Abstract
Objective
In 2017, the Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA) introduced a standardised process to appraise innovativeness of medicines. Innovative medicines are provided speeder market access and dedicated funds. Innovativeness criteria are: unmet therapeutic need, added therapeutic value and quality of the evidence (Grading of Recommendations Assessment, Development and Evaluation method). We investigated the role played by these three criteria on the final decision aimed to understand how the new Italian innovativeness appraisal framework was implemented.
Design
A desk research gathered AIFA’s appraisal reports on innovativeness and data analyses were conducted. No patients were directly involved in this study.
Setting and participants
We scrutinised all 77 appraisal reports available on AIFA’s website (2017–2020).
Primary and secondary outcome measures
The impact of the three domains on final decision was investigated through a series of univariate analyses.
Results
Among 77 appraisal reports on innovativeness available, 49 (64%) and 28 (36%) were for oncology and non-oncology medicines, respectively. The appraisals were equally distributed among ‘fully innovative’ (36%), ‘conditionally innovative’ (30%) and ‘not innovative’ (34%). Added therapeutic value was the most important driver on innovativeness decision, followed by quality of the evidence. Drugs for rare diseases and with paediatric/mixed indications were appraised ‘innovative’ by a larger proportion, but no statistical significance was found.
Conclusions
Despite some limitations, including the moderate number of appraisals, this paper provides an insight into the determinants of innovativeness appraisals for medicines in Italy and the accuracy of the appraisal process. This has important implications in terms of transparency and accountability in the prioritisation process applied to innovative medicines.
Keywords: health policy, health services administration & management, public health, international health services
Strengths and limitations of this study.
This is an original, up-to-date analysis of the new National Drugs Agency appraisals framework for drug innovativeness in the Italian setting
This study was based on a limited number of appraisals, but we systematically considered all the available ones.
The relatively small number of appraisals did not allow to analyse possible different patterns of association between the three innovativeness criteria and the type of innovativeness (ie, fully or conditionally innovative).
Introduction
Market access for pharmaceuticals in Italy is managed by the Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA). AIFA, different from most other European countries’ medicines agencies, has both regulatory competence and access competence.1 The latter includes the negotiation of reimbursement, ex-factory price and managed entry agreements, and the appraisal of innovativeness status, possibly required by the pharmaceutical companies at market launch or autonomously carried out by AIFA.2 Innovativeness status has some advantages from an access perspective, including two dedicated funds (one for cancer medicines and the other for non-cancer medicines) and immediate access to regional markets.
The criteria to get innovativeness status, which can be attributed only to drugs indicated for serious illnesses (life-threatening diseases; diseases producing frequent hospitalisations or causing disabilities that can seriously compromise quality of life), are the unmet therapeutic need, the added therapeutic value and the quality of the evidence (Determina AIFA 519/2017).3
The unmet therapeutic need is rated as:
Maximum: there are no alternatives for that specific indication.
Important: there are a few alternatives, but with no impact on clinically relevant endpoints.
Moderate: there are alternatives with a limited and/or uncertain or unreliable impact on clinically relevant endpoints.
Poor: there are alternatives for the same indication with clinically proven reliable results.
Absent: there are alternatives for the same indication with an important impact on the natural history of the disease.
The added therapeutic value that refers to clinical benefit can be rated as:
Maximum: the new drug has proven larger efficacy than any possible existing alternatives. In this case, the treatment is able to either cure the illness or significantly alter its natural history.
Important: the new drug has a proven larger efficacy measured on clinically relevant endpoints, decreases the risk of invalidating or fatal complications, avoids highly dangerous clinical procedures or has more favourable risk/benefit ratio than any available alternatives. In a subset of patients, the treatment either modifies the natural history of the disease or is beneficial in other clinically significant ways, for example, in terms of quality of life or disease-free intervals, when compared with available alternatives.
Moderate: the new drug has a larger efficacy than any available alternatives, but it is only moderate or only proven in some subsets of patients, with limited impact on the quality of life.
Poor: the new drug has either a limited improvement of efficacy or has been proven on endpoints which are not clinically relevant. Minor advantages, for example, more acceptable administration route.
Absent: the new drug has no relevant benefit when compared with other available treatments.
Endpoint relevance has been specified for cancer medicines, with overall survival (OS) being considered the gold standard, and the lack of OS data needed to justify. The document quotes that progression-free survival, disease-free survival, full response time or other surrogated endpoints (with already established clinical benefits) may be taken into account, according to indication and settings. Toxicity is also considered to evaluate the treatment’s adequacy.
