2010;169:683–696. doi: 10.1016/j.neuroscience.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 49.Melo-Carrillo A, Noseda R, Nir RR, Schain AJ, Stratton J, Strassman AM, et al. Selective Inhibition of Trigeminovascular Neurons by Fremanezumab: A Humanized Monoclonal Anti-CGRP Antibody. J Neurosci. 2017;37:7149–7163. doi: 10.1523/JNEUROSCI.0576-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura-Craig M, Gill BK. Effect of neurokinin A, substance P and calcitonin gene related peptide in peripheral hyperalgesia in the rat paw. Neurosci Lett. 1991;124:49–51. doi: 10.1016/0304-3940(91)90819-f. [DOI] [PubMed] [Google Scholar]
- 51.Sun RQ, Lawand NB, Willis WD. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. Pain. 2003;104:201–208. doi: 10.1016/s0304-3959(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 52.Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol. 2004;92:2859–2866. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- 53.Natura G, von Banchet GS, Schaible HG. Calcitonin gene-related peptide enhances TTX-resistant sodium currents in cultured dorsal root ganglion neurons from adult rats. Pain. 2005;116:194–204. doi: 10.1016/j.pain.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Han JS, Adwanikar H, Li Z, Ji G, Neugebauer V. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol Pain. 2010;6:10. doi: 10.1186/1744-8069-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang X, Wang S, Qin G, Xie J, Tan G, Zhou J, et al. Tyrosine Phosphorylation of NR2B Contributes to Chronic Migraines via Increased Expression of CGRP in Rats. Biomed Res Int. 2017;2017:7203458. doi: 10.1155/2017/7203458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okutsu Y, Takahashi Y, Nagase M, Shinohara K, Ikeda R, Kato F. Potentiation of NMDA receptor-mediated synaptic transmission at the parabrachial-central amygdala synapses by CGRP in mice. Mol Pain. 2017;13:1744806917709201. doi: 10.1177/1744806917709201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang XY, Zhou HR, Wang S, Liu CY, Qin GC, Fu QQ, et al. NR2B-Tyr phosphorylation regulates synaptic plasticity in central sensitization in a chronic migraine rat model. J Headache Pain. 2018;19:102. doi: 10.1186/s10194-018-0935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallai V, Alberti A, Gallai B, Coppola F, Floridi A, Sarchielli P. Glutamate and nitric oxide pathway in chronic daily headache: evidence from cerebrospinal fluid. Cephalalgia. 2003;23:166–174. doi: 10.1046/j.1468-2982.2003.00552.x. [DOI] [PubMed] [Google Scholar]
- 59.Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 60.Summ O, Holland PR, Akerman S, Goadsby PJ. TRPV1 receptor blockade is ineffective in different in vivo models of migraine. Cephalalgia. 2011;31:172–180. doi: 10.1177/0333102410375626. [DOI] [PubMed] [Google Scholar]
- 61.Toriumi H, Shimizu T, Ebine T, Takizawa T, Kayama Y, Koh A, et al. Repetitive trigeminal nociceptive stimulation in rats increases their susceptibility to cortical spreading depression. Neurosci Res. 2016;106:74–78. doi: 10.1016/j.neures.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 62.Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, et al. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci. 2008;28:4904–4917. doi: 10.1523/JNEUROSCI.0233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 64.Liu J, Du J, Yang Y, Wang Y. Phosphorylation of TRPV1 by cyclin-dependent kinase 5 promotes TRPV1 surface localization, leading to inflammatory thermal hyperalgesia. Exp Neurol. 2015;273:253–262. doi: 10.1016/j.expneurol.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Gao Y, Tian Q, Deng Q, Wang Y, Zhou T, et al. TRPV1 SUMOylation regulates nociceptive signaling in models of inflammatory pain. Nat Commun. 2018;9:1529. doi: 10.1038/s41467-018-03974-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mizumura K, Murase S. Role of nerve growth factor in pain. Handb Exp Pharmacol. 2015;227:57–77. doi: 10.1007/978-3-662-46450-2_4. [DOI] [PubMed] [Google Scholar]
