Abstract
Background and Objectives
Cariprazine, a dopamine D3-preferring D3/D2 receptor partial agonist, is approved for the treatment of adults with schizophrenia (1.5–6 mg/day) and manic/mixed (3–6 mg/day) episodes associated with bipolar I disorder. This population pharmacokinetic analysis describes the concentration-time profiles of cariprazine and its two major active metabolites, desmethyl-cariprazine (DCAR) and didesmethyl-cariprazine (DDCAR). Additionally, the potential impact of patient characteristics, creatinine clearance, and cytochrome P450 2D6 (CYP2D6) metabolizer status on the pharmacokinetics of cariprazine and its metabolites was evaluated.
Methods
Data from three phase 1 and ten phase 2/3 studies in adult patients with schizophrenia or bipolar mania were included. Nonlinear mixed-effects pharmacokinetic modeling was performed using the NONMEM software package. Compartmental modeling was performed sequentially with the cariprazine elimination rate used as the DCAR formation rate and likewise the elimination rate of DCAR used with a delay as the DDCAR formation rate.
Results
Cariprazine pharmacokinetics were described by a three-compartment model with zero-order input of the dose to a depot compartment followed by first-order absorption and first-order elimination. DCAR and DDCAR pharmacokinetics were described by two-compartment models with linear elimination. Statistically significant predictors of pharmacokinetic parameters included weight, sex, and race, though differences in exposures were not large enough to require an adjustment in dose. Creatinine clearance was not a statistically significant predictor of drug clearance, and a post hoc analysis found that CYP2D6 metabolizer status was not associated with changes in exposure levels for cariprazine, DCAR, or DDCAR. The median time to 90% of steady state was approximately 1 week for cariprazine and DCAR and 3 weeks for DDCAR.
Conclusions
Population pharmacokinetic modeling provided a quantitative description of the concentration-time profile of cariprazine and its metabolites.
Electronic supplementary material
The online version of this article (10.1007/s13318-020-00650-4) contains supplementary material, which is available to authorized users.
Key Points
| Population compartmental modeling was used to describe the pharmacokinetics of cariprazine and its active metabolites, desmethyl-cariprazine (DCAR) and didesmethyl-cariprazine (DDCAR). |
| Patient characteristics, such as race, sex, weight, CrCL, and CYP2D6 metabolizer status, did not significantly affect the total cariprazine exposure; thus, no dose adjustments were warranted. |
| DDCAR was the most prominent moiety at steady state, representing 64% of total cariprazine exposure, and the median time to 90% steady state was 5 days for cariprazine, 5 days for DCAR, 21 days for DDCAR, and 18 days for total cariprazine. |
Introduction
Cariprazine is a dopamine D3-preferring D3/D2 receptor partial agonist and serotonin 5-HT1A receptor partial agonist [1, 2]. Clinical trials have demonstrated the efficacy and tolerability of cariprazine in adult patients with schizophrenia [3–5] as well as in patients with manic or mixed episodes associated with bipolar I disorder [6–8]. Cariprazine is approved in the US for both indications and in the European Union for the treatment of schizophrenia; further, cariprazine is also approved in the US for the treatment of depressive episodes associated with bipolar I disorder. The pharmacokinetic properties of cariprazine are characterized by relatively slow absorption, multi-exponential disposition, and slow elimination [9]. Cariprazine is extensively metabolized by cytochrome P450 3A4 (CYP3A4) and, to a lesser extent, by cytochrome P450 2D6 (CYP2D6) to 2 active metabolites, desmethyl-cariprazine (DCAR) and didesmethyl-cariprazine (DDCAR). DCAR is further metabolized into DDCAR by CYP3A4 and CYP2D6; then, DDCAR is further metabolized by CYP3A4 to a hydroxylated metabolite, and both DCAR and DDCAR possess similar pharmacologic activity to the parent drug [10–12].
An initial evaluation was performed to assess the population pharmacokinetic profile of cariprazine using 12 clinical trials with either sparse sampling over long treatment periods or extensive sampling over short treatment periods (generally not for a sufficient length of time to capture the elimination phase of the active moieties). The final models included additional data from Study A002-A11 [11], which was specifically designed to assess pharmacokinetics in Japanese patients with schizophrenia during 12 weeks of treatment and 12 weeks post-treatment, using rich sampling to allow better definition of the elimination phase as compared to the earlier studies. This article presents the initial and final population pharmacokinetic models of cariprazine, DCAR, and DDCAR. The influence of patient characteristics, renal function, liver dysfunction classification, and classes of concomitant medications on cariprazine pharmacokinetics was also assessed. In addition, a post hoc analysis was conducted to assess the influence of CYP2D6 metabolizer status on exposure levels of cariprazine, DCAR, DDCAR, and the molar sum of all three (Total CAR).
Methods
Overview
The population pharmacokinetic modeling of cariprazine and its metabolites was performed in two stages (Supplemental Table 1). The initial analysis included data from three phase 1, three phase 2, and six phase 3 trials (Table 1) [3–5, 7, 8, 11, 13–16]. Then, the updated population analysis added an additional schizophrenia study (Study A002-A11; Table 1) with an extensive sampling scheme. Two long-term, open-label schizophrenia studies in non-hospitalized patients (RGH-MD-11 and RGH-MD-17) [14, 15] were used to further validate model fit but were excluded from the final model because of greater uncertainty in dosing records (due to self-reporting).
Table 1.
Studies included in the population pharmacokinetic analysis of cariprazine, DCAR, and DDCAR
| Study number [citation] | Development phase (design) | Study population | Planned number of patients receiving cariprazine | Planned cariprazine doses at time of PK sample collection, mg | Planned duration of active treatment, days |
|---|---|---|---|---|---|
| RGH-MD-01 [25]a | 1 | Schizophrenia | 48 (6 per cohort) | 0.5, 1, 1.5, 2, 3, 3.5, 4, 5, 5.5, 7, 7.5, 9.5, 12.5 | 22 (30 for Cohort G) |
| RGH-MD-02a | 1b | Schizophrenia | 50 | 1.5, 12, 18 | 35 |
| RGH-MD-03 [13]b | 2 (RDBPC) | Schizophrenia | 250 (125 per group) | 4.5, 12 | 42 |
| RGH-MD-04 [4]b | 3 (RDBPC) | Schizophrenia | 300 (150 per group) | 3, 6 | 42 |
| RGH-MD-05 [3]b | 3 (RDBPC) | Schizophrenia | 300 (150 per group) | 4.5, 6, 7.5, 9 | 42 |
| RGH-MD-11 [14] b,c | 3 (open-label) | Schizophrenia | 600 | 3, 6, 9 | 336 |
| RGH-MD-16 [5]b | 2b (RDBPC) | Schizophrenia | 405 (135 per group) | 1.5, 3, 4.5 | 42 |
| RGH-MD-17 [15]b,c | 2b (open-label) | Schizophrenia | 250 | 1.5, 3, 4.5 | 336 |
| RGH-MD-18a | 1b | Schizophrenia | 24 (6 per cohort) | 1.5, 3, 6, 9, 12, 15, 18, 21 | 28 |
| RGH-MD-32 [7]b | 3 (RDBPC) | Bipolar mania | 160 | 3, 6, 9, 12 | 21 |
| RGH-MD-33 [8]b | 3 (RDBPC) | Bipolar mania | 330 (165 per group) | 3, 4.5, 6, 9, 12 | 21 |
| RGH-MD-36 [16]b | 3 (open-label) | Bipolar mania | 400 | 1.5, 3, 6, 9 | 112 |
| A002-A11 [11]a,d | 2/3 (open-label) | Schizophrenia | 30 | 3, 6, 9 | 84 |
DCAR desmethyl-cariprazine, DDCAR didesmethyl-cariprazine, PK pharmacokinetic, RDBPC randomized, double-blind, placebo-controlled trial
aSerial sampling studies: serial sampling following the first dose was done in four studies, three of which included sampling up to 24 h (RGH-MD-01, RGH-MD-02, and RGH-MD-18) and one that included sampling up to 8 h (A002-A11). Serial sampling was also collected after the last dose in all four studies. For RGH-MD-01, serial sampling over a 168-h period was also done after the 22nd or 30th dose (12.5 mg); for RGH-MD-02, serial sampling over a 24-h period after the 29th dose (18 mg); for RGH-MD-18, serial sampling over a 168 to 192-h period was also done after the 28th dose (doses > 15 mg were excluded). For A002-A11, serial sampling over a 4032-h period (168 days) was also done after the 84th dose. In addition, at least one additional 24-h sampling profile was collected on an intervening dose day. Sparse samples were also collected at a variety of days between the serial samples
bSparse sampling studies: sparse sampling was done at various times across the duration of the studies. The first sample was collected on Day 5 (RGH-MD-32 and RGH-MD-33), Day 7 (RGH-MD-11, RGH-MD-17, and RGH-MD-36), or Day 14 (RGH-MD-03, RGH-MD-04, RGH-MD-05, RGH-MD-16). Sampling following the final dose occurred in all of the studies: a sample up to 24 h postdose, at 336–360 h, and 672–696 h (RGH-MD-03, RGH-MD-11, and RGH-MD-17), a sample up to 24 h postdose and at 336–360 h post dose (RGH-MD-04, RGH-MD-05, RGH-MD-16), and up to 24 h postdose and at 168–192 h (RGH-MD-32, RGH-MD-33, and RGH-MD-36)
cStudies RGH-MD-11 and RGH-MD-17 were not included in the development of the models. The final models were applied to these studies to evaluate the ability of the models to predict into longer-term trials
dThe data for this study were only available for the updated (final) model. Because the study included a serial PK sampling design, it will be treated as a Phase 1 study for the purposes of the PK modeling
Cariprazine Dosing and Sample Collection
Cariprazine was administered orally once daily at doses ranging from 0.5 to 21 mg/day across studies; uptitration schedules varied across studies with the majority reaching maximum dose by Day 9. Some studies allowed flexible dosing regimens for adverse events, tolerability issues, or inadequate response. Blood samples were collected to measure cariprazine, DCAR, and DDCAR plasma levels. Pharmacokinetic sampling was variable including full profiles and sparse samples with the majority of samples collected on the first day of dosing and ≥ 7 days after the first dose (Table 1). Plasma concentrations below the lower limit of quantification (LLOQ; 20 pg/ml for cariprazine and DCAR, 20–50 pg/ml for DDCAR) were excluded from analysis.