To appraise the quality of evidence, AIFA has chosen the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method.4 According to this approach, the quality of clinical evidence can be graded as high, moderate, low or very low. The choice of GRADE methodology was aimed at improving the transparency and reproducibility of the appraisal process; this structured and flexible methodological tool provides a systematic approach in the assessment and is meant to minimise biases and improve consistency of the decisions.5
The innovativeness is appraised per indication, and the innovativeness status lasts 3 years. The appraisal model represents a common framework for all indications, even if safeguard clauses are provided for rare indications where the quality of the evidence is more likely to be lower.
The industry usually applies for innovativeness, even if AIFA can proceed to evaluate it regardless of the industry’s application. The innovativeness request is appraised by the AIFA’s Technical-Scientific Committee (CTS). CTS may decide for full innovativeness, conditional innovativeness or non-innovativeness. Conditionally innovative medicines share with fully innovative medicines only the immediate access to regional markets. Conditional innovativeness is granted when the evidence is not sufficiently mature to provide a full innovativeness status and lasts 18 months.
Despite the growing interest in these new criteria and the relevant appraisal process,6 to our knowledge only preliminary descriptive analyses (based on less of 20 innovativeness appraisals updated to 2018) were available,7–9 and no clear and robust evidence emerged on the role played by the three criteria on the final decision, if these criteria have been consistently used over time and if other variables influence the innovativeness status.
Our analyses, based on available innovativeness appraisals updated to July 2020, aim to cover these information gaps and, more in general, to understand how the new Italian innovativeness appraisal framework was implemented.
Methods
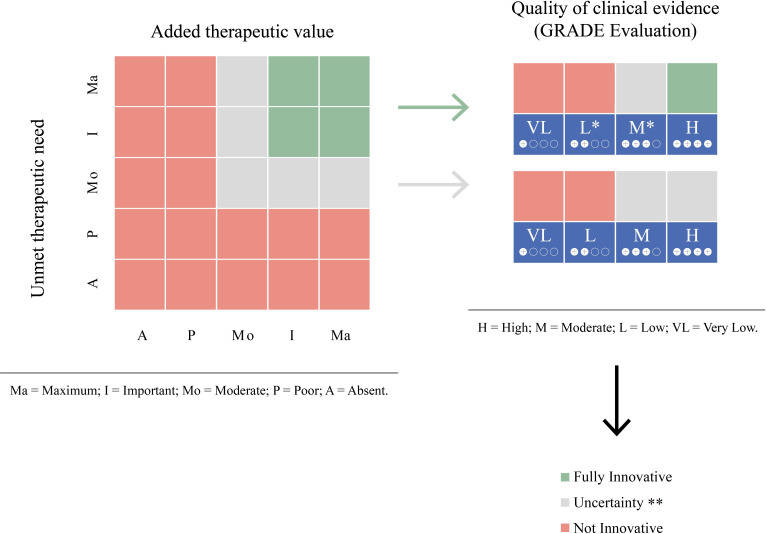
The new decision rule adopted by AIFA (figure 1)10 consists of granting innovativeness if both unmet need and added therapeutic value are graded ‘Maximum’ or ‘Important’ and the quality of evidence is rated ‘High’ (green zone). Conversely, if the unmet need or the added therapeutic value is graded ‘Poor’ or ‘Absent’, or the quality of evidence is rated ‘Low’ or ‘Very Low’ innovativeness will not be granted (red zone). For rare indications, the innovative status may be granted even if the quality of evidence is graded ‘Low’, but the unmet need and the added therapeutic value are both at least ‘Important’. To note, in the intermediate situations (grey zone) there is uncertainty about innovation status, and AIFA decides case by case.
Figure 1.
Criteria used to evaluate innovativeness adopted by the Italian Medicines Agency (adapted from Recchia, 2017).10 *For rare disease there is the following exception: the fully innovative is attributed in the presence of at least important unmet therapeutic need and added therapeutic value in presence of at least low quality of clinical evidence. **The innovativeness appraisal has to be decided on a case-by-case basis. GRADE, Grading of Recommendations Assessment, Development and Evaluation.
Pharmaceutical companies are informed by AIFA on the intended final appraisal and can rebut on appraisals in 10 days. The final appraisal is published on the AIFA’s website, together with a short description of the rationale behind the decision taken (www.aifa.gov.it). These appraisals are written in Italian only. An English version should be desirable to allow greater dissemination of information outside Italy.
Appraisal reports on innovativeness were downloaded from the AIFA’s website11 as of 31 July 2020: 77 appraisal reports were found, 49 and 28 for oncology and non-oncology medicines, respectively.
