Abstract
Background
Omadacycline is a semisynthetic aminomethylcycline antibacterial derived from the tetracycline class. It is approved in the USA to treat adults with acute bacterial skin and skin-structure infections and community-acquired bacterial pneumonia.
Objectives
This phase I, open-label study evaluated the effect of a potential drug–drug interaction of verapamil—a known P-glycoprotein (P-gp) inhibitor—with omadacycline on the pharmacokinetic profile of omadacycline in healthy adults. The safety and tolerability of omadacycline taken alone and in combination with verapamil were also evaluated.
Methods
A single oral dose of 240 mg verapamil extended release (ER) was given 2 h prior to a single oral dose of 300 mg omadacycline.
Results
Ten (83.3%) of the 12 participants enrolled in the study completed the study, and all enrolled participants were included in the safety and pharmacokinetic populations. An increase of 14–25% in systemic exposure to omadacycline was seen when administered following a single oral dose of 240 mg verapamil ER compared with omadacycline alone, as measured by the area under the concentration–time curve (AUC) from time 0 to 24 h after dosing (AUC0–24), from time 0 to the last quantifiable concentration (AUC0–t), from time 0 extrapolated to infinity (AUC0–inf), and by maximum (peak) observed plasma concentration (Cmax). Treatment-emergent adverse events were reported by one participant (nausea and headache).
Conclusions
These findings suggest that, if given with a known P-gp inhibitor, dose adjustment of oral omadacycline is not warranted based on small increases in absorption and systemic exposure. No safety signals were identified.
Electronic supplementary material
The online version of this article (10.1007/s13318-020-00651-3) contains supplementary material, which is available to authorized users.
Key Points
| P-glycoprotein inhibitors have a small effect on the oral absorption of omadacycline. |
| Based on the absence of safety concerns, dose adjustments for oral omadacycline are not required when administered with a known P-glycoprotein inhibitor. |
Introduction
Omadacycline, a semisynthetic aminomethylcycline antibacterial derived from the tetracycline class [1], was approved in the USA in October 2018 to treat acute bacterial skin and skin-structure infections [2, 3] and community-acquired bacterial pneumonia (CABP) [4]. Omadacycline has shown favorable safety and tolerability profiles, demonstrated high clinical success, and was found to be noninferior to comparator antibiotics.
Intravenous (IV) and oral formulations of omadacycline have been evaluated in more than 20 phase I clinical studies [5–7]. Phase I studies have assessed the pharmacokinetic profiles of IV and oral doses of omadacycline, and have demonstrated that a 300 mg oral dose provides comparable exposure to that achieved with a 100 mg IV dose [5–9]. Based on in vitro studies, omadacycline has a low propensity to permeate across the gastrointestinal (GI) tract as a substrate, but not as an inhibitor, of P-glycoprotein (P-gp) [10].
The primary objective of this phase I, open-label study was to evaluate the effect of a potential drug–drug interaction of verapamil—a known P-gp inhibitor—with omadacycline on the pharmacokinetic profile of omadacycline in healthy adults. Verapamil was chosen for this study because it is one of the drugs recommended in the US Food and Drug Administration (FDA) guidance as a strong P-gp inhibitor, meaning that it can be used to assess P-gp contribution in vivo [11]. In addition, omadacycline was shown to be a substrate of P-gp (but not an inhibitor or inducer) in in vitro transporter studies in CaCo cells [12]. In accordance with the decision tree in the FDA guideline, the fact that omadacycline is a P-gp substrate necessitated an in vivo study to clinically assess the impact of a P-gp inhibitor on the exposure of oral omadacycline. Verapamil is also a known calcium channel blocker, and the pharmacokinetics of verapamil have been published [13, 14].
A coprimary objective of the study was to evaluate the effect of a light meal on the pharmacokinetics of omadacycline in healthy adults; these results have been briefly described separately [15]. A secondary objective was to evaluate the safety and tolerability of omadacycline.
Methods
Study Design
This was a phase I, open-label, three-period, single-sequence study in healthy adults. Participants underwent screening evaluations to determine eligibility within 21 days before dosing in period 1, and were admitted to the clinical site on the day before dosing (day −1 of period 1) for baseline evaluations.