Model Development Overview
Models for cariprazine, DCAR, and DDCAR were developed using standard pharmacometric practices for population model development and evaluation of covariates [17], including base structural model development, fitting of the full multivariate model, backward elimination of covariates, model refinement, and model evaluation. Modeling for initial and updated datasets was performed using NONMEM 7.1.2 [18], a nonlinear mixed-effects modeling software package, in combination with SAS 9.2 [19]; the first-order conditional estimation (FOCE) and FOCE with interaction estimation methods were used.
Base Structural Model Development
Initial Dataset
Development of a base structural model for cariprazine, DCAR, and DDCAR was performed sequentially using the Individual Pharmacokinetic Parameters (IPP) method [20] and involved (1) analysis of the initial phase 1 datasets excluding samples following first dose < 1.5 mg, samples following doses of 21 mg, samples collected > 25 h postdose, and DDCAR samples following the first dose; (2) analysis of samples described in item (1) for phase 1, of samples collected within 25 h of dosing, and samples following doses < 21 mg for all phase 2/3 studies (except Studies RGH-MD-11 and RGH-MD-17).
Prior to the data deletions described above, non-linear models for elimination, distribution, and volume of distribution and target-mediated elimination models were also evaluated. The majority of the more complex models failed to minimize, and none improved the biases observed with linear models in the clinical range of the data. Additionally, goodness-of-fit plots for models with samples collected > 25 h postdose indicated the need for additional compartments; however, these samples (122 samples or approximately 4% of the Phase 1 data) could not adequately define the additional parameters (relative standard error [RSE] > 50%). Thus, data exclusions described in items (1) and (2) were made to improve the predictions of concentrations by the model for data from doses and times of clinical relevance. Furthermore, patients without cariprazine pharmacokinetic samples were excluded from DCAR and DDCAR modeling, and patients without DCAR pharmacokinetic samples were excluded from DDCAR modeling since patients would not have the necessary Bayesian parameter estimates of the preceding analytes.
Models for cariprazine, DCAR, and DDCAR were developed separately and sequentially. The final cariprazine model was incorporated as part of the DCAR model with all cariprazine model parameters fixed to the Bayesian estimates for each patient and the cariprazine elimination rate serving as the formation rate of DCAR. The DCAR model was similarly incorporated into the DDCAR model (Fig. 1). This method assumes that 100% of cariprazine is converted to DCAR, and 100% of DCAR is converted to DDCAR. Therefore, all DCAR and DDCAR parameters are apparent values. As cariprazine is minimally excreted as unchanged drug in urine (1.2% of daily administered dose) [12], clearance via the renal pathway was not included in the model.
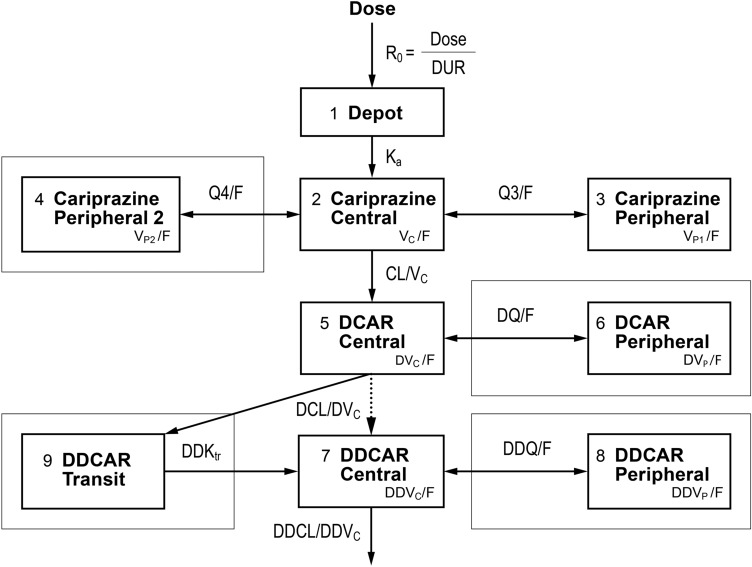
Fig. 1.
Schematic of the base structural cariprazine, DCAR, and DDCAR Pharmacokinetic Models for Evaluation. Box indicates compartments used only in final updated models, and parameter abbreviations are defined in Table 2. DCAR desmethyl-cariprazine, DDCAR didesmethyl-cariprazine
Updated Dataset
The final analysis dataset included all data (including previously excluded data) from the 13 studies with the following exceptions: (1) data following doses ≥ 15 mg/day (> 2 times maximum approved clinical dose [6 mg/day]); (2) outliers, as dictated by the population pharmacokinetic analysis; (3) DDCAR measurable plasma concentrations between the first and second dose (for serial pharmacokinetic studies, 132 of 2118 samples [6%]; for sparse sample studies, 2 of 10,108 samples [0.02%]). Concentrations collected ≥ 25 h postdose were included in the final analysis since addition of the rich sampling data for 12 weeks following the last dose (Study A002-A11) stabilized estimation of terminal elimination parameters.
The final analysis dataset included four studies with serial pharmacokinetic sampling. Three of the studies collected serial samples on Day 2 (1 Cohort), Day 4 (1 Cohort), or Day 5 (2 Cohorts). The last set of serial pharmacokinetic samples was collected on Day 22, 28, 29, 30, or 42. Of the additional nine studies with sparse pharmacokinetic samples, pharmacokinetic sampling began on Day 5, 7, or 14. Thus, there were minimal pharmacokinetic concentrations on Day 25 to assist with estimation of time-varying pharmacokinetic model parameters or non-linear pharmacokinetic model parameters. Additionally, only 2.6% of patients in the model development dataset had a measurable DDCAR concentration prior to Day 2 suggesting that the formation of DDCAR is delayed relative to the other moieties. To demonstrate the impact of the uptitration schedules and pharmacokinetic sampling schedules, a plot of the number of measurable pharmacokinetic samples per dose level and week is shown in Supplemental Fig. 1. Prior to the data deletions described above, linear models, non-linear models for elimination, distribution, and volume of distribution, and target-mediated elimination models were evaluated. The majority of the more complex models failed to minimize, and none improved the biases observed for linear pharmacokinetic models in the clinical dose range of the data. Development of the base structural model for the updated dataset followed similar sequential steps to the initial model (Fig. 1); in brief, steps were: (1) modeling data from Phase 1 Studies and Study A002-A11 as they collected serial pharmacokinetic samples; (2) modeling data from the Combined Phase 1–3 Dataset (Phase 1 Dataset + Phase 2/3 Dataset), which excluded data from RGH-MD-11 and RGH-MD-17 (Model Validation Dataset).