The following data were retrieved from the appraisal reports and inserted into an extraction template:
Final appraisal (‘fully innovative’, ‘conditionally innovative’ or ‘not innovative’).
Rank attributed to the unmet need, the added therapeutic value and the quality of evidence.
-
Variables that may have an influence on the final decision taken by the CTS, including:
The target disease: oncological (solid/haematological) disease or non-oncological disease (infectious/autoimmune/other diseases).
Population: adult, paediatric, mixed.
Rare disease (according to Orphanet): yes or no.
Number of ‘Summaries of Findings’ (SoF) according to the GRADE system that reported the key information concerning the magnitudes of relative and absolute effects of the interventions examined, the amount of available evidence and the certainty (or quality) of available evidence.12
Number of clinical studies considered.
Number of randomised clinical trials (RCT), supporting the application for innovativeness.
Number of observational studies, supporting the application for innovativeness.
Appraisal date.
We first calculated some descriptive statistics: frequencies and percentages for categorical variables; mean and median values, SDs, quartiles and extreme values for continuous variables.
Afterwards, we scrutinised the role played by the above-mentioned variables on the innovativeness appraisal. Fully innovative and conditionally innovative appraisals were merged in a unique category denominated ‘innovative’, given the limited number of appraisal reports. With reference to comparisons between groups (ie, innovative vs non-innovative outcome), categorical data were analysed using a contingency table with the χ2 or Fisher’s exact test, as appropriate. Continuous data were analysed using a Student’s t-test, after checking for normal distribution (based on the Shapiro-Wilk statistic), or a Wilcoxon rank-sum test otherwise.
With reference to the primary aim of this study, that is, the role played by the three domains on innovativeness status (innovativeness vs not innovativeness), we decided a priori to compare groups by using the test for continuous variables that has a higher power to detect possible differences in this set of preliminary analyses. In fact, the Fisher’s exact test has low power to detect associations, that is, the probability of obtaining false-negative conclusions (type II error) is high.
Finally, we developed a recursive algorithm for innovativeness using a determinist approach to scrutinise the role played by the three above-mentioned criteria (unmet need, therapeutic added value, quality of the evidence). This approach was merely data driven and the univariate analyses on the role played by the three domains on innovative status were the starting point to create the decision tree.
Patient and public involvement
No patients were directly involved in this study.
Results
Detailed information for each of the 77 available appraisals is reported in online supplemental table 1.
bmjopen-2020-041259supp001.pdf (139.4KB, pdf)
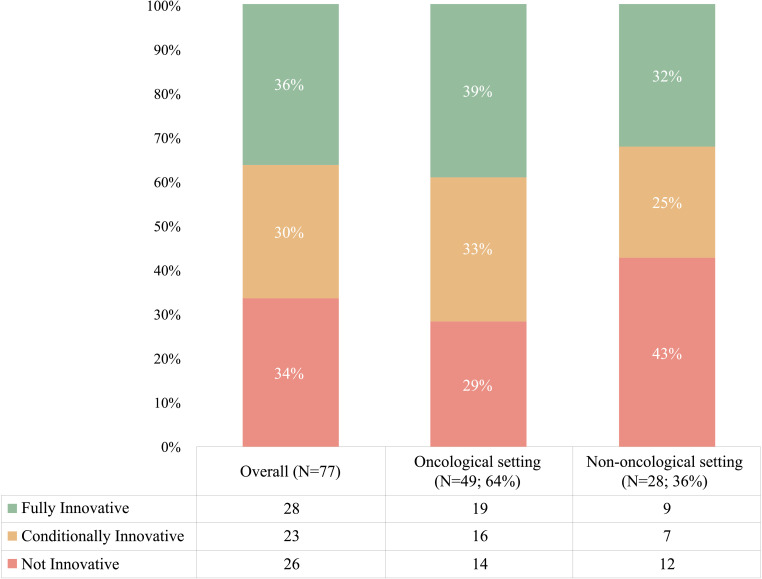
Figure 2 shows that appraisals were equally distributed among ‘fully innovative’ (36% of the total), ‘conditionally innovative’ (30%) and ‘not innovative’ (34%). Cancer medicines were more often appraised as fully innovative (39%) whereas other drugs show a higher proportion of non-innovative status (29% cancer drugs were appraised not innovative, compared with 43% non-cancer treatments), but the difference was not significant (p=0.20).
Figure 2.
Innovative appraisals by the Italian Medicines Agency (2017–2020).
The role played on innovativeness status by the appraisal year, rare disease, target disease, target population, number of SoF, overall number of studies and number of RCT and phase I/II studies is illustrated in table 1.
