Abstract
Background
Insertions in exon 20 (Ex20ins) of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) are relatively insensitive to first‐ and second‐generation EGFR‐tyrosine kinase inhibitors (TKIs) in non‐small cell lung cancer (NSCLC). This study aimed to investigate the immune microenvironment features and efficacy of PD‐1/PD‐L1 blockade of NSCLC with EGFR and HER2 Ex20ins.
Methods
Clinical characteristics, coexisting mutations, and outcomes to EGFR‐TKIs and immune checkpoint blockade were reviewed for NSCLC patients with exon 20 mutations of EGFR or HER2. Data obtained included the molecular spectrum (extended genotyping for mutations in 324 cancer‐related genes), as well as tumor mutational burden (TMB), PD‐L1 protein expression, and the abundance of CD4+ and CD8+ tumor‐infiltrating lymphocytes (TILs).
Results
A total of 1270 NSCLC patients were identified. Of these, 504 (39.7%) cases had EGFR mutations and 6.9% (35/504) of them had EGFR Ex20ins. Meanwhile, 21 (1.7%) cases with HER2 Ex20ins were detected. Comprehensive genomic profiling identified A767_V769dup variant (25.0%) was the most common type in tumors with EGFR Ex20ins. Co‐occurring mutations were not uncommon including TP53 (45%), PIK3CA (20%), CDKN2A (10%), and EGFR amplification (20%). The average TMB was 3.3 mutations/megabase. PD‐L1 expression in patients with EGFR Ex20ins was significantly higher than for those with HER2 mutations (48.6% vs. 19.0%, P = 0.027). High TMB and PD‐L1 expression was independently associated with significantly poor prognosis (P = 0.025, P = 0.045, respectively) while there was no association between CD4+/CD8+ TILs and prognosis in EGFR or HER2 mutant NSCLC. Finally, patients harboring EGFR Ex20ins seemed to be sensitive to PD‐1/PD‐L1 blockage whereas it showed limited efficacy in patients with HER2 Ex20ins.
Conclusions
NSCLC patients with EGFR/HER2 Ex20ins had similar genomic characteristics and distinct immune features. Patients with EGFR Ex20ins had significantly higher PD‐L1 expression than those with HER2 mutations, which may be the potential reason for the different responses to PD‐1/PD‐L1 blockage.
Keywords: EGFR, HER2, immunotherapy, NSCLC, PD‐L1

NSCLC patients with EGFR/HER2 Ex20ins had similar genomic characteristics and distinct immune features when compared to common EGFR mutations. Patients with EGFR Ex20ins had significantly higher PD‐L1 expression than those with HER2 mutations, which may be the potential reason for the different responses to PD‐1/PD‐L1 blockage.
Introduction
Lung cancer is the most common cause of cancer‐related death worldwide, 1 with non‐small cell lung cancer (NSCLC) accounting for 75% of all lung cancer cases. 2 Most patients with NSCLC harboring sensitizing epidermal growth factor receptor (EGFR) mutations confer a high response rate of 70%–80% to EGFR‐tyrosine kinase inhibitors (TKIs) such as erlotinib, gefitinib, and afatinib. 3 , 4 , 5 However, approximately 4%–12% of EGFR‐mutant NSCLC tumors have in‐frame insertions within exon 20, named EGFR Ex20ins, and are generally insensitive to first‐ or second‐generation EGFR‐TKIs due to the modified structures of their kinase domains (except for A763_764insFQEA), 6 , 7 , 8 resulting in a dismal prognosis. 9 , 10
Currently, a family of Ex20ins has been described in the human epidermal growth factor receptor 2 gene (HER2, also known as ERBB2), which consists of 90% of HER2 alterations in NSCLC, and approximately 2%–4% of patients with NSCLC harbor these mutations. 11 , 12 Exon 20 alterations of EGFR and HER2 have similar crystal structures and biological functions, including the α‐C helix (residues 762–766 in EGFR and 770–774 in HER2) and the loop following the α‐C helix (residues 767–774 in EGFR and 775–783 in HER2). 12 , 13 Generally, mutations in exon 20 have shown constitutive phosphorylation of kinase domain, resulting in downstream activation of the PI3K‐AKT and MAPK pathways, which induce tumor initiation and development. 12 Similar to the patients with EGFR Ex20ins, treatment with the EGFR/HER2‐selective TKIs (eg, afatinib or dacomitinib) have reported limited success in patients with exon 20 mutations of HER2. 12 However, several next‐generation EGFR‐TKIs (some with pan‐HER activity) have demonstrated preclinical activity against mutations in exon 20 of either EGFR or HER2 and are in clinical development (TAK‐788, osimertinib, poziotinib). 12 , 14 To date, no EGFR‐TKIs are approved for these patients and their diversity of structures suggests that different insertion events may have divergent responsiveness to various TKIs. 13 Thus, identifying the clinical features and new treatment response is of paramount importance.