- 67.Sarchielli P, Alberti A, Floridi A, Gallai V. Levels of nerve growth factor in cerebrospinal fluid of chronic daily headache patients. Neurology. 2001;57:132–134. doi: 10.1212/wnl.57.1.132. [DOI] [PubMed] [Google Scholar]
- 68.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 69.Holland S, Coste O, Zhang DD, Pierre SC, Geisslinger G, Scholich K. The ubiquitin ligase MYCBP2 regulates transient receptor potential vanilloid receptor 1 (TRPV1) internalization through inhibition of p38 MAPK signaling. J Biol Chem. 2011;286:3671–3680. doi: 10.1074/jbc.M110.154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 72.Liu JF, Wu R, Li JX. Toll of Mental Disorders: TLR-Mediated Function of the Innate Immune System. Neurosci Bull. 2019;35:771–774. doi: 10.1007/s12264-018-00335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wadachi R, Hargreaves KM. Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res. 2006;85:49–53. doi: 10.1177/154405910608500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Min H, Cho WH, Lee H, Choi B, Kim YJ, Lee HK, et al. Association of TRPV1 and TLR4 through the TIR domain potentiates TRPV1 activity by blocking activation-induced desensitization. Mol Pain. 2018;14:1744806918812636. doi: 10.1177/1744806918812636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burstein R, Zhang X, Levy D, Aoki KR, Brin MF. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: therapeutic implications for migraine and other pains. Cephalalgia. 2014;34:853–869. doi: 10.1177/0333102414527648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kosaras B, Jakubowski M, Kainz V, Burstein R. Sensory innervation of the calvarial bones of the mouse. J Comp Neurol. 2009;515:331–348. doi: 10.1002/cne.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schueler M, Messlinger K, Dux M, Neuhuber WL, De Col R. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain. 2013;154:1622–1631. doi: 10.1016/j.pain.2013.04.040. [DOI] [PubMed] [Google Scholar]
- 78.Kayama Y, Shibata M, Takizawa T, Ibata K, Shimizu T, Ebine T, et al. Functional interactions between transient receptor potential M8 and transient receptor potential V1 in the trigeminal system: Relevance to migraine pathophysiology. Cephalalgia. 2018;38:833–845. doi: 10.1177/0333102417712719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang X, Strassman AM, Novack V, Brin MF, Burstein R. Extracranial injections of botulinum neurotoxin type A inhibit intracranial meningeal nociceptors’ responses to stimulation of TRPV1 and TRPA1 channels: Are we getting closer to solving this puzzle? Cephalalgia. 2016;36:875–886. doi: 10.1177/0333102416636843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shimizu T, Shibata M, Toriumi H, Iwashita T, Funakubo M, Sato H, et al. Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-A. Neurobiol Dis. 2012;48:367–378. doi: 10.1016/j.nbd.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 81.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 82.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 83.Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem. 2009;284:1570–1582. doi: 10.1074/jbc.M807270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:1674–1681. doi: 10.1523/JNEUROSCI.3632-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 86.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 87.Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D’Andrea MR, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 88.Abe J, Hosokawa H, Okazawa M, Kandachi M, Sawada Y, Yamanaka K, et al. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res. 2005;136:91–98. doi: 10.1016/j.molbrainres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 89.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 90.Horne DB, Biswas K, Brown J, Bartberger MD, Clarine J, Davis CD, et al. Discovery of TRPM8 Antagonist (S)-6-(((3-Fluoro-4-(trifluoromethoxy)phenyl)(3-fluoropyridin-2-yl)methyl)carbamoy l)nicotinic Acid (AMG 333), a Clinical Candidate for the Treatment of Migraine. J Med Chem. 2018;61:8186–8201. doi: 10.1021/acs.jmedchem.8b00518. [DOI] [PubMed] [Google Scholar]