Table 1.
Variables detected on the appraisal document and innovativeness status (2017–2020)
| All diseases (n=77) | Oncology (n=49) | Non-oncology (n=28) | ||||||||||
| All medicines n (%) |
Innovative* n (%) |
Not innovative n (%) |
P value† | All medicines n (%) |
Innovative* n (%) |
Not innovative n (%) |
P value† | All medicines n (%) |
Innovative* n (%) |
Not innovative n (%) |
P value† | |
| CTS appraisal year | ||||||||||||
| 2017 | 28 (36.4) | 18 (64.3) | 10 (35.7) | 18 (36.7) | 12 (66.7) | 6 (33.3) | 10 (35.7) | 6 (60.0) | 4 (40.0) | |||
| 2018 | 25 (32.5) | 17 (68.0) | 8 (32.0) | 15 (30.6) | 10 (66.7) | 5 (33.3) | 10 (35.7) | 7 (70.0) | 3 (30.0) | |||
| 2019 | 24 (31.2) | 16 (66.7) | 8 (33.3) | 0.96 | 16 (32.7) | 13 (81.2) | 3 (18.8) | 0.57 | 8 (28.6) | 3 (37.5) | 5 (62.5) | 0.37 |
| Rare disease | ||||||||||||
| No | 34 (44.2) | 20 (58.8) | 14 (41.2) | 23 (46.9) | 17 (73.9) | 6 (26.1) | 11 (39.3) | 3 (27.3) | 8 (72.7) | |||
| Yes | 43 (55.8) | 31 (72.1) | 12 (27.9) | 0.22 | 26 (53.1) | 18 (69.2) | 8 (30.8) | 0.72 | 17 (60.7) | 13 (76.5) | 4 (23.5) | 0.02 |
| Disease | ||||||||||||
| Solid tumours | 30 (39.0) | 21 (70.0) | 9 (30.0) | 30 (61.2) | 21 (70.0) | 9 (30.0) | – | – | – | |||
| Haematological malignancies | 19 (24.7) | 14 (73.7) | 5 (26.3) | 19 (38.8) | 14 (73.7) | 5 (26.3) | 0.78 | – | – | – | ||
| Infectious diseases | 5 (6.5) | 3 (60.0) | 2 (40.0) | – | – | – | 5 (17.9) | 3 (60.0) | 2 (40.0) | |||
| Autoimmune diseases | 3 (3.9) | 1 (33.3) | 2 (66.7) | – | – | – | 3 (10.7) | 1 (33.3) | 2 (66.7) | |||
| Other | 20 (26.0) | 12 (60.0) | 8 (40.0) | 0.64 | – | – | – | 20 (71.4) | 12 (60.0) | 8 (40.0) | 0.68 | |
| Population | ||||||||||||
| Adults only | 65 (84.4) | 42 (64.6) | 23 (35.4) | 46 (93.9) | 33 (71.7) | 13 (28.3) | 19 (67.9) | 9 (47.4) | 10 (52.6) | |||
| Paediatric or mixed | 12 (15.6) | 9 (75.0) | 3 (25.0) | 0.74 | 3 (6.1) | 2 (66.7) | 1 (33.3) | 0.99 | 9 (32.1) | 7 (77.8) | 2 (22.2) | 0.22 |
| Mean number SoF (SD) | 3.4 (2.9) | 3.1 (2.6) | 3.8 (3.4) | 0.34 | 2.5 (2.2) | 2.5 (2.2) | 2.4 (2.1) | 0.69 | 4.9 (3.4) | 4.4 (2.8) | 5.5 (4.0) | 0.42 |
| Clinical Studies (n) | ||||||||||||
| 1 | 61 (79.2) | 41 (67.2) | 20 (32.8) | 45 (91.8) | 31 (68.9) | 14 (31.1) | 16 (57.1) | 10 (62.5) | 6 (37.5) | |||
| >1 | 16 (20.8) | 10 (62.5) | 6 (37.5) | 0.72 | 4 (8.2) | 4 (100.0) | 0 (0.0) | 0.31 | 12 (42.9) | 6 (50.0) | 6 (50.0) | 0.51 |
| RCT (n) | ||||||||||||
| 0 | 15 (19.5) | 10 (66.7) | 5 (33.3) | 12 (24.5) | 7 (58.3) | 5 (41.7) | 3 (10.7) | 3 (100.0) | 0 (0.0) | |||
| 1 | 49 (63.6) | 34 (69.4) | 15 (30.6) | 34 (69.4) | 25 (73.5) | 9 (26.5) | 15 (53.6) | 9 (60.0) | 6 (40.0) | |||
| >1 | 13 (16.9) | 7 (53.8) | 6 (46.1) | 0.57 | 3 (6.1) | 3 (100.0) | 0 (0.0) | 0.32 | 10 (35.7) | 4 (40.0) | 6 (60.0) | 0.17 |
| Clinical trials phase I/II (n) | ||||||||||||
| 0 | 59 (76.6) | 38 (64.4) | 21 (35.6) | 37 (75.5) | 28 (75.7) | 9 (24.3) | 22 (78.6) | 10 (45.4) | 12 (54.6) | |||
| ≥1 | 18 (23.4) | 13 (72.2) | 5 (27.8) | 0.54 | 12 (24.5) | 7 (58.3) | 5 (41.7) | 0.29 | 6 (21.4) | 6 (100.0) | 0 (0.0) | 0.02 |
*Innovative status includes fully and conditionally innovative.