On day 1 of each period, after an overnight fast, participants received the following study treatments (there was a washout of at least 4 days between the last dose in one period and the first dose in the next period): period 1 was a single oral dose of 300 mg omadacycline (2 × 150 mg tablets); period 2 was a single oral dose of 240 mg verapamil extended release (ER; 1 × 240 mg capsule) followed by a single oral dose of 300 mg omadacycline (2 × 150 mg tablets) 2 h later; and period 3 was a single oral dose of 300 mg omadacycline (2 × 150 mg tablets) with a light meal. Data for period 3 are not reported in this paper.
Study Population
Eligible participants were healthy, nonsmoking, male and female adults between 18 and 55 years of age (inclusive), with a body mass index of between 18 and 30 kg/m2 (inclusive). Participants were required to meet all inclusion and exclusion criteria and to provide written informed consent. Full details of inclusion and exclusion criteria are included in the Electronic supplementary material (ESM).
Sample Collection and Analysis
Blood samples (4 mL) for pharmacokinetic analysis of omadacycline were collected from all participants at the following time points: before dosing (predose) and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 16, and 24 h after dosing with omadacycline (periods 1 and 2).
Blood samples (4 mL) for pharmacokinetic analysis of verapamil were collected from all participants at the following time points: before dosing (predose) and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 16, and 24 h after dosing with verapamil (period 2 only).
Bioanalytical Assay
For omadacycline, an analytical method was developed and validated for the quantitative determination of omadacycline in human plasma (sodium heparin). A brief description of the method is as follows. Sodium heparin plasma (0.1 mL) was extracted by solid-phase extraction on a 96-well (reverse-phase) platform. Analysis of omadacycline was performed on a liquid chromatography/mass spectrometry system consisting of two Shimadzu LC-10AD vp pumps, a Shimadzu SCL-10AD vp pump controller, a PE 200 autosampler (PerkinElmer Corp.), a Chromolith RP 18e 4.6 × 100 mm column with a Chromolith RP 18e, 4.6 × 5 mm guard column, and a SCIEX API 4000 mass spectrometer. The internal standard used was omadacycline-d6.
The height of the m/z 557.3 → 470.0 peak for omadacycline ion was measured against the height of the m/z 563.4 → 476.3 peak for omadacycline-d6. The peak height data were integrated using the SCIEX program Analyst installed on a Windows XP platform (Analyst, v.1.4.2; Applied Biosystems, Foster City, CA, USA). Concentration data and statistics were obtained by a laboratory information management system (LIMS; Watson LIMS, v.7.2.0.01, Thermo Fisher Scientific, Philadelphia, PA, USA). The concentration range of the validated omadacycline assay was 20–2000 ng/mL, and the inter-run precision and accuracy were ≤ 3.8% over this concentration range. Samples below the lower limit of quantification were treated as zero; samples above the upper limit of quantification were diluted and reanalyzed.
For verapamil, an analytical method was developed and validated for the quantitative determination of verapamil in human plasma (K2EDTA). A brief description of the method is as follows. K2EDTA plasma (0.050 mL) was extracted by protein precipitation and analyzed on a liquid chromatography/mass spectrometry system consisting of two Shimadzu LC-10AD vp pumps, a Quadra-96 model 320 workstation, a Waters XBridge C18 5 micron 2.1 × 30 mm column, and a SCIEX API 4000 mass spectrometer. The internal standard used was verapamil-d6.
The area of the m/z 455.3 → 165.2 peak for verapamil ion was measured against the area of the m/z 461.3 → 165.2 peak for verapamil-d6. The peak area data were integrated by the SCIEX program Analyst installed on a Windows XP platform (Analyst, v.1.4.2; Applied Biosystems). Concentration data and statistics were obtained by a LIMS (Watson LIMS, v.7.2.0.01; Thermo Fisher Scientific). The concentration range of the validated verapamil assay was 0.2–200 ng/mL, and the inter-run precision and accuracy was ≤ 5.7% over this concentration range.
Samples below the lower limit of quantification were treated as zero; samples above the upper limit of quantification were diluted and reanalyzed.
Pharmacokinetic Assessments
The following pharmacokinetic parameters were calculated for omadacycline (periods 1 and 2) and verapamil (period 2 only) from plasma concentration versus actual time data for each participant using noncompartmental analysis: area under the plasma concentration versus time curve (AUC) from time 0 to 24 h after dosing (AUC0–24); AUC from time 0 to the last quantifiable concentration (AUC0–t); AUC from time 0 extrapolated to infinity (AUC0–inf); maximum (peak) observed plasma concentration (Cmax); time to reach Cmax (tmax); terminal elimination half-life (t1/2); and terminal phase rate constant (λz). Details of the pharmacokinetic variables are included in the ESM.