The differential equations used for fitting the base models are shown in Eqs. 1–4 (cariprazine), 5–6 (DCAR), and 7–9 (DDCAR) in the supplemental material (Supplemental Equation Set 1).
Covariate Analysis
Initial Model
The following covariates were selected to evaluate their potential to explain variability in pharmacokinetic parameters of cariprazine, DCAR, and DDCAR: demographics (sex [male, female]; age [continuous]; race [Caucasian/white, African-American/black, Asian, other]; body weight [continuous]); renal function based on creatinine clearance (CrCL; continuous, estimated using the method of Cockcroft and Gault [21]); liver dysfunction classification as defined by the National Cancer Institute Working Group, clinical laboratory measures, and the concomitant administration of medications (e.g., cytochrome P450 [CYP] inhibitors and inducers).
Following development of the appropriate base structural model, Bayesian estimates of relevant parameters were generated for each patient and used to perform a stepwise generalized additive model (GAM). For highly correlated covariates (e.g., body weight [WTKG], body mass index [BMI], ideal body weight [IBW]), only one covariate was selected for the GAM analysis. The covariate model obtained from the GAM analysis was then added to the base pharmacokinetic model and fit to the data in NONMEM. After full multivariate models were selected, error models for interindividual variability (IIV) and residual variability (RV) were evaluated, and following adjustment of the models, a univariate stepwise backward elimination procedure was used to remove nonsignificant covariates until all remaining covariates were significant (α = 0.001). Following backward elimination, models for cariprazine, DCAR, and DDCAR evaluating the effect of all concomitant medication covariates (i.e., CYP inhibitors and inducers) were tested.
Covariate modeling was performed sequentially. The cariprazine model, following backward elimination of covariates, was incorporated as part of the DCAR model with all cariprazine model parameters fixed to the Bayesian estimates for each patient prior to performing the covariate analysis for DCAR. The DCAR model was likewise sequentially incorporated into the DDCAR model following backward elimination of covariates.
Updated Model
The full multivariate models for cariprazine, DCAR, and DDCAR were developed by adding statistically significant covariates from the final initial dataset model (Supplemental Equation Set 2 in supplemental material) to the updated base structural model. For the updated analysis, race was redefined as Caucasian/white, African-American/black, Asian-Indian, Asian-Japanese, and other. Fully updated models were examined to determine whether significant covariates in the initial model remained significant based on the updated dataset. Evaluation of error models and subsequent adjustments followed by a backward elimination procedure to remove nonsignificant covariates were also performed.
Because CrCL was not selected by the GAM analysis for inclusion in the full multivariate initial model, an additional univariate evaluation of CrCL's effect on the clearance parameters of cariprazine, DCAR, and DDCAR was performed (α = 0.001) by adding the CrCL effect on cariprazine, DCAR, and DDCAR apparent clearance (CL/F, DCL/F, and DDCL/F, respectively) to the models with the other statistically significant covariates remaining in the models.
Model Refinement, Evaluation, and Validation
Initial Dataset Model and Updated Dataset Model
Following backward elimination of covariates, the model was further evaluated to detect inadequacies or biases in covariate models and to assure no trends remained. If warranted, additional modifications were made to simplify covariate relationships. Assuming that uncertainty in the final model parameters was small relative to other sources of variability, the adequacy of the final models was evaluated using a simulation-based prediction-corrected visual predictive check (PCVPC) method [22]. Using the analysis dataset and the control streams for the final population pharmacokinetic models for cariprazine, DCAR, and DDCAR, a bootstrap procedure was also performed for each model. Up to 500 bootstrap datasets were created by resampling with replacement, stratified by phase 1 versus phase 2 or 3 data. Each of the final population pharmacokinetic models was estimated for each bootstrap dataset, resulting in distributions for each estimated parameter. The percentage of datasets that minimized successfully was reported, and bootstrap confidence intervals (CIs) were calculated for each parameter. Models terminating in rounding errors with ≥ 2 significant digits and all gradients < 10 were considered successful.
Post-Modeling Assessments
Application of the Final Model to the Model Validation Dataset
To further explore adequacy of the final models, each model was applied to the data from the Model Validation Dataset (data from long-term studies RGH-MD-11 and RGH-MD-17), and population and individual predictions of concentrations were compared to measured concentrations.
Clinical Relevance of Statistically Significant Covariates
Covariates included in final pharmacokinetic models were considered statistically significant and were evaluated to determine if the covariate effect was clinically significant, addressed through summarization and graphical representation of computed individual patient exposure measures following a 6-mg steady-state dose. Geometric mean ratios and 90% CI of the individual exposure measures at steady state (maximum and minimum observed drug concentration [Cmax,ss and Cmin,ss, respectively] and area under the plasma concentration time-curve over a 24-h dosing period [AUC0–24,ss]) were calculated for each covariate of interest. Percent change in relevant pharmacokinetic exposures was used to assess clinical significance of covariates.
CYP2D6 Metabolizer Status
Using a hypothetical steady-state dose of 6 mg/day for all individuals and their Bayesian parameter estimates (including CL/F), the final pharmacokinetic models for cariprazine, DCAR, and DDCAR were used to compute Cmax,ss, Cmin,ss, and AUC0–24,ss at steady state for each patient; values for Total CAR (sum of cariprazine, DCAR, and DDCAR in nM) were calculated for each individual.
To test the statistical significance of CYP2D6 metabolizer status, an analysis of variance (ANOVA) was performed using the final model-predicted and dose-normalized steady-state pharmacokinetic exposures based on Bayesian post hoc apparent clearance parameters (CL/F, DCL/F, and DDCL/F) for a subset of patients for whom a blood sample for genomic testing was collected (RGH-MD-04, RGH-MD-05, RGH-MD-11, and RGH-MD-36) and the metabolizer status was known.
Results
Patients
Of the 2199 study participants included in the updated model dataset, 66% were men, 81% were 18–49 years of age, 19% were 50–65 years of age, 46% were Caucasian/white, and 35% were African-American/black (Supplemental Table 2). The mean (standard deviation [SD]) age was 39.2 (10.8) years, and the mean (SD) WTKG was 78.9 (18.7) kg. A total of 1802 (82%) patients had normal renal function (CrCL ≥ 90 ml/min), 377 (17%) had mild impairment (60 ≤ CrCL ≤ 89 ml/min), and 20 (1%) had moderate impairment (30 ≤ CrCL ≤ 59 ml/min). Of 908 patients with known CYP2D6 metabolizer genotype status, 868 (96%) were classified as extensive metabolizers (EM; defined as ultra, extensive, or intermediate metabolizers) while 40 (4%) were classified as poor metabolizers (PM).
Basic Pharmacokinetic Models
Initial Model
In the initial dataset, 492 (3.4%) cariprazine, 1341 (9.2%) DCAR, and 1324 (9.2%) DDCAR samples with postdose concentrations < LLOQ were excluded from the model development dataset (percentages based on samples remaining at time of exclusion). Furthermore, 482 (4.0%) cariprazine, 462 (3.9%) DCAR, and 472 (4.2%) DDCAR samples collected > 25 h postdose were excluded. The final pooled dataset used in the initial population pharmacokinetic modeling included 11,412 cariprazine, 11,189 DCAR and 10,286 DDCAR samples from 2049, 2044, and 2002 patients, respectively. Cariprazine disposition was well described by a two-compartment model with zero-order input of the dose followed by first-order absorption and first-order elimination. With elimination of cariprazine and DCAR acting as the formation rate of DCAR and DDCAR, respectively, a one-compartment model with first-order elimination described the disposition of both DCAR and DDCAR. Because models were developed sequentially, all cariprazine parameter estimates for the DCAR model and all cariprazine and DCAR parameter estimates for the DDCAR model were fixed to their individual Bayesian values.
Updated Model
In the build of the final pooled dataset, 436 (3.2%) cariprazine, 1223 (8.8%) DCAR, and 368 (2.9%) DDCAR samples with post-dose concentrations < LLOQ were excluded from the model development dataset (percentages based on samples remaining at the time of exclusion). The final pooled dataset for the updated model included 13,227 cariprazine, 12,462 DCAR, and 12,092 DDCAR samples from 2199, 2180, and 2140 patients, respectively. As adding the rich sampling data for 12 weeks following the last dose (Study A002-A11) stabilized the estimation of the terminal elimination parameters, concentrations collected > 25 h postdose were reincluded in the updated model.