†Comparisons between innovative and non-innovative outcomes were performed using a contingency table with the χ2 or Fisher’s exact test, as appropriate for categorical data. Continuous data were analysed using a Student’s t-test, after checking for normal distribution (based on the Shapiro-Wilk statistic), or a Wilcoxon rank-sum test otherwise.
CTS, Technical-Scientific Committee; RCT, randomised clinical trial; SoF, Summaries of Findings.
No significant association between innovativeness evaluation and the factors examined emerged when all types of disease were considered together. A similar proportion of appraisals was evaluated innovative with (66.1%) or without (66.7%) RCT evidence in support. Rare disease and paediatric/mixed indications were appraised innovative by a larger proportion, although not statistically significant. Furthermore, rarity of disease and type of disease did not seem to be determinant for the innovativeness evaluation. In the non-oncological setting, rare disease status (p=0.02) and availability of one or more phase I/II studies (p=0.02) were more frequently reported in the innovative indication group. Non-oncological forms have a higher number of RCTs supporting them compared with oncological ones (more than one RCT supporting 36% of non-oncological ones compared with approximately 6% of oncological ones).
As a second step, we investigated the role of each of the three domains on appraisals. Table 2 shows the association between unmet therapeutic need, added therapeutic need and quality of evidence and the final appraisal.
Table 2.
Role played by the three domains on innovativeness status (2017–2020)
| All medicines | Innovative‡ | Not innovative | P value* | ||||
| Unmet therapeutic need | |||||||
| n | 77 | 51 | 26 | ||||
| Maximum (scale=1) | 10 | (13.0%) | 7 | (70.0%) | 3 | (30.0%) | |
| Important (scale=2) | 30 | (39.0%) | 22 | (73.3%) | 8 | (26.7%) | |
| Moderate (scale=3) | 32 | (41.6%) | 22 | (68.7%) | 10 | (31.2%) | |
| Poor (scale=4) | 5 | (6.5%) | 0 | (0.0%) | 5 | (100.0%) | |
| Range | 1–4 | 1–3 | 1–4 | ||||
| Mean (SD) | 2.4 | (0.8) | 2.3 | (0.7) | 2.7 | (0.9) | 0.09 |
| Median (IQR) | 2 | (2–3) | 2 | (2–3) | 3 | (2–3) | |
| Added therapeutic value | |||||||
| n | 76† | 51 | 25† | ||||
| Maximum (scale=1) | 0 | (0.0%) | 0 | (0.0%) | 0 | (0.0%) | |
| Important (scale=2) | 25 | (32.9%) | 24 | (96.0%) | 1 | (4.0%) | |
| Moderate (scale=3) | 31 | (40.8%) | 27 | (87.1%) | 4 | (12.9%) | |
| Poor (scale=4) | 19 | (25.0%) | 0 | (0.0%) | 19 | (100.0%) | |
| Very poor (scale=5) | 1 | (1.3%) | 0 | (0.0%) | 1 | (100.0%) | |
| Range | 2–5 | 2–3 | 2–5 | ||||
| Mean (SD) | 2.9 | (0.8) | 2.5 | (0.5) | 3.8 | (0.6) | <0.01 |
| Median (IQR) | 3 | (2–4) | 3 | (2–3) | 4 | (4–4) | |
| Quality of clinical evidence (GRADE evaluation) |
|||||||
| n | 77 | 51 | 26 | ||||
| High (scale=1) | 11 | (14.3%) | 10 | (90.9%) | 1 | (9.1%) | |
| Moderate (scale=2) | 42 | (54.5%) | 28 | (66.7%) | 14 | (33.3%) | |
| Low (scale=3) | 18 | (23.4%) | 11 | (61.1%) | 7 | (38.9%) | |
| Very low (scale=4) | 6 | (7.8%) | 2 | (33.3%) | 4 | (66.7%) | |
| Range | 1–4 | 1–4 | 1–4 | ||||
| Mean (SD) | 2.2 | (0.8) | 2.1 | (0.8) | 2.5 | (0.8) | 0.03 |
| Median (IQR) | 2 | (2–3) | 2 | (2–3) | 2 | (2–3) | |
*Comparisons between innovative and non-innovative outcomes were performed using a Student’s t-test, after checking for normal distribution (based on the Shapiro-Wilk statistic), or a Wilcoxon rank-sum test otherwise.