Safety and Tolerability Assessments
Safety and tolerability were assessed by monitoring and recording adverse events (AEs), clinical laboratory test results (hematology, serum chemistry, and urinalysis), vital sign measurements, 12-lead electrocardiogram (ECG) results, and physical examination findings.
Planned Sample Size and Statistical Analysis
The planned sample size (n) was 12 participants. Although the sample size was not based on a formal calculation of statistical power, it was considered sufficient to assess the safety and pharmacokinetics of omadacycline. The pharmacokinetic parameters were summarized by treatment using the following descriptive statistics: n, mean, standard deviation (SD), median, geometric mean (AUC and Cmax only), coefficient of variation, minimum, and maximum. A linear mixed-effect model (SAS PROC MIXED) with treatment as a fixed effect and participant as a random effect was fitted to the natural log-transformed pharmacokinetic parameters AUC0–24, AUC0–t, AUC0–inf, and Cmax to estimate effects and construct confidence intervals (CI) to use when comparing omadacycline with verapamil (treatment B, test treatment) to omadacycline alone (treatment A, reference treatment). Point estimates and 90% CIs for differences on the log scale were exponentiated to obtain estimates for the ratios of geometric means and respective 90% CIs on the original scale (criterion interval, 80–125%). No adjustment was made for multiplicity. A forest plot was used to determine the ratio of the geometric least squares mean and the 90% CI. For tmax, the Wilcoxon signed-rank test was performed.
Treatment-emergent AEs (TEAEs) were defined as any events that were not present before study drug exposure or any events that were already present but worsened in intensity or frequency after exposure to the study drug. All TEAEs were summarized by treatment, system organ class, preferred term, relationship to study drug, and severity. All AEs, TEAEs, serious AEs (including deaths), and AEs that led to early discontinuation were presented in data listings. Safety data summaries were provided at the study level for clinical laboratory test results and ECG measurements and at the treatment level (by treatment received) for vital sign measurements (blood pressure and heart rate).
Results
Participant Demographics and Baseline Clinical Characteristics
Twelve male participants aged 20–45 years were enrolled in the study (Table 1). Ten participants (83.3%) completed the study and two (16.7%) were discontinued from the study for failing to meet all criteria for continued participation (i.e., they had an out-of-range heart rate at check-in for period 2). All 12 enrolled participants (100%) were included in the safety and pharmacokinetic populations in period 1; as two subjects discontinued the study, the ten remaining subjects were included in period 2. Baseline demographics and clinical characteristics remained consistent.
Table 1.
Participant demographics and baseline clinical characteristics
| Characteristic | Period 1 (N = 12) | Period 2 (N = 10)a |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 34.4 (7.7) | 36.6 (6.1) |
| Median | 34.0 | 36.0 |
| Minimum, maximum | 20, 45 | 27, 45 |
| Sex, n (%) | ||
| Male | 12 (100.0) | 10 (100.0) |
| Race, n (%) | ||
| White | 5 (41.7) | 5 (50) |
| Black or African American | 5 (41.7) | 5 (50) |
| Asian | 1 (8.3) | 0 |
| Multiracial | 1 (8.3) | 0 |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 5 (41.7) | 5 (50) |
| Not Hispanic or Latino | 7 (58.3) | 5 (50) |
| Height (cm) | ||
| Mean (SD) | 178.3 (6.1) | 178.6 (4.3) |
| Median | 179.4 | 179.4 |
| Minimum, maximum | 166.2, 188.1 | 171.8, 185.3 |
| Weight (kg) | ||