Immune checkpoint inhibitors (ICIs) such as programmed cell death‐1 (PD‐1)/programmed cell death ligand‐1 (PD‐L1) antibodies have led to unprecedented durable clinical benefit for NSCLC, but response rates are low for patients with targetable driver mutations. 15 Biomarker studies have revealed a significant correlation between PD‐L1 expression and the likelihood of a response to PD‐1 inhibitors, whereas EGFR mutation appears to be a negative predictive factor. 16 , 17 Given that most sensitizing EGFR mutations are not responsive to PD‐1 blockade either exon 19 deletions or L858R in exon 21; however, it is unclear whether such treatment is also without benefit in patients with uncommon EGFR mutations. 18 Currently, few reports with integrated analyses interpret the underlying mechanism of the response to PD‐1/PD‐L1 inhibitors in the Ex20ins subgroup. Therefore, there is a substantial clinical need to identify new therapies to overcome the innate drug resistance of NSCLC tumors harboring exon 20 insertions in EGFR or HER2.
The present study aimed to evaluate the genomic and immune characteristics of NSCLC patients with EGFR or HER2 exon 20 mutations from a large dataset with molecular spectrum, tumor mutational burden (TMB), PD‐L1 expression, the CD4+ and CD8+ tumor‐infiltrating lymphocytes (TILs) infiltration, as well as the efficacy of immune checkpoint inhibitor and TKIs.
Methods
Patients
Among 1270 NSCLC patients, 35 patients carrying EGFR Ex20ins and 21 patients harboring HER2 Ex20ins in Zhejiang Cancer Hospital between April 2016 and September 2018 were retrospectively enrolled. Clinical data were obtained from the electronic medical record database. Tumor response was examined by computed tomography (CT) and evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The study protocol was approved by the ethics committee of Zhejiang Cancer Hospital.
Immunohistochemistry
Immunohistochemistry (IHC) analyses were carried out according to standard protocols. Immunohistochemical staining for PD‐L1 was performed using Dako PD‐L1 IHC 22C3 pharmDx kit and a Dako Autostainer Link 48 with standard antigen retrieval methods. The antibodies used for TILs infiltration evaluation were antihuman CD4 (clone: B468A1, diluted at 1:200, Santa Cruz, Texas, USA) and antihuman CD8 (clone 144B, diluted at 1:100, Abcam, Cambridge, UK). All IHC stained sections were reviewed by two independent pathologists who were blinded to the clinical information.
Tumors with ≥1% of tumor cells stained in membrane were considered positive for PD‐L1. 7 PD‐L1 immunohistochemistry (Clone 22C3) was graded by a tumor positive score (TPS) system. Consistent with a previous study, 19 our study examined CD4 and CD8 staining on lymphocytes as the proportion of positive cells among all nucleated cells, and soring was recorded as negative (<10%) or positive (≥10%).
Genomic analysis
Collected formalin‐fixed, paraffin‐embedded tumor specimens underwent histological review, and only those containing sufficient tumor cells as shown by hematoxylin‐eosin staining were subjected to nucleic acid extraction. A total of 31 patients including 20 cases with EGFR mutation and 11 cases with HER2 mutation received comprehensive genomic profiling (CGP) testing to determine the genomic status, which targets 324 cancer‐associated genes. Patient samples were evaluated for genomic alterations, including base pair substitutions, insertions/deletions (indels), copy number alterations, and rearrangements. TMB was characterized in 31 individuals as the number of somatic base substitution or indel alterations per megabase (Mb) per previously described methods. 20 In line with a previous study, 8 the cutoff value of TMB was five mutations/Mb in patients with Ex20ins. In addition, the remaining 15 patients with EGFR Ex20ins and 10 with HER2 mutation were tested for cancer‐associated genes (EGFR/ALK/ROS1/KRAS/NRAS/BRAF/RET/HER2/PIK3CA/MET) by polymerase chain reaction. Tumor specimens and genomic DNA were isolated using the AMRS DNA Sample Preparation Kit (Amoy Diagnostics, Xiamen, People's Republic of China) following the manufacturer's instructions.
Statistical analysis
Statistical analyses were performed using SPSS version 20.0 (Chicago, IL) and GraphPad Prism (version 7.01). The chi‐square test or Fisher's exact test were used to assess the significance among categorical variables. The survival curves were generated by Kaplan‐Meier estimator and the Cox proportional hazards regression model was adopted to determine the hazard ratio (HR). Variables of univariate analysis with P < 0.1 were included in the multivariate Cox regression analysis. Statistical significance was considered as P ≤ 0.05 using a two‐sided test.
Results
Clinical characteristics
Among 1270 NSCLC patients, 504 (39.7%) cases had EGFR mutations. A total of 35 cases with EGFR Ex20ins and 21 cases harboring HER2 Ex20ins were identified. Of the patients with EGFR/HER2 Ex20ins, the median age was 56 years old (range: 29–80 years); 57.1% (32 patients) were female, 71.4% (40 patients) were younger than 65 years, 32.1% (18 patients) were current or former smokers, 85.7% (48 patients) were diagnosed with stage IV, 46.4% (26 patients) had family history, 50.0% (28 patients) previously received TKIs, 89.3% (50 patients) previously received chemotherapy, 53.6% (30 patients) were given third‐line or beyond therapy, and 91.1% (51 patients) had a performance status (PS) score of 0 to 1. No differences on above baseline characteristics were found between EGFR and HER2 mutant NSCLC (P > 0.05, Table 1). Clinical demographics of this cohort are summarized in Table 1.
Table 1.