†For one rating (10—Nivolumab), the added therapeutic value was reported as ‘not assessable’.
‡Innovative status includes fully and conditionally innovative.
GRADE, Grading of Recommendations Assessment, Development and Evaluation.
A significant difference between innovative and not innovative outcomes was found both for the added therapeutic value (p<0.01) and the quality of evidence domains (p=0.03). For innovative and non-innovative indications, the added therapeutic value had an average score of 2.5 (between ‘Moderate’ and ‘Important’) and 3.8 (between ‘Poor’ and ‘Moderate’), respectively. The quality of evidence for innovative and non-innovative medicines had an average score of 2.1 (‘Moderate’) and 2.5 (between ‘Low’ and ‘Moderate’), respectively. The average scores of unmet need for innovative and not innovative evaluations were not significantly different (p=0.09), being respectively equal to 2.3 and 2.7 (both between ‘Moderate’ and ‘Important’).
Taking into account the above-mentioned univariate findings, where added therapeutic value (p<0.01) and quality of evidence (p=0.03) were associated to innovativeness status, a data-driven decision tree using a deterministic approach was developed (online supplemental figure 1). The decision tree did not explicate all the appraisals’ final decision but accounted for 63 out of 77 cases (82%). As for the other 14 appraisals, eight of them were either ‘conditionally innovative’ or ‘not innovative’ because they had ‘moderate’ added therapeutic value and a ‘low’ GRADE evaluation. The other six cases were given either a ‘full’ or a ‘conditioned’ innovativeness because they had a ‘moderate’ added therapeutic value along with a ‘high’ GRADE evaluation. When the final assessment was uncertain, it was not possible to discern factors determining the final appraisal, nor to find out the driver from the characteristics of the indication, such as the disease (oncological or non-oncological) or the rarity of the disease. Finally, we found that for ultrarare diseases (≤1 patient per 100 000 people) very low quality of evidence was not an impediment to obtain innovativeness.
bmjopen-2020-041259supp002.pdf (181.3KB, pdf)
Discussion
The present study analysed the new AIFA approach to appraise innovativeness for medicines. The appraisal process relies on three criteria: unmet therapeutic need, added therapeutic value and quality of clinical evidence assessed with GRADE method. Despite the growing interest in this new appraisal process, there is still no evidence on the role played by the three criteria on the final decision, if these criteria have been consistently used overtime and if other variables do influence the innovativeness status. We found that added therapeutic value was the most influential parameter, followed by quality of evidence, whereas unmet therapeutic need had a quite limited impact on the final appraisal. It seems that a high-unmet therapeutic need is perceived as a prerequisite of innovativeness that drives the decision to apply for innovativeness, instead of being the driver of the appraisal process. Notwithstanding in five cases the unmet need had a poor rating, since its evaluation is not straightforward.13 We investigated the potential role of other variables—namely the characteristics of the drugs and the evidence provided—that is, whether there is a systematic correlation between these variables and innovativeness status. Some relationships were found: for example, a larger proportion of drugs for rare diseases were appraised innovative. However, the statistical significance of these relationships is not reached. We have also investigated the general accuracy of the appraisal process. Despite the high level of discretion left to the Scientific Committee in appraising the unmet need and the added therapeutic value, this process looked generally coherent.