The final analysis dataset included four studies with serial pharmacokinetic sampling. Three of the studies collected serial samples on Day 2 (1 Cohort), Day 4 (1 Cohort), or Day 5 (2 Cohorts). The last set of serial pharmacokinetic samples was collected on Day 22, 28, 29, 30, or 42. Of the additional nine studies with sparse pharmacokinetic samples, pharmacokinetic sampling began on Day 5, 7, or 14. Thus, there were minimal pharmacokinetic concentrations on Days 2–5 to assist with estimation of time-varying pharmacokinetic model parameters or non-linear pharmacokinetic model parameters. Additionally, only 2.6% of patients in the model development dataset had a measurable DDCAR concentration prior to Day 2 suggesting that the formation of DDCAR is delayed relative to the other moieties. To demonstrate the impact of the uptitration schedules and pharmacokinetic sampling schedules, plots of the number of measurable pharmacokinetic samples per dose level and per week are shown in Supplemental Fig. 1. Prior to the data deletions described above, linear models, non-linear models for elimination, distribution, and volume of distribution, and target-mediated elimination models were evaluated. The majority of the more complex models failed to minimize, and none improved the biases observed for linear pharmacokinetic models in the clinical dose range of the data.
The additions to the dataset allowed additional compartments to be added to the initial dataset models, which more fully described the disposition of each moiety. For the updated model, cariprazine disposition was well described by a three-compartment model with zero-order input of the dose followed by first-order absorption and first-order elimination. With cariprazine elimination acting as the formation rate of DCAR, a two-compartment model with first-order elimination described the disposition of DCAR. With DCAR elimination acting as the formation rate of DDCAR to a transit compartment with first-order transfer to the central compartment to delay the formation of DDCAR (only 2.6% of patients in the model development dataset had a measurable concentration prior to Day 2), a two-compartment model with first-order elimination described the disposition of DDCAR. Fixed parameters were used for DCAR and DDCAR models as described for the initial model. As mentioned previously, the schedule of pharmacokinetic sample collection included minimal samples during early accumulation (Days 25), and more complex time-varying or non-linear pharmacokinetic models could not be fit to the data to describe the early accumulation. Thus, the base models included a first dose shift parameter (FD; 1 for concentrations following the first dose, 0 otherwise) on the apparent central volume of distribution (Vc/F), apparent peripheral volume (Vp/F), and apparent first distribution clearance (Q3/F) parameters of cariprazine and on Vc/F of DCAR to correct model mis-specification in predicting accumulation following multiple doses with basic linear models.
Covariate Analysis
Initial Model
Following backward elimination and model refinement, covariate analysis indicated that the following covariates were statistically significant (p < 0.001) predictors of IIV: IBW and race on CL/F; IBW on cariprazine apparent central volume of distribution (VC/F); age, WTKG, race, and sex on DCL/F; WTKG on DCAR VC (DVC/F); IBW and race on DDCL/F; and age, WTKG, and race on DDCAR VC (DDVC/F).
Updated Model
The full multivariate model for the updated dataset included statistically significant covariates from the initial model with a separate category of race added for Japanese patients. For practical use, IBW was changed to WTKG in all IBW-based covariate effects prior to backward elimination, as both models performed similarly. Backward elimination of covariates for the updated cariprazine model showed race to be a statistically significant predictor of CL/F (p < 0.001) and WTKG to be a statistically significant predictor of VC (p < 0.001). WTKG was not a significant predictor of CL/F (p = 0.012); however, to assist with scaling cariprazine pharmacokinetic to the pediatric population, weight as a predictor of CL/F was retained in the model. For the updated DCAR model, backward elimination of covariates showed race, WTKG, and sex to be statistically significant predictors of DCL/F (p < 0.001) and WTKG to be a statistically significant predictor of DVC/F (p < 0.001). Backward elimination of covariates for the updated DDCAR model showed race to be a statistically significant predictor of DDCL/F (p < 0.001) and WTKG and race to be statistically significant predictors of DDVC/F (p < 0.001). The effects of sex, race, and WTKG on apparent clearance and volume are shown in Supplemental Figs. 2–8.
The additional univariate covariate analysis showed that CrCL was not a statistically significant predictor of clearance for cariprazine, DCAR, or DDCAR.
Final Models
For final models of the initial dataset, parameter equations are listed in the supplemental material (Equations A1–6 in Supplemental Equation Set 2), and parameter estimates, standard errors, and IIV values are provided in Supplemental Table 3.
The parameter equations for the final cariprazine, DCAR, and DDCAR models of the updated dataset are described in Supplemental Equation Set 3 of the supplementary materials. Measured concentration-time profiles of typical individual patients from studies RGH-MD-01 and A002-A11 (studies with rich pharmacokinetic sampling) are provided for comparison (Supplemental Figs. 9–10). Parameter estimates, standard errors, and, where applicable, IIV values for updated final models are provided in Table 2, and goodness-of-fit plots are provided in Supplemental Fig. 11.
Table 2.
Pharmacokinetic parameter estimates and standard errors for the final models
| Parameter | Description/factors (units) | Estimate (bootstrap 90% CI)a | RSE% | IIV (bootstrap 90% CI)a,h |
|---|---|---|---|---|
| Cariprazine | ||||
| DUR | Duration of zero-order absorption process (h) | 2.57 (2.35, 2.79) | 2.32 | NE |
| Ka | First-order absorption rate constant (h−1) | 0.352 (0.32, 0.39) | 3.95 | 118% CVb (99.5, 138) |
| CL/F | Apparent elimination clearancec (l/h) | 21.5 (21.1, 21.8) | 1.13 | 32.4% CV (30.7, 33.9) |
| Power of WTKG | 0.0946 (0.0341, 0.161) | 38.5 | ||
| Proportional shift for race = black | − 0.0907 (− 0.114, − 0.0639) | 17.1 | ||
| Proportional shift for race = Asiand | − 0.178 (− 0.211, − 0.142) | 12.5 | ||
| Proportional shift for race = Japanesed | − 0.111 (− 0.178, − 0.0401) | 52.5 | ||
| VC/F | Apparent central volumee (l) | 266 (241, 288) | 2.68 | 109% CVb (90.2, 125) |
| Proportional shift for first dose | 2.84 | FIXEDf | ||
| Power of WTKG | 1.66 (1.40, 1.96) | 3.83 | ||
| Q3/F | Apparent first distribution clearance (l/h) | 0.431 | FIXEDf | NE |
| Proportional shift for first dose | 39.4 | FIXEDf | ||
| VP1/F | Apparent first peripheral volume (l) | 149 | FIXEDf | NE |
| Proportional shift for first dose | 2.61 | FIXEDf | ||
| Q4/F | Apparent second distribution clearance (l/h) | 100 | FIXEDf | NE |
| VP2/F | Apparent second peripheral volume (l) | 501 | FIXEDf | NE |
| DCAR | ||||
| DCL/F | Apparent DCAR elimination clearanceg (l/h) | 77.3 (75.3, 79.4) | 1.70 | 42.4% CV (40.7, 44.1) |
| Power of WTKG | 0.578 (0.488, 0.648) | 9.18 | ||
| Proportional shift for race = black | 0.249 (0.203, 0.292) | 11.0 | ||
| Proportional shift for race = Asiand | − 0.0861 (− 0.135, − 0.0432) | 39.0 | ||