The clinicopathological factors in NSCLC patients with EGFR or HER2 Ex20ins
| Parameters | All cases (N = 56) | EGFR Ex20ins | HER2 EX20ins | P‐value |
|---|---|---|---|---|
| Gender | ||||
| Male | 24 (42.9%) | 15 (42.9%) | 9 (42.9%) | 1.000 |
| Female | 32 (57.1%) | 20 (57.1%) | 12 (57.1%) | |
| Age, year | ||||
| <65 | 40 (71.4%) | 22 (62.9%) | 18 (85.7%) | 0.067 |
| ≥65 | 16 (28.6%) | 13 (37.1%) | 3 (14.3%) | |
| Smoking status | ||||
| Never | 38 (67.9%) | 25 (71.4%) | 13 (61.9%) | 0.460 |
| Ever/current | 18 (32.1%) | 10 (28.6%) | 8 (38.1%) | |
| Performance status | ||||
| 0–1 | 51 (91.1%) | 31 (88.6%) | 20 (95.2%) | 0.717 |
| 2 | 5 (8.9%) | 4 (11.4%) | 1 (4.8%) | |
| Family history | ||||
| Yes | 26 (46.4%) | 13 (37.1%) | 13 (61.9%) | 0.072 |
| No | 30 (53.6%) | 22 (62.9%) | 8 (38.1%) | |
| Disease stage | ||||
| IIIb | 8 (14.3%) | 4 (11.4%) | 4 (19.0%) | 0.430 |
| IV | 48 (85.7%) | 31 (88.6%) | 17 (81.0%) | |
| PD‐L1 status | ||||
| <1% | 36 (64.3%) | 18 (51.4%) | 17 (81.0%) | 0.027 |
| ≥1% | 20 (35.7%) | 17 (48.6%) | 4 (19.0%) | |
| CD4 expression | ||||
| Negative | 34 (60.7%) | 22 (62.9%) | 12 (57.1%) | 0.672 |
| Positive | 22 (39.3%) | 13 (37.1%) | 9 (42.9%) | |
| CD8 expression | ||||
| Negative | 22 (39.3%) | 14 (40.0%) | 8 (38.1%) | 0.888 |
| Positive | 34 (60.7%) | 21 (60.0%) | 13 (61.9%) | |
| Lines of treatment | ||||
| First/second | 26 (46.4%) | 15 (42.9%) | 10 (47.6%) | 0.729 |
| Third/posterior line | 30 (53.6%) | 20 (57.1%) | 11 (52.4%) | |
| Previous TKI therapy | ||||
| Yes | 28 (50.0%) | 15 (42.9%) | 13 (61.9%) | 0.168 |
| No | 28 (50.0%) | 20 (57.1%) | 8 (38.1%) | |
| Previous chemotherapy therapy | ||||
| Yes | 50 (89.3%) | 30 (85.7%) | 20 (95.2%) | 0.265 |
| No | 6 (10.7%) | 5 (14.3%) | 1 (4.8%) | |
| Total | 56 | 35 (62.5%) | 21 (37.5%) | |
TKI, tyrosine kinase inhibitors.
Genomic characteristics
CGP identified A767_V769dup variant was the most frequent, comprising 25.0% of EGFR Ex20ins cases. In addition, EGFR Ex20ins patients had co‐occurring mutations including TP53 (45%), PIK3CA (20%), CDKN2A (10%), and EGFR amplification (20%). Figure 1a shows the subtype of EGFR Ex20ins variants among NSCLC and Fig 1b indicates the distribution of EGFR Ex20ins in patients with concurrent mutations. Notably, the average TMB of EGFR/HER2 Ex20ins was 3.3 mutations/Mb;and eight cases (25.8%) had TMB more than five mutations/Mb. Further multivariate analysis suggested that TMB was an independent prognostic factor after adjusting for clinicopathological factors (HR = 4.95, 95% confidence interval [CI]: 1.46–6.84, P = 0.025, Table 2). With regard to HER2 Ex20ins mutants, A775_G776insYVMA variant was the most common type, making up 80.9% of HER2‐mutant cases. The most common concurrent mutations were TP53 and PIK3CA.
Figure 1.
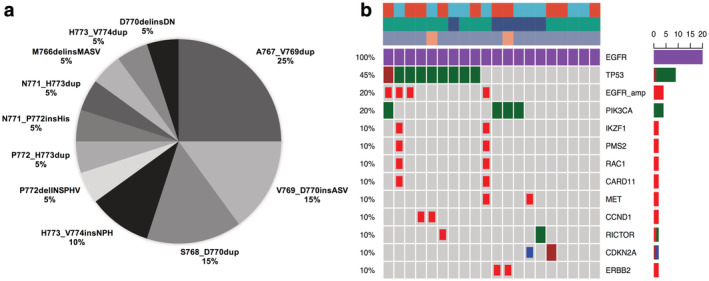
(a) The subtype of EGFR Ex20ins variants among NSCLC. (b) The distribution of EGFR Ex20ins in NSCLC patients with concurrent mutations. Somatic gene mutations detected with comprehensive genomic profiling testing covering 324 genes in lung cancer specimens positive for exon 20 mutation of EGFR. Each column corresponds to one of the 20 patients. Alterations:  , Missense;
, Missense;  , CN_del;
, CN_del;  , CN_amp;
, CN_amp;  , Indel;
, Indel;  , Splice_site. Gender:
, Splice_site. Gender:  , Male;
, Male;  , Female. Age:
, Female. Age:  , <65;
, <65;  , >=65. Stage:
, >=65. Stage:  , III;
, III;  , IV.