Relying on a structured, transparent and replicable value framework to appraise new medicines is a much debated topic. Value frameworks for health technologies have been investigated by the literature14 and huge efforts have been made to define clinical value frameworks in specific therapeutic areas, such as cancer drugs.15 Despite there is a general consensus that unmet need and clinical value are important value domains, it is still a matter of debate whether a threshold for minimum clinical value (meaningful clinical benefit) should be set and used by regulatory authorities,16 as well as how other domains should be considered (eg, patient-reported outcomes and acceptability to patients) and how different domains could be aggregated to support operationally pricing based on value.17 18
Other European countries have relied on a formal appraisal of added therapeutic value. This is done, for example, in France and Germany where all new drugs and indications are appraised and added therapeutic value is ranked in five and six levels, respectively.1 Ranks are used for price/discount negotiations. In France, the absolute benefit is ranked too and used to take decisions on reimbursement (introduction in the positive list and copayment). There is evidence on the coherence between ranks attributed in the two countries to the same medicine,19 consistency between these rankings and other way of measuring added value by Health Tecnhology Assessment (HTA) organisations (eg, between the added therapeutic value rank in France and quality-adjusted life years gained in England20) and scientific societies21 and the role played by the added therapeutic value in price/discount negotiation.22 Italy is the only country in Europe where (1) innovativeness status is appraised on the grounds of a ranked unmet need, added therapeutic value and quality of the evidence, (2) innovative medicines are provided a speeder market access and dedicated funds, and (3) added therapeutic value rank is not used in price negotiation. As a consequence, our results, besides being the first one published on the Italian case, cannot be fully compared with that of our countries.
The study has some limitations. First, it is based on a quite small number of appraisals. This did not allow to analyse possible different patterns of association between the three innovativeness criteria and the type of innovativeness (ie, fully or conditionally innovative). Only the availability of a larger number of innovativeness appraisals will allow to address this issue.
As already mentioned, innovativeness appraisals can be requested by the companies or spontaneously carried out by AIFA. The information on the applicant was not available and no stratified analysis could be performed, despite it would have been very interesting. We could analyse only the final appraisal published by AIFA, but we did not have any access to the applications submitted by the companies. This implies that the results of the present study cannot be considered a predictor of the response by AIFA to the applicant. However, our analysis was aimed at evaluation of the key drivers and the consistency of the AIFA decision-making process, rather than the comparison of applications submitted by the companies and final decision of AIFA.
Despite the above-mentioned limitations, our analysis has some important implications. Companies are pushed to provide solutions with an added therapeutic value and a high quality of evidence, since the latter are the driver of innovativeness, which brings important advantages for market access. We are aware that investments by the pharmaceutical companies are taken globally, but the more HTA agencies insist on clear and transparent criteria to appraise new medicines, the higher will be the impact on the management of pipelines by the pharmaceutical companies.
The new process implemented by AIFA is also consistent with the need to rely on a prespecified value framework enhancing transparency, accountability and, because of its intrinsic consistency, predictability of innovativeness appraisals.
Last but not least, prioritisation of access through innovativeness is managed transparently, on the grounds of quite objective criteria and providing the whole stakeholders with the rationale of decision taken.
Conclusion
To date, the new Italian innovativeness appraisal framework looked generally coherent and can be considered an important step towards a more transparent and evidence-based management of access to medicines in Italy. In the future, the process could be further enhanced, for example, including in a more structured framework patient-reported outcome measures, which role is still debated, whereas at present the appraisal process mostly relies on clinical variables, and proving for an interaction between innovativeness (and its domains) appraisals and price negotiation.
Supplementary Material
Acknowledgments
The authors thank L Benedan for her editorial assistance and M Iannantuoni for providing graphic design assistance.
Footnotes
Contributors: CG, PB and CJ designed the study and developed the methods. CG and PB reviewed the literature. CG performed data analysis. CG, PB and CJ contributed to data interpretation. CG prepared the tables and figures. CG and CJ drafted the manuscript. PB provided critical review of the manuscript. All authors have reviewed and approved the final version of the manuscript for publication.
Funding: This research was partially funded by a grant from Celgene to Statinfo.