| Proportional shift for race = Japanesed | − 0.145 (− 0.218, − 0.0574) | 55.1 | ||
| Proportional shift for sex = female | − 0.160 (− 0.190, − 0.130) | 11.4 | ||
| DVC/F | Apparent DCAR central volumee (l) | 128 (106, 150) | 6.56 | 115% CVb (103, 130) |
| Proportional shift for first dose | 1.27 | FIXEDf | ||
| Power of WTKG | 1.18 (0.604, 1.74) | 23.7 | ||
| DQ/F | Apparent DCAR distribution clearance (l/h) | 78.5 (60.9, 105) | 4.04 | NE |
| DVP/F | Apparent DCAR peripheral volume (l) | 347 (292, 411) | 2.85 | NE |
| Proportional shift for first dose | 0.535 | FIXEDf | ||
| DDCAR | ||||
| DDCL/F | Apparent DDCAR elimination clearancec (l/h) | 9.24 (8.93, 9.57) | 1.72 | 57.4% CV (54.3, 60.3) |
| Power of WTKG | 0.427 (0.317, 0.526) | 10.7 | ||
| Proportional shift for race = black | 0.547 (0.481, 0.636) | 8.98 | ||
| Proportional shift for race = Asiand | − 0.194 (− 0.242, − 0.140) | 14.0 | ||
| Proportional shift for race = Japanesed | − 0.156 (− 0.249, − 0.0407) | 28.6 | ||
| DDVC/F | Apparent DDCAR central volumec (l/h) | 1310 (1260, 1360) | 1.19 | 77.8% CV (73.0, 82.7) |
| Power of WTKG | 0.881 (0.747, 1.02) | 8.93 | ||
| Proportional shift for race = black | 0.676 (0.580, 0.811) | 9.10 | ||
| Proportional shift for race = Asiand | − 0.240 (− 0.299, − 0.159) | 28.0 | ||
| Proportional shift for race = Japanesed | 0.0888 (− 0.0312, 0.249) | 65.1 | ||
| DDQ/F | Apparent DDCAR distribution clearance (l/h) | 0.386 | FIXEDf | NE |
| DDVP/F | Apparent DDCAR peripheral volume (l) | 258 | FIXEDf | NE |
| DDKtr | Rate constant delaying DDCAR formation (h−1) | 0.0269 | FIXEDf | NE |
CI confidence interval, CV coefficient of variance, DCAR desmethyl-cariprazine, DDCAR didesmethyl-cariprazine, IIV interindividual variability, NE not estimated, RSE relative standard error, WTKG body weight
aThe 5th to 95th percentile of the estimates from fitting the model to 500 bootstrap datasets. Minimization was successful for 74.6% (373), 91.6% (458), and 91.6% (458) of the datasets for cariprazine, DCAR, and DDCAR, respectively
bIIV in Ka, VC, and DVc were estimated for Phase 1 patients only
cIn white, 79-kg WTKG patients
dAsian patients were mainly from studies conducted in India and Japanese patients were from Study A002-A11 only
eIn 79-kg WTKG patients
fBecause the Phase 2/3 study designs did not collect samples following the first dose, all shifts in the parameters for the first dose were fixed to the Phase 1 base model values. Additional parameters were fixed to the Phase 1 model values after determination that the Phase 2/3 sampling design did not support their estimation. Due to the differences in sampling designs, residual variability was estimated separately for the Phase 1 studies (full profile) versus the Phase 2/3 studies (sparse sampling)
gIn white, male, 79-kg WTKG patients
hThe eta shrinkage for CL/F, VC/F, and Ka was 8.53%, 7.56%, and 76.5%, respectively. The eta shrinkage for DCL/F and DVC/F was 7.04% and 66.2%, respectively. The eta shrinkage for DDCL/F and DDVc/F was 5.02% and 7.86%, respectively. Thus, diagnostic plots were not used to guide covariate analysis
iThe condition number for the cariprazine model, DCAR model, and DDCAR model was 33.2, 14.8, and 83.0, respectively
The final models included an FD parameter on the Vc/F, Vp/F, and Q3/F parameters of cariprazine and on the Vc/F of DCAR to correct model mis-specification in predicting accumulation following multiple doses with basic linear models.
All parameters were well estimated (RSE < 50%), except for the effect of Japanese race on CL/F and DCL/F. The higher RSE for this race effect may be related to the small number of Japanese subjects (n = 37 [1.7%] of the model development dataset). The bootstrap CIs indicate the estimate of this covariate effect was highly variable; however, CIs do not contain zero, which indicates a statistically significant difference in CL/F and DCL/F for Japanese patients compared with Caucasian patients.
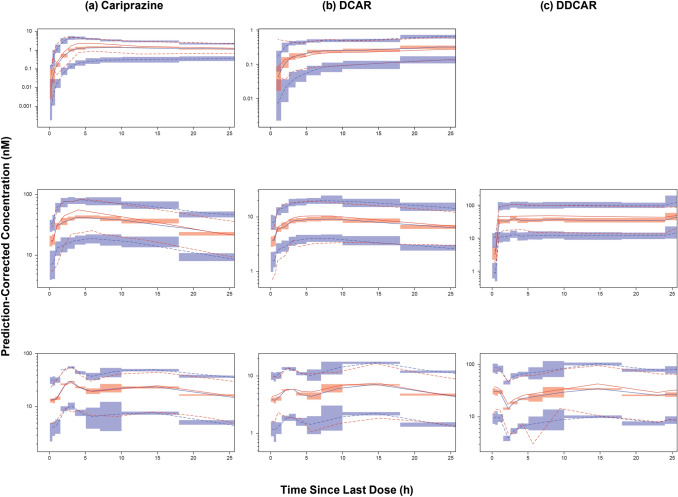
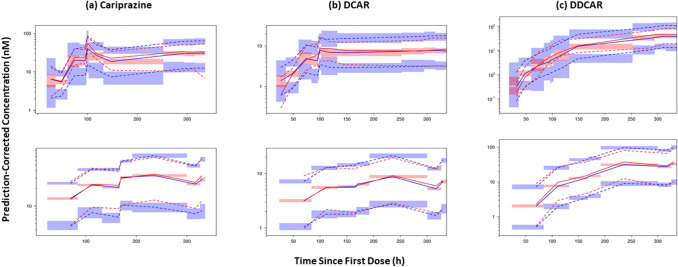
The evaluation of the final model and the PCVPC for the first 24 h postdose are described in Fig. 2, for the first 2 weeks following time since first dose in Fig. 3, and for the full range of time since last dose described in Supplemental Fig. 12.
Fig. 2.
Prediction-corrected visual predictive check (PCVPC) of the final updated models. Visual predictive check of final models for the 0–24 h time since last dose profiles for a cariprazine, b DCAR, and c DDCAR. PCVPC plots of phase 1 studies (top, single dose [not available for DDCAR model]; middle, multiple dose) and phase 3 studies (bottom, multiple dose) are shown separately. Red and blue lines denote observed data and predictions, respectively; solid lines denote median, dashed lines represent 5th and 95th percentiles; shaded areas represent 95% CI of prediction percentiles. CI confidence interval, DCAR desmethyl-cariprazine, DDCAR didesmethyl-cariprazine
Fig. 3.
Prediction-corrected visual predictive check (PCVPC) of the final updated models. Visual predictive check of final models for 0–336 h since first dose for a cariprazine, b DCAR, and c DDCAR. PCVPC plots of phase 1 studies (top, multiple dose) and phase 3 studies (bottom, multiple dose) are shown separately. Red and blue lines denote observed data and predictions, respectively; solid lines denote median, dashed lines represent 5th and 95th percentiles; shaded areas represent 95% CI of prediction percentiles. CI confidence interval, DCAR desmethyl-cariprazine, DDCAR didesmethyl-cariprazine
Post-Modeling Assessments
Application of the Final Model to the Model Validation Dataset
PCVPCs for final models were generated using patients, doses, and sample times from 1-year studies (Model Validation Dataset; RGH-MD-11 and RGH-MD-17); plots are shown in Supplemental Fig. 13. PCVPCs of each model show good correspondence between median simulated and observed data for the first 168 h postdose. This is true for all 3 moieties except for an overprediction bias at 168 h postdose for DDCAR. The final models underpredicted samples collected more than 168 h postdose; however, the amount of observed data for this time period was less than the amount of observed data prior to 168 h. The behavior of the observed 5th and 95th percentiles exhibits high variability, which suggests that recorded dosing history may have been more unreliable (related to dosing compliance) for longer treatment durations in continuation studies (validation studies).
Clinical Relevance of Statistically Significant Covariates
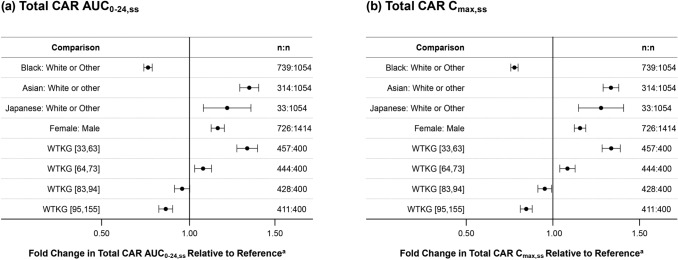
Body weight, race, and sex were statistically significant predictors of pharmacokinetic model parameters; this resulted in lower Total CAR pharmacokinetic exposures (Cmax,ss and AUC0–24,ss) for patients who were black and higher exposures for patients who were Asian and Japanese compared with patients who were Caucasian and higher exposures for females versus males (Fig. 4). Total CAR pharmacokinetic exposures were also higher for patients with lower body weight (< 74 kg) and lower for patients with higher body weight (> 82 kg) compared with patients in the reference weight range (74–82 kg); thus, exposure increased as body weight decreased. All covariate-related changes in exposures were within 36% of the relevant comparator group (white or other for race, male for sex, and weight range of 74 to 82 kg for the body weight categories).