- 91.Gobel H, Schmidt G, Soyka D. Effect of peppermint and eucalyptus oil preparations on neurophysiological and experimental algesimetric headache parameters. Cephalalgia. 1994;14:228–234. doi: 10.1046/j.1468-2982.1994.014003228.x. [DOI] [PubMed] [Google Scholar]
- 92.Borhani Haghighi A, Motazedian S, Rezaii R, Mohammadi F, Salarian L, Pourmokhtari M, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010;64:451–456. doi: 10.1111/j.1742-1241.2009.02215.x. [DOI] [PubMed] [Google Scholar]
- 93.Green BG, McAuliffe BL. Menthol desensitization of capsaicin irritation. Evidence of a short-term anti-nociceptive effect. Physiol Behav. 2000;68:631–639. doi: 10.1016/s0031-9384(99)00221-8. [DOI] [PubMed] [Google Scholar]
- 94.Takashima Y, Ma L, McKemy DD. The development of peripheral cold neural circuits based on TRPM8 expression. Neuroscience. 2010;169:828–842. doi: 10.1016/j.neuroscience.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Freilinger T, Anttila V, de Vries B, Malik R, Kallela M, Terwindt GM, et al. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat Genet. 2012;44:777–782. doi: 10.1038/ng.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chasman DI, Schurks M, Anttila V, de Vries B, Schminke U, Launer LJ, et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet. 2011;43:695–698. doi: 10.1038/ng.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anttila V, Winsvold BS, Gormley P, Kurth T, Bettella F, McMahon G, et al. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet. 2013;45:912–917. doi: 10.1038/ng.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gavva NR, Sandrock R, Arnold GE, Davis M, Lamas E, Lindvay C, et al. Reduced TRPM8 expression underpins reduced migraine risk and attenuated cold pain sensation in humans. Sci Rep. 2019;9:19655. doi: 10.1038/s41598-019-56295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kayama Y, Shibata M, Takizawa T, Ibata K, Nakahara J, Shimizu T, et al. Signaling Pathways Relevant to Nerve Growth Factor-induced Upregulation of Transient Receptor Potential M8 Expression. Neuroscience. 2017;367:178–188. doi: 10.1016/j.neuroscience.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 100.Burgos-Vega CC, Ahn DD, Bischoff C, Wang W, Horne D, Wang J, et al. Meningeal transient receptor potential channel M8 activation causes cutaneous facial and hindpaw allodynia in a preclinical rodent model of headache. Cephalalgia. 2016;36:185–193. doi: 10.1177/0333102415584313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ling YH, Chen SP, Fann CS, Wang SJ, Wang YF. TRPM8 genetic variant is associated with chronic migraine and allodynia. J Headache Pain. 2019;20:115. doi: 10.1186/s10194-019-1064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem. 1999;274:7325–7333. doi: 10.1074/jbc.274.11.7325. [DOI] [PubMed] [Google Scholar]
- 103.Shibasaki K. TRPV4 activation by thermal and mechanical stimuli in disease progression. Lab Invest. 2020;100:218–223. doi: 10.1038/s41374-019-0362-2. [DOI] [PubMed] [Google Scholar]
- 104.Barabas ME, Kossyreva EA, Stucky CL. TRPA1 is functionally expressed primarily by IB4-binding, non-peptidergic mouse and rat sensory neurons. PLoS One. 2012;7:e47988. doi: 10.1371/journal.pone.0047988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 107.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 108.Koivisto A, Jalava N, Bratty R, Pertovaara A. TRPA1 antagonists for pain relief. Pharmaceuticals (Basel) 2018;11:117. doi: 10.3390/ph11040117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nassini R, Materazzi S, Benemei S, Geppetti P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol. 2014;167:1–43. doi: 10.1007/112_2014_18. [DOI] [PubMed] [Google Scholar]
- 110.Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De Siena G, et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain. 2012;135:376–390. doi: 10.1093/brain/awr272. [DOI] [PubMed] [Google Scholar]