Competing interests: CG is a senior consultant at Statinfo. CJ and PB have received a consultant fee from Celgene as scientific consultants for the project.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository. Appraisal reports on innovativeness are publicly downloadable from the AIFA’s website at https://www.aifa.gov.it/farmaci-innovativi.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Panteli D, Arickx F, Cleemput I, et al. Pharmaceutical regulation in 15 European countries review. Health Syst Transit 2016;18:1–122. [PubMed] [Google Scholar]
- 2.Jommi C, Minghetti P. Pharmaceutical pricing policies in Italy : Babar Z-U-D, Pharmaceutical prices in the 21st century. Adis, Cham: Springer, 2015: 131–50. [Google Scholar]
- 3.AIFA Criteri per la classificazione dei farmaci innovativi e dei farmaci oncologici innovativi ai sensi dell’articolo 1, comma 402, della legge 11 dicembre 2016, n. 232 2017;519. [Google Scholar]
- 4.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortinguerra F, Tafuri G, Trotta F, et al. Using grade methodology to assess innovation of new medicinal products in Italy. Br J Clin Pharmacol 2020;86:93–105. 10.1111/bcp.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scavone C, Capuano A, Rossi F. New criteria of Italian medicine agency for the Attribution of therapeutic innovation: viewpoint of the pharmacologist. G Ital di Farm e Farm 2017;9:5–12. [Google Scholar]
- 7.Urbinati D, Rova A, Cioni L. PHP83 - THE IMPACT OF THE NEW INNOVATIVE ALGORITHM IN ITALY. Value in Health 2018;21:S164 10.1016/j.jval.2018.09.977 [DOI] [Google Scholar]
- 8.Sligh S. PHP274 - ITALY’S NEW PHARMACEUTICAL INNOVATION RANKING SYSTEM: KEY CRITERIA FOR SUCCESSFULLY ACHIEVING INNOVATIVE STATUS. Value in Health 2018;21:S196 10.1016/j.jval.2018.09.1168 [DOI] [Google Scholar]
- 9.Meremetidis A, Ferrario M, Giuliani G. PHP61 - THE NEW AIFA INNOVATION FRAMEWORK TO RECOGNIZE INNOVATIVE DRUGS: A PRELIMINARY ANALYSIS OF KEY DRIVERS OF EVALUATION. Value in Health 2018;21:S160 10.1016/j.jval.2018.09.955 [DOI] [Google Scholar]
- 10.Di Marzio S. E l’AIFA tracciò la strada dell’innovatività. AboutPharma 2017;148:28–30. [Google Scholar]
- 11.Agenzia Italiana del Farmaco. Farmaci innovativi.
- 12.The GRADE Working Group Grade Handbook. Handbook for grading the quality of evidence and the strength of recommendations using the grade approach, 2013. [Google Scholar]
- 13.Vreman RA, Heikkinen I, Schuurman A, et al. Unmet medical need: an introduction to definitions and stakeholder perceptions. Value Health 2019;22:1275–82. 10.1016/j.jval.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 14.Towse A, Barnsley P. Approaches to identifying, measuring, and aggregating elements of value. Int J Technol Assess Health Care 2013;29:360–4. 10.1017/S0266462313000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherny NI, de Vries EGE, Dafni U, et al. Comparative assessment of clinical benefit using the ESMO-magnitude of clinical benefit scale version 1.1 and the ASCO value framework net health benefit score. J Clin Oncol 2019;37:336–49. 10.1200/JCO.18.00729 [DOI] [PubMed] [Google Scholar]
- 16.Grössmann N, Del Paggio JC, Wolf S, et al. Five years of EMA-approved systemic cancer therapies for solid tumours-a comparison of two thresholds for meaningful clinical benefit. Eur J Cancer 2017;82:66–71. 10.1016/j.ejca.2017.05.029 [DOI] [PubMed] [Google Scholar]
- 17.Jommi C, Armeni P, Costa F, et al. Implementation of value-based pricing for medicines. Clin Ther 2020;42:15–24. 10.1016/j.clinthera.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 18.Sussex J, Towse A, Devlin N. Operationalizing value-based pricing of medicines : a taxonomy of approaches. Pharmacoeconomics 2013;31:1–10. 10.1007/s40273-012-0001-x [DOI] [PubMed] [Google Scholar]
- 19.Ruof J, Schwartz FW, Schulenburg J-M, et al. Early benefit assessment (EBA) in Germany: analysing decisions 18 months after introducing the new AMNOG legislation. Eur J Health Econ 2014;15:577–89. 10.1007/s10198-013-0495-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drummond M, de Pouvourville G, Jones E, et al. A comparative analysis of two contrasting European approaches for rewarding the value added by drugs for cancer: England versus France. Pharmacoeconomics 2014;32:509–20. 10.1007/s40273-014-0144-z [DOI] [PubMed] [Google Scholar]
- 21.Li J, Vivot A, Alter L, et al. Appraisal of cancer drugs: a comparison of the French health technology assessment with value frameworks of two oncology societies. Expert Rev Pharmacoecon Outcomes Res 2020;20:405–9. 10.1080/14737167.2019.1635458 [DOI] [PubMed] [Google Scholar]
- 22.Theidel U, von der Schulenburg J-MG. Benefit assessment in Germany: implications for price discounts. Health Econ Rev 2016;6:1–12. 10.1186/s13561-016-0109-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041259supp001.pdf (139.4KB, pdf)
bmjopen-2020-041259supp002.pdf (181.3KB, pdf)