Fig. 4.
Geometric mean ratios and 90% confidence intervals of 6 mg/day steady-state exposures for the updated dataset, by covariate comparison. aReference for race is white or other (n = 1054), for sex is male (n = 1414), and for WTKG [range] is WTKG [74, 82] (n = 400). AUC0–24,ss area under the plasma concentration-time curve over a 24-h period at steady state, Cmax,ss maximum observed concentration at steady state, Total CAR molar sum of cariprazine, desmethyl-cariprazine (DCAR), DDCAR didesmthyl-cariprazine, WTKG body weight in kilograms
CYP2D6 Metabolizer Status
There were 908 patients with known CYP2D6 metabolizer status and model-predicted pharmacokinetic parameters for cariprazine, DCAR, and DDCAR. This subset included 40 patients classified as PM and 868 classified as EM. The ANOVA of the updated model-predicted pharmacokinetic exposures for this subset showed that none of the cariprazine, DCAR, or DDCAR CL/F values or exposures (Cmin,ss, Cmax,ss, or AUC0–24,ss) for the PM and EM patient groups were associated with a statistically significant difference (α = 0.05). Mean exposures for CYP2D6 PM patients were within ± 10% of mean exposures for CYP2D6 EM patients. While the updated model described the concentration-time course for up to 240 h postdose, as compared to the 24 h for the initial model, the findings of the CYP2D6 assessment were similar for the two models [23, 24].
Simulations
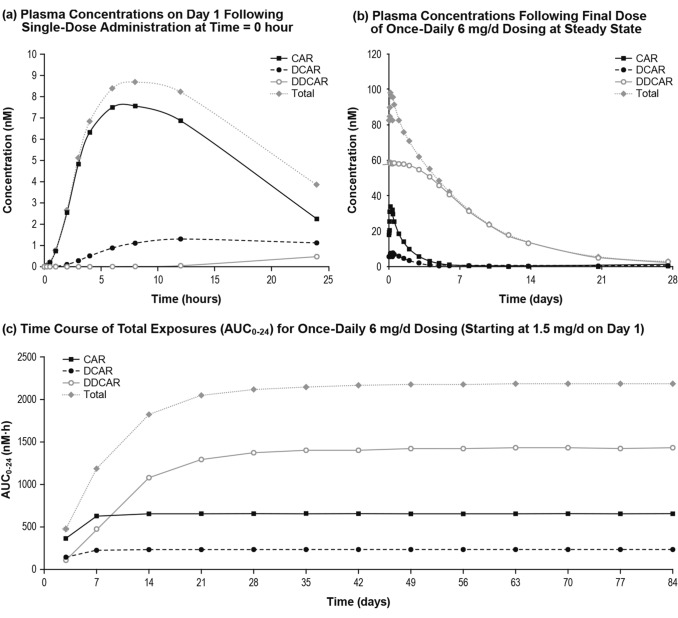
A simulation of an overall typical patient (79 kg, Caucasian/white adult male) who was administered a 6 mg/day dose was conducted to illustrate concentration-time profiles (Fig. 5a) and depict the decline in plasma concentration after last dose (Fig. 5b). Time to steady state is illustrated in Fig. 5c. Simulations of the overall typical patient support that cariprazine was the prominent active moiety after the first 6-mg dose on Day 1 (Fig. 5a), while DDCAR was the prominent moiety at steady state (Fig. 5b). The final models predicted that for an overall typical patient initiating cariprazine treatment with an up-titration schedule at 1.5 mg/day incremental doses to a final dose of 6 mg/day, the AUC0–24,ss value for Total CAR would achieve 90% of the steady-state AUC0–24,ss value by Day 21 of dosing (Fig. 5c).
Fig. 5.
Predicted plasma exposures for a typical patient following administration of cariprazine 6 mg/day. Simulations involved: a single dose at 6 mg/day; b final dose at 6 mg/day under steady-state conditions; c uptitration to 6 mg/day by Day 4 as follows: 1.5 mg on Day 1, 3 mg on Day 2, 4.5 mg on Day 3, and 6 mg/day thereafter. AUC0–24,ss area under the plasma concentration-time curve over a 24-h period at steady state. CAR cariprazine, DCAR desmethyl-cariprazine, DDCAR didesmethyl-cariprazine, Total CAR sum of CAR and major metabolites (DCAR and DDCAR)
For the overall typical patient, the predicted partial area from the start of the terminal phase to tlast (time of last measurable concentration as determined by LLOQ values) is only 5.7% for cariprazine, 4.9% for DCAR, 3.8% for DDCAR, and 3.4% for Total CAR of (AUC0–tlast). Because percentages of exposures during the terminal phase are low, time to steady state was better reflected by effective half-life rather than terminal half-life (computed from the slope of the last 4 predicted measurable concentrations; mean terminal half-life [SD] was 10.1 [0.7] days for cariprazine, 5.9 [2.8] days for DCAR, 19.1 [9.3] days for DDCAR, and 18.9 [9.2] days for Total CAR). In other words, time to steady state for cariprazine is mainly determined by the first and second disposition phase and for the metabolites is mainly determined by the first disposition phase rather than the terminal elimination phase. Therefore, effective half-life is more clinically meaningful.
Median time to 90% of steady state was 5 days for cariprazine, 5 days for DCAR, 21 days for DDCAR, and 18 days for Total CAR, which translated into a mean (SD) effective half-life (time to achieve 90% steady state divided by 3.32 [11]) of 1.5 (0.7) days for cariprazine, 1.5 (0.7) days for DCAR, 7.0 (8.7) days for DDCAR, and 5.8 (8.5) days for Total CAR (Table 3).
Table 3.
Summary statistics of predicted steady-state pharmacokinetic parameters for cariprazine and its major metabolites at 6 mg/day dose
| Predicated exposures, and half-life | Cariprazine (n = 2599) | DCAR (n = 2580) | DDCAR (n = 2539) | Total CAR (n = 2539) |
|---|---|---|---|---|
| Cmax,ss (nM) | ||||
| Mean (SD) | 37.6 (13.5) | 8.8 (4.1) | 65.6 (106.6) | 112.0 (111.7) |
| Median | 35.4 | 8.1 | 55.3 | 100.8 |
| Min, max | 11.7, 246.6 | 1.1, 51.7 | 1.3, 4833.5 | 19.5, 4944.0 |
| Cmin,ss (nM) | ||||
| Mean (SD) | 22.0 (12.7) | 6.8 (3.8) | 65.6 (106.6) | 94.4 (111.1) |
| Median | 19.9 | 6.1 | 55.3 | 83.0 |
| Min, max | 2.5, 223.8 | 0.7, 47.1 | 1.3, 4833.5 | 9.0, 4927.1 |
| AUC0–24,ss (nM·h) | ||||
| Mean (SD) | 690.2 (313.0) | 188.7 (94.9) | 1573.8 (2558.3) | 2452.0 (2671.1) |
| Median | 641.0 | 171.1 | 1325.9 | 2180.6 |
| Min, max | 148.6, 5609.1 | 21.5, 1193.8 | 27.5, 115,996.3 | 344.1, 118,434.6 |
| Effective t1/2 (day) | ||||
| Mean (SD) | 1.5 (0.7) | 1.5 (0.7) | 7.0 (8.7) | 5.8 (8.5) |
| Median | 1.5 | 1.5 | 6.3 | 5.4 |
| Min, max | 0.3, 10.5 | 0.6, 10.2 | 1.5, 370.2 | 0.9, 367.5 |
AUC0–24,ss area under the plasma concentration-time curve over a 24-h period at steady state, Cmax,ss maximum plasma concentration, Cmin,ss minimum plasma concentration, DCAR desmethyl-cariprazine, DDCAR didesmethyl-cariprazine, SD standard deviation, t½ half-life, Total CAR sum of cariprazine and major metabolites (DCAR and DDCAR)
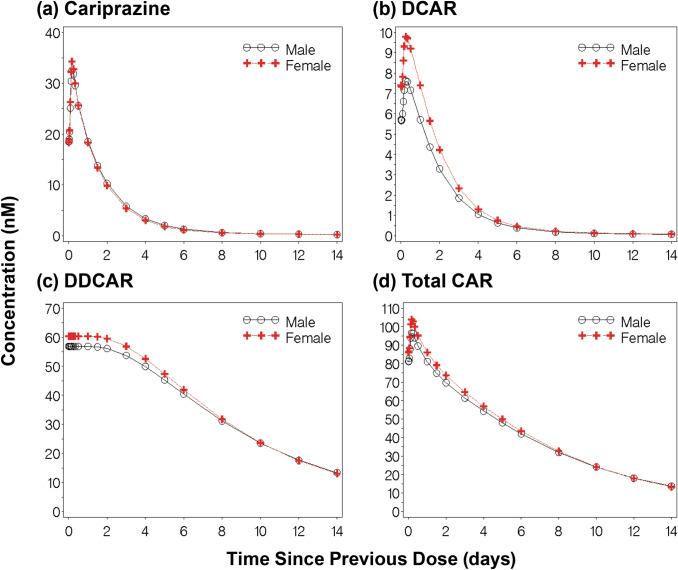
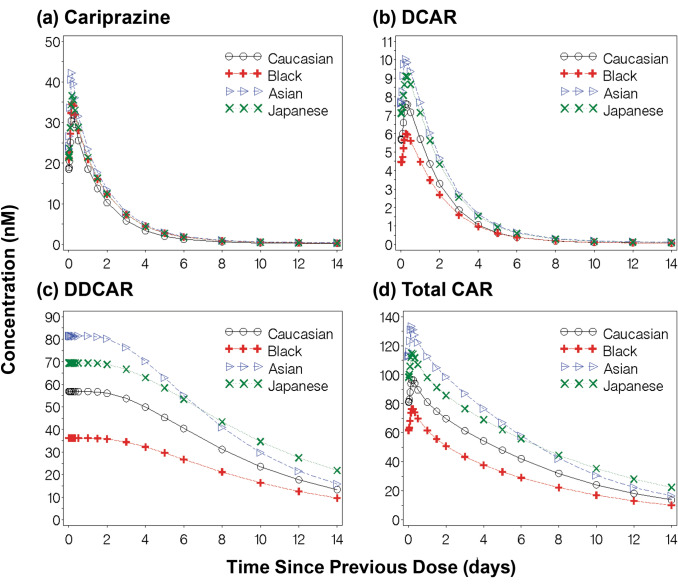
To illustrate differences in cariprazine, DCAR, DDCAR, and Total CAR concentrations due to covariates, 14-day concentration-time profiles following steady-state dosing were generated for a typical Caucasian male (84 kg) and female (73 kg) patient (Fig. 6) and for a typical male Caucasian (84 kg), black (87 kg), Asian (60 kg), and Japanese (78 kg) patient (Fig. 7). Despite an 11 kg difference in body weight, there were minimal differences in exposures between male and female patients; the most observable differences were seen in the slightly higher DCAR and DDCAR exposures in the first 7 days post-steady state dosing.