- 111.Jiang L, Wang Y, Xu Y, Ma D, Wang M. The Transient Receptor Potential Ankyrin Type 1 Plays a Critical Role in Cortical Spreading Depression. Neuroscience. 2018;382:23–34. doi: 10.1016/j.neuroscience.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 112.Jiang L, Ma D, Grubb BD, Wang M. ROS/TRPA1/CGRP signaling mediates cortical spreading depression. J Headache Pain. 2019;20:25. doi: 10.1186/s10194-019-0978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kheradpezhouh E, Choy JMC, Daria VR, Arabzadeh E. TRPA1 expression and its functional activation in rodent cortex. Open Biol. 2017;7:160314. doi: 10.1098/rsob.160314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Warfvinge K, Edvinsson L. Distribution of CGRP and CGRP receptor components in the rat brain. Cephalalgia. 2019;39:342–353. doi: 10.1177/0333102417728873. [DOI] [PubMed] [Google Scholar]
- 115.Shatillo A, Koroleva K, Giniatullina R, Naumenko N, Slastnikova AA, Aliev RR, et al. Cortical spreading depression induces oxidative stress in the trigeminal nociceptive system. Neuroscience. 2013;253:341–349. doi: 10.1016/j.neuroscience.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 116.Sato H, Shibata M, Shimizu T, Shibata S, Toriumi H, Ebine T, et al. Differential cellular localization of antioxidant enzymes in the trigeminal ganglion. Neuroscience. 2013;248:345–358. doi: 10.1016/j.neuroscience.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 117.Trevisan G, Benemei S, Materazzi S, De Logu F, De Siena G, Fusi C, et al. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain. 2016;139:1361–1377. doi: 10.1093/brain/aww038. [DOI] [PubMed] [Google Scholar]
- 118.De Logu F, Nassini R, Materazzi S, Carvalho Goncalves M, Nosi D, Rossi Degl’Innocenti D, et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat Commun. 1887;2017:8. doi: 10.1038/s41467-017-01739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain. 2011;152:38–44. doi: 10.1016/j.pain.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kunkler PE, Zhang L, Johnson PL, Oxford GS, Hurley JH. Induction of chronic migraine phenotypes in a rat model after environmental irritant exposure. Pain. 2018;159:540–549. doi: 10.1097/j.pain.0000000000001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Silberstein SD. Preventive migraine treatment. Continuum (Minneap Minn) 2015;21:973–989. doi: 10.1212/CON.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol Ther. 2008;120:157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 123.Marone IM, De Logu F, Nassini R, De Carvalho Goncalves M, Benemei S, Ferreira J, et al. TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain. 2018;141:2312–2328. doi: 10.1093/brain/awy177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koroleva K, Mustafina A, Yakovlev A, Hermann A, Giniatullin R, Sitdikova G. Receptor Mechanisms Mediating the Pro-Nociceptive Action of Hydrogen Sulfide in Rat Trigeminal Neurons and Meningeal Afferents. Front Cell Neurosci. 2017;11:226. doi: 10.3389/fncel.2017.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Benemei S, De Logu F, Li Puma S, Marone IM, Coppi E, Ugolini F, et al. The anti-migraine component of butterbur extracts, isopetasin, desensitizes peptidergic nociceptors by acting on TRPA1 cation channel. Br J Pharmacol. 2017;174:2897–2911. doi: 10.1111/bph.13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nagatomo K, Kubo Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc Natl Acad Sci U S A. 2008;105:17373–17378. doi: 10.1073/pnas.0809769105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Andersson DA, Gentry C, Alenmyr L, Killander D, Lewis SE, Andersson A, et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Delta(9)-tetrahydrocannabiorcol. Nat Commun. 2011;2:551. doi: 10.1038/ncomms1559. [DOI] [PubMed] [Google Scholar]
- 128.Mirrasekhian E, Nilsson JLA, Shionoya K, Blomgren A, Zygmunt PM, Engblom D, et al. The antipyretic effect of paracetamol occurs independent of transient receptor potential ankyrin 1-mediated hypothermia and is associated with prostaglandin inhibition in the brain. FASEB J. 2018;32:5751–5759. doi: 10.1096/fj.201800272R. [DOI] [PubMed] [Google Scholar]