, IV.
Table 2.
Univariate and multivariate Cox regression analyses of prognostic factors for survival in NSCLC patients with EGFR or HER2 Ex20ins
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Parameters | HR (95% CI) | P‐value | HR (95% CI) | P‐value |
| Gender | ||||
| Male | 1.00 | 1.00 | ||
| Female | 0.38 (0.13–1.09) | 0.073 | 0.22 (0.11–1.43) | 0.052 |
| Age, year | ||||
| <65 | 1.00 | |||
| ≥65 | 0.80 (0.26–2.45) | 0.702 | ||
| Smoking status | ||||
| Never | 1.00 | |||
| Ever/current | 1.73 (0.65–4.62) | 0.276 | ||
| Performance status | ||||
| 0–1 | 1.00 | |||
| 2 | 0.89 (0.31–2.59) | 0.835 | ||
| Family history | ||||
| Yes | 1.00 | |||
| No | 0.51 (0.18–1.39) | 0.188 | ||
| Disease stage | ||||
| IIIb | 1.00 | |||
| IV | 2.92 (0.27–3.99) | 0.350 | ||
| PD‐L1 expression | ||||
| <1% | 1.00 | 1.00 | ||
| ≥1% | 4.67 (1.80–12.1) | 0.002 | 6.21 (1.05–8.22) | 0.045 |
| CD4 expression | ||||
| No | 1.00 | |||
| Yes | 0.70 (0.25–1.98) | 0.506 | ||
| CD8 expression | ||||
| No | 1.00 | |||
| Yes | 0.46 (0.18–1.17) | 0.104 | ||
| TMB | ||||
| Low (<5 mutation/Mb) | 1.00 | 1.00 | ||
| High (≥5 mutation/Mb) | 6.7 (1.59–8.41) | 0.019 | 4.95 (1.46–6.84) | 0.025 |
CI, confidence interval; HR, hazard ratio; Mb, megabase; TMB, tumor mutational burden.
Immune microenvironment feature
PD‐L1 staining as well as the presence and absence of CD4+ and CD8 + TILs were used to show the tumor immune microenvironment feature (Fig 2). In total, 48.6% (17/35) of the NSCLC patients showed positive PD‐L1 expression in TCs of EGFR Ex20ins tumors; this percentage was markedly higher than that in HER2‐mutant patients (48.6% vs. 19.0%, P = 0.027), whereas the distribution of CD4+ or CD8+ TILs was similar in these two groups (P = 0.672, P = 0.888, Table 1). Among the patients with EGFR and HER2 Ex20ins, the median OS was significantly shorter in the PD‐L1‐positive group than in the PD‐L1‐negative group (12.0 vs. 28.6 months, P = 0.001, Fig 3) and PD‐L1 was identified as an independent predictor (HR = 6.21, 95% CI: 1.05–8.22, P = 0.045, Table 2). Neither the infiltration of CD4+ TILs nor that of CD8+ TILs showed prognostic value in EGFR and HER2 mutant NSCLC (P = 0.503, P = 0.095, respectively; Fig 3). Moreover, no relationship between PD‐L1 expression and TILs infiltration was found in patients with Ex20ins (P = 0.888, P = 0.672, respectively).
Figure 2.
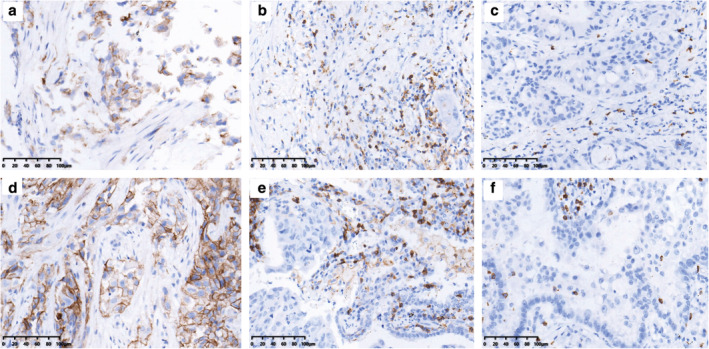
The expression of PD‐L1 and infiltration of CD4+, CD8+ T cells within NSCLC patients harboring Ex20ins of EGFR or HER2. NSCLC tumors with EGFR Ex20ins: (a) Positive expression of PD‐L1; (b) presence of CD4+ TILs; (c) presence of CD8+ TILs. NSCLC tumors with HER2 Ex20ins: (d) Positive expression of PD‐L1; (e) presence of CD4+ TILs; and (f) presence of CD8+ TILs. (*200).
Figure 3.