Fig. 6.
Predicted plasma exposures for a typical male and female patient following administration of cariprazine. Fourteen-day concentration-time profiles (a cariprazine; b DCAR; c DDCAR; d Total CAR) following steady-state dosing for a typical male (84 kg, Caucasian) and typical female (73 kg, Caucasian) patient. DCAR desmethyl-cariprazine, DDCAR didesmethyl-cariprazine, Total CAR molar sum of cariprazine, DCAR, and DDCAR
Fig. 7.
Predicted plasma exposures for a typical Caucasian, Black, Asian, or Japanese male patient following administration of cariprazine. Fourteen-day concentration-time profiles (a cariprazine; b DCAR; c DDCAR; d Total CAR) following steady-state dosing for a typical Caucasian (84 kg), Black (87 kg), Asian (60 kg), or Japanese (78 kg) male patient. DCAR desmethyl-cariprazine, DDCAR didesmethyl-cariprazine, Total CAR molar sum of cariprazine, DCAR, and DDCAR
Discussion
The model development for cariprazine showed that the disposition of cariprazine was well described by a three-compartment model with zero-order input of the dose to the depot compartment, followed by first-order absorption and first-order elimination, and DCAR and DDCAR were best described by two-compartment models with a first-order elimination rate of cariprazine and DCAR (with a delay) serving as the formation rate of DCAR and DDCAR, respectively.
Evaluation of final models showed that the PCVPC for the first 24 h postdose showed good correspondence between observed and model-predicted percentiles for multiple-dose Phase 2/3 data. For the Phase 1 data, there was underprediction bias for peak cariprazine concentrations following the first dose, multiple doses, and multiple-dose DDCAR concentrations (median only); however, the number of patients and samples from the seven phase 2/3 studies in the final model development dataset (outpatients) far exceeded the data from the three phase 1 studies (generally hospitalized patients) so it is expected that the model would fit the sparse pharmacokinetic data at the expense of the serial pharmacokinetic data. Additional PCVPC plots for the full range of the concentration-time profile since the previous dose course showed similar findings for the first 240 h post-previous dose, after which the amount of observed data was small. The PCVPC of the final models for the first 2 weeks of dosing (0–336 h after the first dose) showed that the first dose of cariprazine was underpredicted and the first dose of DCAR was overpredicted. For the remainder of the 2 weeks, the predictions for phase 1 studies (serial sampling) indicated that cariprazine had a continued trend (observed within the 95% CI of prediction) of underprediction that diminished as time increased; DCAR and DDCAR observations and predictions generally corresponded well. For the remainder of the 2 weeks, the predictions for phase 2/3 studies (sparse sampling) indicated that the predicted and observed concentrations of cariprazine and DCAR corresponded closely with observations generally within the 95% CI of the prediction intervals. For DDCAR, there was a trend for underprediction with the observed and predicted concentrations generally parallel and the difference diminishing as time increased. Thus, the linear pharmacokinetic models with shifts for parameters after the first dose of cariprazine, DCAR, and DDCAR adequately predicted the data for the first 2 weeks of dosing. Therefore, the simplified linear pharmacokinetic models with a shift in distributional and volume parameters after the first dose were able to adequately predict the time to steady state for each moiety.
The noted under-prediction bias was also considered acceptable because the primary goal of the analysis was to best describe data observed in the clinical setting (multiple dosing). In fact, predictions of key exposure parameters for cariprazine, DCAR, and DDCAR by the final population pharmacokinetic models are consistent with values obtained by non-compartmental methods (Supplemental Table 4) [11]. In addition, dose linearity at steady state in the dose range of 3–9 mg/day was also demonstrated based on pharmacokinetic parameters determined by non-compartmental methods, suggesting that use of linear pharmacokinetics models within the clinical dose range in these population pharmacokinetic analyses was appropriate.
Covariate analyses showed that WTKG, race, and sex were statistically significant predictors of one or more pharmacokinetic parameters across the three analyte models. However, geometric mean ratios of steady-state Total CAR exposures comparing covariate groups were all within a limited range (Fig. 4). The effect of sex likely offsets differences related to body weight, and the anticipated washout of drug would be similar regardless of sex. Even though body weight may confound effects, there were observable differences in steady-state concentrations of the typical patient in each race category. The anticipated time for washout of the drug may be faster for male patients who are black and possibly longer for male patients who are Japanese compared to other male patients. For analysis involving race covariates, it should be noted that only Japanese patients were enrolled in Study A002-A11; therefore, all effects shown with the Japanese race may be confounded with a possible study effect.
Given the recommended dosing range of 1.5–6 mg/day for the treatment of schizophrenia and 3 to 6 mg/day for patients with manic or mixed episodes associated with bipolar I disorder and that efficacy and safety have been established over the dose range of 1.5–9 mg/day for schizophrenia and 3–12 mg/day for bipolar mania (with recommended incremental dose adjustments of 1.5 mg/day), a ≥ 20% change in Total CAR pharmacokinetic exposures related to changes in covariate values was not considered to be clinically relevant. Renal impairment (mild and moderate) was not associated with any significant difference, which is consistent with the fact that the renal pathway contributes minimally to the elimination of cariprazine and its metabolites [12]. Similarly, CYP2D6's poor metabolizer status (n = 40) was not associated with any clinically relevant change in pharmacokinetic exposures. Thus, no dose adjustment is needed in patients with mild or moderate renal impairment or based on CYP2D6 status.
Conclusions
The pharmacokinetics of cariprazine and its active metabolites, DCAR and DDCAR, were described with population compartmental modeling. Based upon findings of this analysis, any impact of patient characteristics, such as race, sex, weight, CrCL, and CYP2D6 metabolizer status, on the active moiety (total cariprazine) exposure does not warrant dose adjustment.