| Mean (SD) | 78.9 (11.1) | 81.2 (8.4) |
| Median | 80.4 | 80.4 |
| Minimum, maximum | 53.5, 99.8 | 71.3, 99.8 |
| Body mass index (kg/m2) | ||
| Mean (SD) | 24.7 (2.6) | 25.4 (2.0) |
| Median | 24.6 | 25.1 |
| Minimum, maximum | 19.4, 29.1 | 23.0, 29.1 |
SD standard deviation
aAll 12 enrolled participants (100%) were included in the safety and pharmacokinetic populations in period 1; as two subjects discontinued the study, the ten remaining subjects were included in period 2
Pharmacokinetics
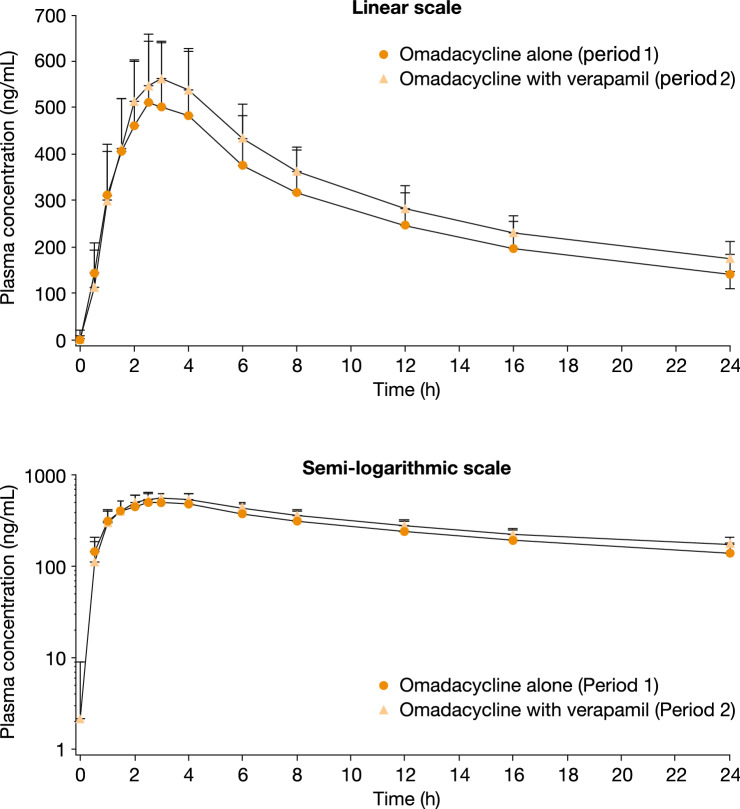
The overall shapes of the mean omadacycline plasma concentration–time profiles obtained after a single oral dose of omadacycline alone (period 1) and after a single oral dose of verapamil followed by a single oral dose of omadacycline (period 2) were similar (Fig. 1). Based on visual inspection, the Cmax of omadacycline was attained within approximately 3 h postdose for both treatment periods, with concentrations measurable up to 24 h (i.e., the last sampling time) in all instances. In period 2, Cmax of verapamil was attained within approximately 12 h postdose, with concentrations measurable from 2 h after administration up to 24 h (i.e., the last sampling time) (data not shown).
Fig. 1.
Mean (SD) concentration of omadacycline versus time (pharmacokinetic population)
Exposure to omadacycline in plasma was higher when omadacycline was administered following verapamil (period 2), compared with the administration of omadacycline alone (period 1) (Table 2). There were no appreciable differences in the t1/2 of omadacycline between period 1 (arithmetic mean 12.7 h) and period 2 (arithmetic mean 14.0 h), and the median tmax was 2.8 h for both treatment periods.
Table 2.
Descriptive summary of plasma pharmacokinetic parameters of omadacycline by treatment (pharmacokinetic population)
| Parameter (unit) | Period 1 Omadacycline alone (N = 12) |
Period 2 Omadacycline with verapamil (N = 10) |
|---|---|---|
| AUC0–24 (ng·h/mL)a | 6189.2 (26.8) | 7269.4 (14.0) |
| AUC0–t (ng·h/mL)a | 6189.2 (26.8) | 7271.7 (14.0) |
| AUC0–inf (ng·h/mL)a | 8691.0 (28.7) | 10,775.2 (17.8) |
| Cmax (ng/mL)a | 534.3 (23.7) | 595.7 (12.6) |
| tmax (h)b | 2.8 (1.5, 4.0) | 2.8 (1.5, 4.0) |
| t1/2 (h)c | 12.7 (12.1) | 14.0 (10.3) |
| λz (l/h)c | 0.1 (11.2) | 0.05 (10.7) |
AUC0–inf estimates are not precise, as %AUCextrap was greater than 20% in all instances
AUC area under the plasma concentration versus time curve, AUC0–24 AUC from time 0 to 24 h after dosing, AUC0–t AUC from time 0 to the last quantifiable concentration, AUC0–inf AUC from time 0 extrapolated to infinity, Cmax maximum (peak) observed plasma concentration, %AUCextrap AUC extrapolated from time t to infinity as a percentage of total AUC, tmax time to reach Cmax, t1/2 terminal elimination half-life, λz, terminal phase rate constant
aGeometric mean (arithmetic coefficient of variation) values are presented
bMedian (minimum, maximum) values are presented
cArithmetic mean (arithmetic coefficient of variation) values are presented
The statistical analysis of plasma pharmacokinetic parameters for omadacycline is summarized in Table 3. Following a single oral dose of verapamil (period 2), the AUC measures (AUC0–24, AUC0–t, and AUC0–inf) were 18–25% higher and the Cmax of omadacycline was 14% higher than those obtained following administration of omadacycline alone (period 1). For the tested pharmacokinetic parameters (AUC0–24, AUC0–t, AUC0–inf, and Cmax), a statistically significant effect of a single oral dose of verapamil ER on the pharmacokinetics of omadacycline was confirmed, as the 90% CIs of the geometric least squares mean ratios (test/reference) were not contained within the criterion interval of 80–125%.