- 129.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 131.Delany NS, Hurle M, Facer P, Alnadaf T, Plumpton C, Kinghorn I, et al. Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2. Physiol Genomics. 2001;4:165–174. doi: 10.1152/physiolgenomics.2001.4.3.165. [DOI] [PubMed] [Google Scholar]
- 132.Liedtke W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J Physiol. 2005;567:53–58. doi: 10.1113/jphysiol.2005.088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lindy AS, Parekh PK, Zhu R, Kanju P, Chintapalli SV, Tsvilovskyy V, et al. TRPV channel-mediated calcium transients in nociceptor neurons are dispensable for avoidance behaviour. Nat Commun. 2014;5:4734. doi: 10.1038/ncomms5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100(Suppl 2):14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liedtke W. TRPV4 as osmosensor: a transgenic approach. Pflugers Arch. 2005;451:176–180. doi: 10.1007/s00424-005-1449-8. [DOI] [PubMed] [Google Scholar]
- 136.Moore C, Liedtke WB. Osmomechanical-sensitive TRPV channels in mammals. Neurobiology of TRP Channels. 2nd ed. Boca Raton (FL): CRC Press/Taylor & Francis; 2017, 85–94. [PubMed]
- 137.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 138.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol. 2004;286:C195–C205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 139.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garcia-Elias A, Mrkonjic S, Jung C, Pardo-Pastor C, Vicente R, Valverde MA. The TRPV4 channel. Handb Exp Pharmacol. 2014;222:293–319. doi: 10.1007/978-3-642-54215-2_12. [DOI] [PubMed] [Google Scholar]
- 141.White JP, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol Rev. 2016;96:911–973. doi: 10.1152/physrev.00016.2015. [DOI] [PubMed] [Google Scholar]
- 142.Dias FC, Alves VS, Matias DO, Figueiredo CP, Miranda ALP, Passos GF, et al. The selective TRPV4 channel antagonist HC-067047 attenuates mechanical allodynia in diabetic mice. Eur J Pharmacol. 2019;856:172408. doi: 10.1016/j.ejphar.2019.172408. [DOI] [PubMed] [Google Scholar]
- 143.Kanju P, Chen Y, Lee W, Yeo M, Lee SH, Romac J, et al. Small molecule dual-inhibitors of TRPV4 and TRPA1 for attenuation of inflammation and pain. Sci Rep. 2016;6:26894. doi: 10.1038/srep26894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen Y, Yang C, Wang ZJ. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience. 2011;193:440–451. doi: 10.1016/j.neuroscience.2011.06.085. [DOI] [PubMed] [Google Scholar]
- 145.Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, et al. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008;134:2059–2069. doi: 10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen Y, Williams SH, McNulty AL, Hong JH, Lee SH, Rothfusz NE, et al. Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. Pain. 2013;154:1295–1304. doi: 10.1016/j.pain.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen Y, Kanju P, Fang Q, Lee SH, Parekh PK, Lee W, et al. TRPV4 is necessary for trigeminal irritant pain and functions as a cellular formalin receptor. Pain. 2014;155:2662–2672. doi: 10.1016/j.pain.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moore C, Cevikbas F, Pasolli HA, Chen Y, Kong W, Kempkes C, et al. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc Natl Acad Sci U S A. 2013;110:E3225–E3234. doi: 10.1073/pnas.1312933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Swain SM, Romac JM, Shahid RA, Pandol SJ, Liedtke W, Vigna SR, et al. TRPV4 channel opening mediates pressure-induced pancreatitis initiated by Piezo1 activation. J Clin Invest. 2020;130:2527–2541. doi: 10.1172/JCI134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li L, Liu C, Chen L, Chen L. Hypotonicity modulates tetrodotoxin-sensitive sodium current in trigeminal ganglion neurons. Mol Pain. 2011;7:27. doi: 10.1186/1744-8069-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu L, Chen L, Liedtke W, Simon SA. Changes in osmolality sensitize the response to capsaicin in trigeminal sensory neurons. J Neurophysiol. 2007;97:2001–2015. doi: 10.1152/jn.00887.2006. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Y, Wang YH, Ge HY, Arendt-Nielsen L, Wang R, Yue SW. A transient receptor potential vanilloid 4 contributes to mechanical allodynia following chronic compression of dorsal root ganglion in rats. Neurosci Lett. 2008;432:222–227. doi: 10.1016/j.neulet.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 153.Rajasekhar P, Poole DP, Liedtke W, Bunnett NW, Veldhuis NA. P2Y1 Receptor Activation of the TRPV4 Ion Channel Enhances Purinergic Signaling in Satellite Glial Cells. J Biol Chem. 2015;290:29051–29062. doi: 10.1074/jbc.M115.689729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kanju P, Liedtke W. Pleiotropic function of TRPV4 ion channels in the central nervous system. Exp Physiol. 2016;101:1472–1476. doi: 10.1113/EP085790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Z, Zhou L, An D, Xu W, Wu C, Sha S, et al. TRPV4-induced inflammatory response is involved in neuronal death in pilocarpine model of temporal lobe epilepsy in mice. Cell Death Dis. 2019;10:386. doi: 10.1038/s41419-019-1612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, et al. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience. 2007;148:876–892. doi: 10.1016/j.neuroscience.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 157.Konno M, Shirakawa H, Iida S, Sakimoto S, Matsutani I, Miyake T, et al. Stimulation of transient receptor potential vanilloid 4 channel suppresses abnormal activation of microglia induced by lipopolysaccharide. Glia. 2012;60:761–770. doi: 10.1002/glia.22306. [DOI] [PubMed] [Google Scholar]
- 158.Lu KT, Huang TC, Tsai YH, Yang YL. Transient receptor potential vanilloid type 4 channels mediate Na–K–Cl-co-transporter-induced brain edema after traumatic brain injury. J Neurochem. 2017;140:718–727. doi: 10.1111/jnc.13920. [DOI] [PubMed] [Google Scholar]
- 159.Ferrari MD, Klever RR, Terwindt GM, Ayata C, van den Maagdenberg AM. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65–80. doi: 10.1016/S1474-4422(14)70220-0. [DOI] [PubMed] [Google Scholar]
- 160.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123(Pt 8):1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 161.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 162.Levy D, Strassman AM. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J Neurophysiol. 2002;88:3021–3031. doi: 10.1152/jn.00029.2002. [DOI] [PubMed] [Google Scholar]
- 163.Wei X, Edelmayer RM, Yan J, Dussor G. Activation of TRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia. 2011;31:1595–1600. doi: 10.1177/0333102411427600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Duan J, Xie J, Deng T, Xie X, Liu H, Li B, et al. Exposure to both formaldehyde and high relative humidity exacerbates allergic asthma by activating the TRPV4-p38 MAPK pathway in Balb/c mice. Environ Pollut. 2020;256:113375. doi: 10.1016/j.envpol.2019.113375. [DOI] [PubMed] [Google Scholar]
- 165.Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hassler SN, Ahmad FB, Burgos-Vega CC, Boitano S, Vagner J, Price TJ, et al. Protease activated receptor 2 (PAR2) activation causes migraine-like pain behaviors in mice. Cephalalgia. 2019;39:111–122. doi: 10.1177/0333102418779548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Poole DP, Amadesi S, Veldhuis NA, Abogadie FC, Lieu T, Darby W, et al. Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. J Biol Chem. 2013;288:5790–5802. doi: 10.1074/jbc.M112.438184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lawhorn BG, Brnardic EJ, Behm DJ. Recent advances in TRPV4 agonists and antagonists. Bioorg Med Chem Lett. 2020;30:127022. doi: 10.1016/j.bmcl.2020.127022. [DOI] [PubMed] [Google Scholar]
- 169.Dedov VN, Roufogalis BD. Mitochondrial calcium accumulation following activation of vanilloid (VR1) receptors by capsaicin in dorsal root ganglion neurons. Neuroscience. 2000;95:183–188. doi: 10.1016/s0306-4522(99)00423-6. [DOI] [PubMed] [Google Scholar]
- 170.Szoke E, Seress L, Szolcsanyi J. Neonatal capsaicin treatment results in prolonged mitochondrial damage and delayed cell death of B cells in the rat trigeminal ganglia. Neuroscience. 2002;113:925–937. doi: 10.1016/s0306-4522(02)00208-7. [DOI] [PubMed] [Google Scholar]