DDCAR was the most prominent moiety at steady-state, representing 64% of Total CAR. The median time to 90% steady state was 5 days for cariprazine, 5 days for DCAR, 21 days for DDCAR, and 18 days for Total CAR. Time to steady state for cariprazine and its major active metabolites is mainly determined by the early disposition phases rather than the terminal elimination phase; therefore, effective half-life is more clinically meaningful. The mean (SD) effective half-life was 1.5 (0.7) days for cariprazine, 1.5 (0.7) days for DCAR, 7.0 (8.7) days for DDCAR, and 5.8 (8.5) days for Total CAR. The simulated mean plasma concentrations decreased by approximately 50% within 1 week after the last dose for DDCAR and within 1 day for cariprazine and DCAR. Plasma concentration decreased by approximately 90% within 1 week for cariprazine and DCAR and within 4 weeks for DDCAR. These values are consistent with previously reported data. Overall, the population pharmacokinetic models adequately described the pharmacokinetics of cariprazine and its major active metabolites, which can be applied for simulations in pharmacokinetic predictions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank David J. Jaworowicz Jr., PhD, for assistance with pharmacokinetic modeling. Writing and editorial assistance was provided to the authors by Krystina Neuman, PhD, and Katharine Fang, PhD, of Prescott Medical Communications Group (Chicago, IL), a contractor of AbbVie. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
Declarations
Funding
Financial support for this work was provided by Forest Research Institute, Inc., an Allergan affiliate, (prior to its acquisition by AbbVie), NJ, USA.
Conflict of interest
Antonia Periclou and Timothy Carrothers are employees of and stockholders in AbbVie. Parviz Ghahramani and Tatiana Khariton were employees of Forest Research Institute, Inc., an Allergan affiliate, at the time of the study. Parviz Ghahramani is currently employed by Inncelerex. Margit Kapás is an employee of and stockholder in Gedeon Richter Plc. Cognigen Corporation, a Simulations Plus Company, was contracted to perform the analysis; Luann Phillips is an employee of Cognigen Corporation.
Ethics approval
All clinical trials described in this study were in accordance with the 1964 Helsinki declaration (and its amendments), and the details of the Ethics Committee or institutional review board which approved the study.
Informed consent
Written informed consent was obtained from all patients before enrollment. The authors all consent to have this manuscript published in the journal.
Availability of data and material
Qualified researchers engaged in rigorous, independent scientific research can request access to our clinical trial data. Access will be provided following receipt of a research proposal and Statistical Analysis Plan (SAP), review of the proposal and SAP and execution of a Data Use Agreement (DUA).
Code availability
Cognigen will share selected NONMEM codes as related to this analysis. To request access, the researcher must sign a code use agreement that states the researcher will not use the code for any commercial purposes.
Author contributions
AP, LP, PG, MK, TC, and TK wrote the manuscript, designed the research, and analyzed the data. LP performed the research (modeling).
References
- 1.Kiss B, Horvath A, Nemethy Z, Schmidt E, Laszlovszky I, Bugovics G, Fazekas K, Hornok K, Orosz S, Gyertyan I, Agai-Csongor E, Domany G, Tihanyi K, Adham N, Szombathelyi Z. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328–340. doi: 10.1124/jpet.109.160432. [DOI] [PubMed] [Google Scholar]
- 2.Girgis RR, Slifstein M, D'Souza D, Lee Y, Periclou A, Ghahramani P, Laszlovszky I, Durgam S, Adham N, Nabulsi N, Huang Y, Carson RE, Kiss B, Kapás M, Abi-Dargham A, Rakhit A. Preferential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [11C]-(+)-PHNO. Psychopharmacology. 2016 doi: 10.1007/s00213-016-4382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kane JM, Zukin S, Wang Y, Lu K, Ruth A, Nagy K, Laszlovszky I, Durgam S. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367–373. doi: 10.1097/JCP.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 4.Durgam S, Cutler AJ, Lu K, Migliore R, Ruth A, Laszlovszky I, Nemeth G, Meltzer HY. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J Clin Psychiatry. 2015;76(12):e1574–1582. doi: 10.4088/JCP.15m09997. [DOI] [PubMed] [Google Scholar]
- 5.Durgam S, Starace A, Li D, Migliore R, Ruth A, Nemeth G, Laszlovszky I. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2–3):450–457. doi: 10.1016/j.schres.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 6.Durgam S, Starace A, Li D, Migliore R, Ruth A, Nemeth G, Laszlovszky I. The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial. Bipolar Disord. 2015;17(1):63–75. doi: 10.1111/bdi.12238. [DOI] [PubMed] [Google Scholar]
- 7.Sachs GS, Greenberg WM, Starace A, Lu K, Ruth A, Laszlovszky I, Nemeth G, Durgam S. Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, phase III trial. J Affect Disord. 2015;174:296–302. doi: 10.1016/j.jad.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Calabrese JR, Keck PE, Jr, Starace A, Lu K, Ruth A, Laszlovszky I, Nemeth G, Durgam S. Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. J Clin Psychiatry. 2015;76(3):284–292. doi: 10.4088/JCP.14m09081. [DOI] [PubMed] [Google Scholar]
- 9.Citrome L. Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opin Drug Metab Toxicol. 2013;9(2):193–206. doi: 10.1517/17425255.2013.759211. [DOI] [PubMed] [Google Scholar]
- 10.Mészáros GP, Agai-Csongor E, Kapás M. Sensitive LC-MS/MS methods for the quantification of RGH-188 and its active metabolites, desmethyl- and didesmethyl-RGH-188 in human plasma and urine. J Pharm Biomed Anal. 2008;48(2):388–397. doi: 10.1016/j.jpba.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T, Kubota T, Iwakaji A, Imada M, Kapas M, Morio Y. Clinical pharmacology study of cariprazine (MP-214) in patients with schizophrenia (12-week treatment) Drug Des Devel Ther. 2016;10:327–338. doi: 10.2147/DDDT.S95100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vraylar [package insert]. Irvine: Allergan; 2017.
- 13.Durgam S, Litman RE, Papadakis K, Li D, Nemeth G, Laszlovszky I. Cariprazine in the treatment of schizophrenia: a proof-of-concept trial. Int Clin Psychopharmacol. 2016;31(2):61–68. doi: 10.1097/YIC.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutler AJ, Durgam S, Wang Y, Migliore R, Lu K, Laszlovszky I, Nemeth G. Evaluation of the long-term safety and tolerability of cariprazine in patients with schizophrenia: results from a 1-year open-label study. CNS Spectr. 2017 doi: 10.1017/S1092852917000220. [DOI] [PubMed] [Google Scholar]
- 15.Durgam S, Greenberg WM, Li D, Lu K, Laszlovszky I, Nemeth G, Migliore R, Volk S. Safety and tolerability of cariprazine in the long-term treatment of schizophrenia: results from a 48-week, single-arm, open-label extension study. Psychopharmacology. 2017;234(2):199–209. doi: 10.1007/s00213-016-4450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ketter TA, Sachs GS, Durgam S, Lu K, Starace A, Laszlovszky I, Nemeth G. The safety and tolerability of cariprazine in patients with manic or mixed episodes associated with bipolar I disorder: a 16-week open-label study. J Affect Disord. 2018;225:350–356. doi: 10.1016/j.jad.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services Guidance for Industry: Population Pharmacokinetics. 1999 https://www.fda.gov/downloads/Drugs/.../Guidances/UCM072137.pdf. Accessed 9 Mar 2012.
- 18.ICON Development Solutions. NONMEM [computer program]. Version 7, Level 1.2. Ellicott City. 2010.
- 19.SAS Institute Inc. SAS [computer program]. Version 9.2. Cary: SAS Institute; 2009.
- 20.Zhang L, Beal SL, Sheiner LB. Simultaneous vs. sequential analysis for population PK/PD data I: best-case performance. J Pharmacokinet Pharmacodyn. 2003;30(6):387–404. doi: 10.1023/b:jopa.0000012998.04442.1f. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khariton T, Phillips L, Bihorel S, Fiedler-Kelly J, Kapás M, Periclou A, Ghahramani P. A population PK model for cariprazine and the metabolites. In: Poster presented at the American conference on pharmacometrics, Fort Lauderdale, May 12–15; 2013.
- 24.Periclou A, Phillips L, Bihorel S, Ghahramani P, Kapás M, Carrothers T, Khariton T. Characterization of population pharmacokinetics of cariprazine and its major metabolites. In: Poster presented at the 169th annual meeting of the American Psychiatric Association, Atlanta, May 14–18; 2016.
- 25.Ereshefsky L, Gage A, Yu B, Kapás M, Andor G, Németh G, Laszlovszky I, Bolton G. Phase I study of RGH-188 in schizophrenic patients. Int J Neuropsychopharmacol. 2008;11:140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.