Table 3.
Statistical analysis of plasma pharmacokinetic parameters for omadacycline (pharmacokinetic population)
| Parameter (unit) | Treatment | N | Geometric LS mean | Treatment comparison | Ratio of geometric LS mean (%) | 90% CI of ratio |
|---|---|---|---|---|---|---|
| AUC0–24 (ng·h/mL) | Period 1 | 12 | 6189.2 | Period 2/Period 1 | 118.5 | (103.4, 135.9) |
| Period 2 | 10 | 7335.4 | ||||
| AUC0–t (ng·h/mL) | Period 1 | 12 | 6189.2 | Period 2/Period 1 | 118.6 | (103.4, 135.9) |
| Period 2 | 10 | 7337.8 | ||||
| AUC0–inf (ng·h/mL) | Period 1 | 12 | 8691.0 | Period 2/Period 1 | 124.6 | (108.5, 143.1) |
| Period 2 | 10 | 10,828.0 | ||||
| Cmax (ng/mL) | Period 1 | 12 | 534.3 | Period 2/Period 1 | 113.6 | (101.3, 127.4) |
| Period 2 | 10 | 606.8 |
A linear mixed-effect model with treatment as a fixed effect and participant as a random effect was fitted to the natural log-transformed pharmacokinetic parameters
AUC0–inf estimates are not precise, as %AUCextrap was greater than 20% in all instances
Period 1: omadacycline alone
Period 2: omadacycline with verapamil
AUC area under the plasma concentration versus time curve, AUC0–24 AUC from time 0 to 24 h after dosing, AUC0–t AUC from time 0 to the last quantifiable concentration, AUC0–inf AUC from time 0 extrapolated to infinity, CI confidence interval, Cmax maximum (peak) observed plasma concentration, LS least squares, %AUCextrap AUC extrapolated from time t to infinity as a percentage of total AUC
Safety
Ten participants completed all three treatment periods. Two participants completed period 1 but were discontinued at baseline for period 2 due to heart rate > 90 beats/minute, which exceeded the protocol-specified range for continued participation (i.e., 55–90 beats/minute). These heart rate values were not considered to be related to omadacycline because they occurred 3 days after the single omadacycline dose in period 1, and the immediately preceding values (at 24 h postdose) were within the permitted range.
The treatments were safe and well tolerated in periods 1 and 2. Overall, one participant (8.3%) experienced a total of three TEAEs during the study (nausea was reported during periods 1 and 2, and headache during period 2). All TEAEs were mild in severity and assessments performed by the investigator indicated that they were related to the study drug. No deaths, serious AEs, or TEAEs leading to study discontinuation were reported, and all TEAEs were resolved by the end of the study. There were no clinically relevant findings in the clinical laboratory test results, vital sign measurements, physical examination findings, or 12-lead ECG results.
Discussion
The results of this phase I drug interaction study show that systemic exposure to omadacycline increased when omadacycline was administered following a single oral dose of 240 mg verapamil ER. Compared with omadacycline alone, verapamil dosing increased the geometric mean of the AUC0–inf of omadacycline by 18–25%, and Cmax was increased by approximately 14%. Verapamil did not appear to affect the rate of absorption of omadacycline, since the median tmax was the same (2.8 h) in both reported treatment periods. It also appears that verapamil did not affect the elimination t1/2 of omadacycline, although the t1/2 estimates in this study may not be precise because the sampling time was 24 h.