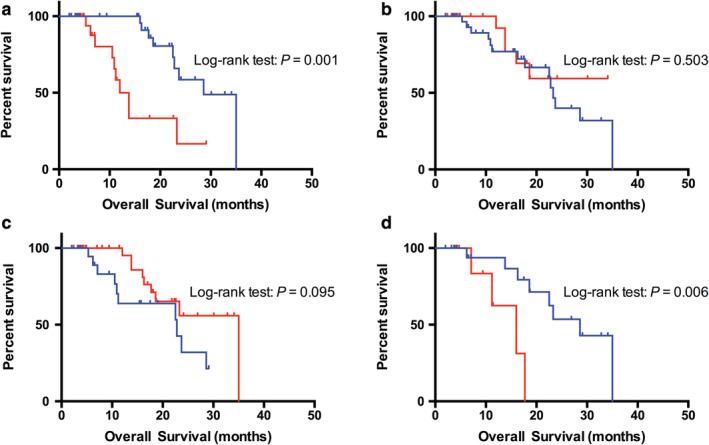
Kaplan‐Meier curves of the median overall survival (OS) of Ex20ins NSCLC patients stratified by PD‐L1 expression, TIL infiltration and TMB data. (a) NSCLC patients with PD‐L1 expression had a median OS time of 12.0 months, which was shorter than the median OS time of patients with negative expression (12.0 vs. 28.6 months, P = 0.001)  , PD‐L1 Negative;
, PD‐L1 Negative;  , PD‐L1 Positive. (b,c) Neither the infiltration of CD4+ TILs nor that of CD8+ TILs showed prognostic value in EGFR or HER2 mutant NSCLC (P = 0.503, P = 0.095, respectively)
, PD‐L1 Positive. (b,c) Neither the infiltration of CD4+ TILs nor that of CD8+ TILs showed prognostic value in EGFR or HER2 mutant NSCLC (P = 0.503, P = 0.095, respectively)  , CD4 Negative;
, CD4 Negative;  , CD4 Positive;
, CD4 Positive;  , CD8 Negative;
, CD8 Negative;  , CD8 Positive. (d) NSCLC patients with TMB‐high exhibited a median OS time of 16.0 months, which was shorter than those in TMB‐low group (16.0 vs. 28.6 months, P = 0.006)
, CD8 Positive. (d) NSCLC patients with TMB‐high exhibited a median OS time of 16.0 months, which was shorter than those in TMB‐low group (16.0 vs. 28.6 months, P = 0.006)  , TMB‐low;
, TMB‐low;  , TMB‐high.
, TMB‐high.
Treatment and survival outcomes
Median OS for the entire population with EGFR or HER2 exon 20 mutations was 23.3 months (95% CI: 18.1–28.2). There was no survival difference between EGFR exon 20 insertion and HER‐2 exon 20 insertion (23.3 vs. 35.0 months, P = 0.168, Fig 4a), and half of the patients had previously received EGFR‐TKIs. Specifically, 15 patients (42.9%) with tumors expressing an Ex20ins of EGFR were treated with targeted therapeutic strategies (eg, icotinib, gefitinib or afatinib) with an objective response rate (ORR) of 13.3%, and 61.9% of HER2‐mutant cases received pyrotinib or afatinib with an ORR of 11.1%. Progression‐free survival (PFS) was similar between both groups (2.6 vs. 1.0 months; P = 0.989, Fig 4b). Although the efficacy of TKIs was dismal, one patient with HER2 Ex20ins had a partial response (PR) to pyrotinib with a PFS of 9.0 months. Additionally, most patients (N = 40) received carboplatin or cisplatin/pemetrexed with an ORR of 30.0%, and were then treated with docetaxel with an ORR of 10.0% when their disease progressed. The efficacy of chemotherapy was similar between NSCLC patients with Ex20ins and wild‐type. 3 , 4
Figure 4.

Kaplan‐Meier curves of the median OS among NSCLC patients with Ex20ins (a)  , EGFR 20ins;
, EGFR 20ins;  , HER2 20ins, and progression‐free survival (PFS) of Ex20ins NSCLC patients to TKIs treatment (b)
, HER2 20ins, and progression‐free survival (PFS) of Ex20ins NSCLC patients to TKIs treatment (b)  , EGFR 20ins;
, EGFR 20ins;  , HER2 20ins. There was no survival difference between EGFR exon 20 insertion and HER‐2 exon 20 insertion (23.3 vs. 35.0 months, P = 0.168). Also, PFS was similar between population with EGFR and HER2 Ex20ins who received TKI therapy (2.6 vs. 1.0 months, P = 0.989).
, HER2 20ins. There was no survival difference between EGFR exon 20 insertion and HER‐2 exon 20 insertion (23.3 vs. 35.0 months, P = 0.168). Also, PFS was similar between population with EGFR and HER2 Ex20ins who received TKI therapy (2.6 vs. 1.0 months, P = 0.989).
To evaluate the clinical response to PD‐1/PD‐L1 blockade based on immune microenvironment status, 15 patients given immunotherapy including nine patients with EGFR Ex20ins and six with HER2 Ex20ins were included in the present study. Among EGFR mutants, the ORR was 22.2% (2/9),and one patient experienced stable disease with a PFS of 10.0 months. Both outcomes were confirmed by subsequent imaging. As for HER2‐mutant cases treated with PD‐1 inhibitor, the ORR was 0.0% (0/6). Notably, one patient experienced a disease hyperprogression (HPD) combined with a serious interstitial pneumonia, which was the cause of his death. The PFS for all individuals during immunotherapy and the representative CT images are shown in Fig 5.
Figure 5.