Plasma analysis of verapamil was performed to confirm measurable concentrations of the drug throughout the 24-h interval for period 2; the ER formulation of verapamil was used, so it was not possible to accurately estimate its half-life or AUC0–inf. Plasma concentrations are a reasonable surrogate, as the interaction with omadacycline occurs due to P-gp within the mucosal lining of the GI tract, where verapamil concentrations are plausibly much higher than in blood.
The results from this study show that P-gp influences the oral absorption of omadacycline, but the effect is small. The oral bioavailability of omadacycline under fasting control conditions was 34.5%, and inhibition of P-gp increased its bioavailability by as much as 25.0% to an estimated value of 43.1% (Fig. 1). However, no clinically relevant differences in drug concentrations were observed that would warrant a dose adjustment of omadacycline when coadministered with known P-gp inhibitors. All participants in this study were male, and although some sex differences in P-gp expression have been observed in rats [16], there is no evidence of differences between men and women in terms of P-gp content in the GI tract [17].
Single oral doses of 300 mg omadacycline alone and 300 mg omadacycline plus 240 mg verapamil ER were safe and well tolerated by healthy adults. There were no clinically relevant findings in the clinical laboratory test results, vital sign measurements, physical examination findings, or 12-lead ECG results. The safety findings in the current study are similar to those reported in other published omadacycline pharmacokinetic and safety studies [7, 8, 12].
Conclusions
In conclusion, a statistically significant effect of a single oral dose of verapamil ER on the pharmacokinetics of omadacycline was confirmed. However, a dose adjustment of oral omadacycline if given with a known P-gp inhibitor is not warranted based on a small increase in absorption and systemic exposure, and no safety signals were identified.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author Contributions
All authors contributed to the study conception and design, material preparation, data collection, and analysis. All authors reviewed and commented on previous versions of the manuscript, and approved the final manuscript.
Declarations
Funding
This work was funded by Paratek Pharmaceuticals, Inc., King of Prussia, PA. Medical writing and editorial assistance was provided by Theresa Singleton, PhD, and Linda Edmondson of Innovative Strategic Communications, LLC, which was funded by Paratek Pharmaceuticals, Inc.
Open Access
Open access publication of this manuscript was sponsored by Paratek Pharmaceuticals, Inc.
Conflict of Interest
A.M. is an employee of Paratek Pharmaceuticals, Inc. P.C.M. is a former employee of, has received consulting fees from, and is a shareholder of Paratek Pharmaceuticals, Inc. E.T. is a former employee and current shareholder of Paratek Pharmaceuticals, Inc. S.B. is a consultant to Paratek Pharmaceuticals Inc. in matters relating to clinical pharmacology and/or drug disposition, including pharmacokinetics, aspects in study design, and data interpretation. S.C. is a consultant to Paratek Pharmaceuticals Inc. T.L.H. has no conflicts of interest to declare.
Consent to participate
Written and signed informed consent was obtained from each study participant before any protocol-specific assessment was performed.
Consent to publish
Not applicable; patient level data are not included in this manuscript.
Code availability
Not-applicable.
Ethical approval and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board or independent ethics committee for each study center, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written and signed informed consent was obtained from each study participant before any protocol-specific assessment was performed. The study was described by investigators and written information was given to each participant.
Data availability
Paratek Pharmaceuticals has a commitment to ensure that access to clinical trial data is available to regulators, researchers, and trial participants, when permitted, feasible and appropriate. Requests for de-identified patient-level data may be submitted to medinfo@paratekpharma.com for review. Datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The Food and Drug Administration 2019 Advisory Committee report on omadacycline can be accessed here: https://www.fda.gov/media/115089/download.