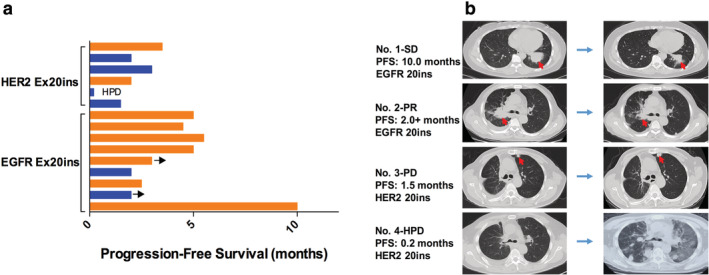
(a) Swimmer plot for duration of disease stability or response to immune checkpoint inhibitors in patients with exon 20 mutation of EGFR or HER2. A total of 15 patients received immunotherapy including nine patients with EGFR Ex20ins and six with HER2 Ex20ins. Bar length indicates the duration of immunotherapy treatment for each patient, with the response observed before treatment failure indicated on the right. The origin corresponds to treatment start date, and the arrow indicates an ongoing response at the time of data censoring  , PD‐L1 ≥1%;
, PD‐L1 ≥1%;  , PD‐L1 <1%. (b) Computed tomography scans of the thorax performed prior (baseline) and after anti‐PD‐1 treatment onset in four patients. The red arrow shows the lung lesion.
, PD‐L1 <1%. (b) Computed tomography scans of the thorax performed prior (baseline) and after anti‐PD‐1 treatment onset in four patients. The red arrow shows the lung lesion.
Discussion
To our knowledge, this study is the first to report the genomic and immune microenvironment features in NSCLC patients with Ex20ins of EGFR or HER2. In our dataset, EGFR Ex20ins alterations were identified in 35 cases (6.9% of EGFR‐mutant NSCLC and 2.8% of all NSCLC), and HER2 mutations were detected in 1.7% of NSCLC patients. EGFR and HER2 mutations were associated with younger population, female sex, and never‐smoker status, which was similar to previous observations in patients with sensitizing EGFR mutations. 21 Overall, patients with EGFR or HER2 Ex20ins had similar clinical characteristics to those with common EGFR mutations.
CGP identified A767_V769dup variant was the most common mutant type in lung tumors with EGFR Ex20ins. Co‐occurring mutations were not uncommon including TP53 (45.0%), PIK3CA (20.0%), CDKN2A (10.0%), and EGFR amplification (20.0%). In addition, A775_G776insYVMA variant was the most common type among HER2 Ex20ins and had high frequency concurrent mutations including TP53 and PIK3CA. In line with previous studies, 7 , 8 , 9 the most common coexisting genomic alteration of Ex20ins was TP53, with the mutation of PIK3CA being considered actionable as a result of its association with the therapeutic efficacy of PI3K‐AKT‐mTOR pathway inhibitors. 22 Notably, comutational landscape was analgous between EGFR Ex20ins and sensitizing EGFR mutation.
In light of the advent of immunotherapy for the treatment of NSCLC, there is an increasing knowledge about the limited efficacy of PD‐1/PD‐L1 inhibitors in patients with EGFR‐mutant NSCLC. 15 , 23 , 24 , 25 To date, PD‐L1 is the best biomarker to guide immunotherapy. Our study explored the distribution of PD‐L1 expression in patients with EGFR and HER2 Ex20ins as well as the infiltration of TILs. PD‐L1 expression in patients with EGFR Ex20ins was significantly higher than for those with HER2 mutations (48.6% vs. 19.0%, P = 0.027). Likewise, a previous study reported that exon 20 mutants had higher PD‐L1 expression (48%) than HER2‐mutant (23%) or sensitizing EGFR‐mutant (22%) lung cancers. 26 Meanwhile, this study identified that EGFR exon 20 mutants had a higher PFS and ORR to immunotherapy than those with common EGFR and HER2 mutations (PFS: 4.0 vs. 1.9 vs. 1.9 months; ORR: 19% vs. 0% vs. 9%; P < 0.05), 26 which may ascribe to high PD‐L1 expression. Nevertheless, a recent article showed that 81.7% of lung adenocarcinoma patients with EGFR Ex20ins had positive PD‐L1 expression (>1%). 7 The higher PD‐L1 expression might be influenced by diverse populations, PD‐L1 antibody assay, and limited tissue samples; however, results from larger studies are still warranted.
Since TILs density are considered to act as a prognostic parameter and a predictive marker for the efficacy of PD‐1/PD‐L1 inhibitors, 27 we surveyed its relevance in NSCLC patients with Ex20ins. The distribution of CD4+ and CD8+ TILs was similar between EGFR and HER2 mutant groups. However, neither the infiltration of CD4+ TILs nor that of CD8+ TILs showed prognostic value in our cohort, which was consistent with previous observations. 7 Moreover, no relationship between PD‐L1 expression and TILs infiltration was found in patients with Ex20ins.
In addition to PD‐L1 expression and TILs infiltration, other features such as TMB also appear to associated with clinical benefit from PD‐1/PD‐L1 inhibitors. 28 In this study, mean TMB of Ex20ins was 3.3 mutations/Mb. TMB measured by CGP is comparable to measurements by whole exome sequencing and typically higher in smoking associated lung cancer due to tobacco carcinogenesis. 8 Jonathan et al. reported the TMB of patients with EGFR Ex20ins was relatively low by analyzing 263 cases (mean 4.3, range 0–40.3 mutations/Mb). 8 Likewise, median TMB was lowest (2.6 mutation/Mb) in cases with del19/L858R, and highest (5.2 mutation/Mb) in cases with G719X. 29 In addition, EGFR‐mutated lung cancer has been previously shown to have a lower TMB compared with EGFR wild‐type lung cancer. 30 Collectively, TMB has also been reported to be low in EGFR‐mutant NSCLC cases, such as EGFR Ex20ins, probably reflecting non‐tobacco associated carcinogenesis. 8
For EGFR and HER2 exon 20 insertions, a rigid placement of the α‐C helix in the inward and the phosphate‐binding loop (P‐loop) into the drug‐binding pocket, caused steric hindrance of the drug‐binding pocket from two directions, increasing the difficulty of drug binding. 12 NSCLC patients with Ex20ins rarely achieve clinical benefit from EGFR‐TKIs, with an ORR around 11% and PFS of 2.0 months. 31 Due to limited efficacy, clinical trials focusing on lung cancers harboring Ex20ins are substantially necessary. A recent clinical trial studying TAK‐788 (EGFR/HER2 exon 20 inhibitor) showed an ORR of 43%, disease control rate of 86%, and median PFS of 7.3 months. 14 Another phase II trial of poziotinib in 44 patients with Ex20ins was evaluated, which showed an ORR of 43.0%, and median PFS of 5.5 months, 12 but the updated ORR of 14.8% among 115 patients is dismal. In addition, a study from China evaluated pyrotinib in the treatment of HER2‐mutated NSCLC with an ORR of 53%. 11 In the present study, one patient harboring a HER2 Ex20ins received pyrotinib experienced a PR with PFS of 9.0 months. In addition, a number of new antibody‐conjugated drugs have also emerged. For example, T‐DM1 treatment in patients with HER2‐mutant lung cancer obtained an ORR of 44% and 5.0 months of median PFS. 32 At present, the application of new compounds targeting EGFR and HER2 exon 20 mutations is promising.
However, no EGFR‐TKIs are currently approved for these patients and their diversity of structures suggests that different insertion events may have divergent responsiveness to TKIs. Immune checkpoint inhibition with PD‐1 and PD‐L1 antibodies has revolutionized the treatment landscape of NSCLC. 27 However, patients with EGFR‐mutant NSCLC may be insensitive to PD‐1/PD‐L1 blockade. A recent meta‐analysis showed no OS benefit compared to chemotherapy in this population. 15 In our cohort, 15 cases received PD‐1/PD‐L1 inhibitors. Nine patients harboring a EGFR Ex20ins mutation showed two PR, whereas patients with HER2 mutation showed limited response to immunotherapy. What is more, one patient experienced HPD together with serious interstitial pneumonia. In line with the efficacy showed previously, 26 patients with HER2 Ex20ins seemed to have a poor response to the PD‐1/PD‐L1 blockade when compared with those with EGFR Ex20ins. Notably, a clinical trial of PD‐1 inhibitor treating NSCLC patients with EGFR or HER2 Ex20ins is being carried out in our center, and further research conclusions are expected.
Limitations to our work include its retrospective nature as it involved a limited number of patients. Thus, it is certain that prospective, preferably multiregion, studies must follow in order to draw definitive conclusions. Moreover, a CGP test was not applied to every individual due to sample accessibility. Finally, the IHC scores for PD‐L1 expression and CD4+/CD8+ TILs infiltration could be affected by specimen type and antibody assay.
In conclusion, our study confirmed that lung tumors harboring EGFR or HER2 Ex20ins have clinical and genomic characteristics which resemble those carrying sensitizing EGFR oncogenes. Additionally, it is likely that patients with EGFR Ex20ins will benefit from exposure to immunotherapy, a finding based on PD‐L1 expression; while PD‐1/PD‐L1 inhibitors showed limited efficacy in HER2 mutants, which might benefit from new therapeutic strategies such as poziotinib or pyrotinib. Taken together, to maximize this effort, it is important to consider the execution of multinational studies in clinical trials in order to assess efficacy, highlighting the possibility of new therapeutic strategies in this patient subset.
Disclosure
None of the authors reported a conflict of interest related to the study.
Acknowledgments
This study was supported by a grant from the Basic Public Foundation of Zhejiang Province of China (No. LGF18H160017).