References
- 1.Honeyman L, Ismail M, Nelson ML, Bhatia B, Bowser TE, Chen J, et al. Structure-activity relationship of the aminomethylcyclines and the discovery of omadacycline. Antimicrob Agents Chemother. 2015;59(11):7044–7053. doi: 10.1128/AAC.01536-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrahamian FM, Sakoulas G, Tzanis E, Manley A, Steenbergen J, Das AF, et al. Omadacycline for acute bacterial skin and skin structure infections. Clin Infect Dis. 2019;69(Supplement 1):S23–S32. doi: 10.1093/cid/ciz396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Riordan W, Cardenas C, Shin E, Sirbu A, Garrity-Ryan L, Das AF, et al. Once-daily oral omadacycline versus twice-daily oral linezolid for acute bacterial skin and skin structure infections (OASIS-2): a phase 3, double-blind, multicentre, randomised, controlled, non-inferiority trial. Lancet Infect Dis. 2019;19(10):1080–1090. doi: 10.1016/S1473-3099(19)30275-0. [DOI] [PubMed] [Google Scholar]
- 4.Stets R, Popescu M, Gonong JR, Mitha I, Nseir W, Madej A, et al. Omadacycline for community-acquired bacterial pneumonia. N Engl J Med. 2019;380(6):517–527. doi: 10.1056/NEJMoa1800201. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka SK, Steenbergen J, Villano S. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg Med Chem. 2016;24(24):6409–6419. doi: 10.1016/j.bmc.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Sun H, Ting L, Machineni S, Praestgaard J, Kuemmell A, Stein DS, et al. Randomized, open-label study of the pharmacokinetics and safety of oral and intravenous administration of omadacycline to healthy subjects. Antimicrob Agents Chemother. 2016;60(12):7431–7435. doi: 10.1128/AAC.01393-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bundrant LA, Tzanis E, Garrity-Ryan L, Bai S, Chitra S, Manley A, et al. Safety and pharmacokinetics of the aminomethylcycline antibiotic omadacycline administered to healthy subjects in oral multiple-dose regimens. Antimicrob Agents Chemother. 2018;62(2):e01487-17. [DOI] [PMC free article] [PubMed]
- 8.Berg JK, Tzanis E, Garrity-Ryan L, Bai S, Chitra S, Manley A, et al. Pharmacokinetics and safety of omadacycline in subjects with impaired renal function. Antimicrob Agents Chemother. 2018;62(2):e02057-17. [DOI] [PMC free article] [PubMed]
- 9.Villano S, Steenbergen J, Loh E. Omadacycline: development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections. Future Microbiol. 2016;11:1421–1434. doi: 10.2217/fmb-2016-0100. [DOI] [PubMed] [Google Scholar]
- 10.Flarakos J, Du Y, Gu H, Wang L, Einolf HJ, Chun DY, et al. Clinical disposition, metabolism and in vitro drug-drug interaction properties of omadacycline. Xenobiotica. 2017;47(8):682–696. doi: 10.1080/00498254.2016.1213465. [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. Drug–drug interactions. FDA draft guidance for industry. Silver Spring: US FDA; 2012.
- 12.Flarakos J, Du Y, Gu H, Wang L, Einolf HJ, Chun DY, et al. Clinical disposition, metabolism and in vitro drug-drug interaction properties of omadacycline. Xenobiotica. 2017;47(8):682–696. doi: 10.1080/00498254.2016.1213465. [DOI] [PubMed] [Google Scholar]
- 13.Hamann SR, Blouin RA, McAllister RG., Jr Clinical pharmacokinetics of verapamil. Clin Pharmacokinet. 1984;9(1):26–41. doi: 10.2165/00003088-198409010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Mattila J, Mäntylä R, Taskinen J, Männistö P. Pharmacokinetics of sustained-release verapamil after a single administration and at steady state. Eur J Drug Metab Pharmacokinet. 1985;10(2):133–138. doi: 10.1007/BF03189707. [DOI] [PubMed] [Google Scholar]
- 15.Rodvold KA, Pai MP. Pharmacokinetics and pharmacodynamics of oral and intravenous omadacycline. Clin Infect Dis. 2019;69(Supplement 1):S16–S22. doi: 10.1093/cid/ciz309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afonso-Pereira F, Dou L, Trenfield SJ, Madla CM, Murdan S, Sousa J, et al. Sex differences in the gastrointestinal tract of rats and the implications for oral drug delivery. Eur J Pharm Sci. 2018;30(115):339–344. doi: 10.1016/j.ejps.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 17.Paine MF, Ludington SS, Chen ML, Stewart PW, Huang SM, Watkins PB. Do men and women differ in proximal small intestinal CYP3A or P-glycoprotein expression? Drug Metab Dispos. 2005;33(3):426–433. doi: 10.1124/dmd.104.002469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Paratek Pharmaceuticals has a commitment to ensure that access to clinical trial data is available to regulators, researchers, and trial participants, when permitted, feasible and appropriate. Requests for de-identified patient-level data may be submitted to medinfo@paratekpharma.com for review. Datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The Food and Drug Administration 2019 Advisory Committee report on omadacycline can be accessed here: https://www.fda.gov/media/115089/download.