References
- 1. Miller KD, Nogueira L, Mariotto AB et al Cancer treatment and survivorship statistics. CA Cancer J Clin 2019; 69: 363–85. [DOI] [PubMed] [Google Scholar]
- 2. Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet 2000; 355: 479–85. [DOI] [PubMed] [Google Scholar]
- 3. Shi Y, Zhang L, Liu X et al Icotinib versus gefitinib in previously treated advanced non‐small‐cell lung cancer (ICOGEN): A randomised, double‐blind phase 3 non‐inferiority trial. Lancet Oncol 2013; 14: 953–61. [DOI] [PubMed] [Google Scholar]
- 4. Yang JC, Wu YL, Schuler M et al Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐lung 3 and LUX‐lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141–51. [DOI] [PubMed] [Google Scholar]
- 5. Klughammer B, Brugger W, Cappuzzo F et al Examining treatment outcomes with erlotinib in patients with advanced non‐small cell lung cancer whose tumors harbor uncommon EGFR mutations. J Thorac Oncol 2016; 11: 545–55. [DOI] [PubMed] [Google Scholar]
- 6. Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non‐small‐cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012; 13: e23–31. [DOI] [PubMed] [Google Scholar]
- 7. Cardona AF, Rojas L, Zatarain‐Barron ZL et al EGFR exon 20 insertion in lung adenocarcinomas among Hispanics (geno1.2‐CLICaP). Lung Cancer 2018; 125: 265–72. [DOI] [PubMed] [Google Scholar]
- 8. Riess JW, Gandara DR, Frampton GM et al Diverse EGFR exon 20 insertions and co‐occurring molecular alterations identified by comprehensive genomic profiling of NSCLC. J Thorac Oncol 2018; 13: 1560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arcila ME, Nafa K, Chaft JE et al EGFR exon 20 insertion mutations in lung adenocarcinomas: Prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther 2013; 12: 220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pan Y, Zhang Y, Li Y et al Prevalence, clinicopathologic characteristics, and molecular associations of EGFR exon 20 insertion mutations in east Asian patients with lung adenocarcinoma. Ann Surg Oncol 2014; 21 (Suppl 4): S490–6. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Jiang T, Qin Z et al HER2 exon 20 insertions in non‐small‐cell lung cancer are sensitive to the irreversible pan‐HER receptor tyrosine kinase inhibitor pyrotinib. Ann Oncol 2019; 30: 447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robichaux JP, Elamin YY, Tan Z et al Mechanisms and clinical activity of an EGFR and HER2 exon 20‐selective kinase inhibitor in non‐small cell lung cancer. Nat Med 2018; 24: 638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takeda M, Sakai K, Hayashi H et al Clinical characteristics of non‐small cell lung cancer harboring mutations in exon 20 of EGFR or HER2. Oncotarget 2018; 9: 21132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Janne PA, Neal JW, Camidge DR et al Antitumor activity of TAK‐788 in NSCLC with EGFR exon 20 insertions. J Clin Oncol 2019; 37: 9007. [Google Scholar]
- 15. Lee CK, Man J, Lord S et al Checkpoint inhibitors in metastatic EGFR‐mutated non‐small cell lung Cancer‐a meta‐analysis. J Thorac Oncol 2017; 12: 403–7. [DOI] [PubMed] [Google Scholar]
- 16. Takada K, Toyokawa G, Tagawa T et al PD‐L1 expression according to the EGFR status in primary lung adenocarcinoma. Lung Cancer 2018; 116: 1–6. [DOI] [PubMed] [Google Scholar]
- 17. Liang W, Guo M, Pan Z et al Association between certain non‐small cell lung cancer driver mutations and predictive markers for chemotherapy or programmed death‐ligand 1 inhibition. Cancer Sci 2019; 110: 2014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gainor JF, Shaw AT, Sequist LV et al EGFR mutations and ALK rearrangements are associated with low response rates to PD‐1 pathway blockade in non‐small cell lung cancer: A retrospective analysis. Clin Cancer Res 2016; 22: 4585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Su S, Dong ZY, Xie Z et al Strong programmed death ligand 1 expression predicts poor response and de novo resistance to EGFR tyrosine kinase inhibitors among NSCLC patients with EGFR mutation. J Thorac Oncol 2018; 13: 1668–75. [DOI] [PubMed] [Google Scholar]
- 20. Chalmers ZR, Connelly CF, Fabrizio D et al Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017; 9: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lohinai Z, Hoda MA, Fabian K et al Distinct epidemiology and clinical consequence of classic versus rare EGFR mutations in lung adenocarcinoma. J Thorac Oncol 2015; 10: 738–46. [DOI] [PubMed] [Google Scholar]
- 22. Janku F, Wheler JJ, Naing A et al PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early‐phase clinical trials. Cancer Res 2013; 73: 276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tu HY, Ke EE, Yang JJ et al A comprehensive review of uncommon EGFR mutations in patients with non‐small cell lung cancer. Lung Cancer 2017; 114: 96–102. [DOI] [PubMed] [Google Scholar]
- 24. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387: 1540–50. [DOI] [PubMed] [Google Scholar]
- 25. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Negrao MV, Reuben A, Robichaux JP et al Association of EGFR and HER‐2 exon 20 mutations with distinct patterns of response to immune checkpoint blockade in non‐small cell lung cancer. J Clin Oncol 2018; 36: 9052. [Google Scholar]
- 27. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015; 27: 450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reck M, Schenker M, Lee KH et al Nivolumab plus ipilimumab versus chemotherapy as first‐line treatment in advanced non‐small‐cell lung cancer with high tumour mutational burden: Patient‐reported outcomes results from the randomised, open‐label, phase III CheckMate 227 trial. Eur J Cancer 2019; 116: 137–47. [DOI] [PubMed] [Google Scholar]
- 29. Ou S‐HI, Ali SM, Bogart J et al Characterization of 1,233 NSCLCs with non‐del19/L858R EGFR mutations (EGFRm) using comprehensive genomic profiling (CGP). J Clin Oncol 2018; 36: 9040. [Google Scholar]
- 30. Dong ZY, Zhong WZ, Zhang XC et al Potential predictive value of TP53 and KRAS mutation status for response to PD‐1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res 2017; 23: 3012–24. [DOI] [PubMed] [Google Scholar]
- 31. Naidoo J, Sima CS, Rodriguez K et al Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: Clinical outcomes and response to erlotinib. Cancer 2015; 121: 3212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peters S, Stahel R, Bubendorf L et al Trastuzumab emtansine (T‐DM1) in patients with previously treated HER2‐overexpressing metastatic non‐small cell lung cancer: Efficacy, safety, and biomarkers. Clin Cancer Res 2019; 25: 64–72. [DOI] [PubMed] [Google Scholar]


